Altered cAMP-Dependent Protein Kinase A in Platelets of Patients With Obsessive-Compulsive Disorder
Abstract
OBJECTIVE: The purpose of this study was to assess cAMP-dependent protein kinase A in patients with obsessive-compulsive disorder (OCD). METHOD: The levels and the activity of protein kinase A were evaluated in whole platelets obtained from 12 unmedicated patients with OCD and 15 healthy comparison subjects. RESULTS: The immunolabeling of protein kinase A regulatory subunits type I and II were significantly greater but that of the catalytic subunit significantly lower in patients with OCD than in healthy subjects. The cAMP-stimulated activity in patients with OCD was significantly lower than that in healthy subjects. CONCLUSIONS: These data suggest a possible role of protein kinase A in the pathophysiology of OCD.
There is evidence for the involvement of cAMP signaling in both the mechanism of action of antidepressant medications and the pathophysiology of affective disorders (1, 2).
Protein phosphorylation has a key role in cAMP signaling because most of the intracellular events mediated by cAMP occur through the activation of protein kinase A, a serine-threonine kinase, which in turn phosphorylates specific substrate proteins (3). Animal studies have shown that protein kinase A itself and some of its substrates are altered following administration of antidepressants, including selective serotonin reuptake inhibitors (SSRIs) (4–6). Moreover, alterations in the cAMP-dependent phosphorylation system have been reported in patients with affective disorders (7–10). However, it remains to be elucidated whether these abnormalities are specifically related to affective disorders or may also be present in other psychiatric disorders. To address this question, in this study we examined the protein kinase A in platelets from unmedicated patients with obsessive-compulsive disorder (OCD) and healthy comparison subjects.
METHOD
The study group included 12 male patients at the Department of Neuroscience, University of Turin. All patients met DSM-III-R criteria for OCD and had been assessed with the Yale-Brown Obsessive Compulsive Scale (their mean total score was 22.1, SD=3.0). They had no current comorbid axis I or axis III illnesses and had never received fluoxetine or other SSRIs. The comparison group included 15 physically healthy men who had no history of any mental or medical disorders. All subjects had been free of any drug treatment for at least 1 month before the study.
The two groups did not significantly differ in age (mean age of the patients with OCD was 30.67 years, SD=4.8; mean age of the healthy subjects was 31.07 years, SD=6.6). After a complete description of the study to the subjects, written informed consent was obtained.
A morning blood sample (50 ml) was obtained from each subject. Platelets were isolated and processed as described previously (7, 10). Platelet proteins were subjected in duplicate to SDS-PAGE and transferred to nitrocellulose membranes. Patients’ samples were always run on the same gel with healthy subjects. Immunoblotting experiments were performed as described elsewhere (10). Briefly, membranes were incubated in TBST buffer pH 7.6 containing 1% of BSA in the presence of monoclonal antibodies against the regulatory subunits type I and type II and the catalytic subunit of protein kinase A (dilution: 1/500) and actin (dilution: 1/3,500).
Protein kinase A activity was measured as described elsewhere (8). Assay was carried out at 30°C in a final volume of 50 µl incubation mixture containing 100 mM Pipes pH 6.9, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 20 µl/ml aprotinin, 20 µl/ml leupeptin, 0.5 mM PMS-F, 0.25 mg/ml BSA, 75 µM Kemptide, 10 µg platelet proteins, 1 mM IBMX, 1 µM cAMP, and 10 µM PKI as appropriate. Following a 1-minute preincubation period, the reaction was initiated by addition of 100 µM γ-32P[ATP] (100 cpm/pmol) and terminated after 1 minute by addition of 10% TCA. The mixture (20 µl) was spotted onto P81 phosphocellulose paper. The filters were washed several times with 75 mM of phosphoric acid. Incorporated radioactivity was measured by liquid scintillation.
Comparisons between groups were performed by using two-tailed Student’s t tests followed by Bonferroni correction when appropriate. Data are expressed as means and standard deviations.
RESULTS
Figure 1 shows representative immunoblots of protein kinase A subunits and actin in whole platelet homogenates from two patients with OCD and two healthy subjects. The immunolabeling of regulatory subunits type I and II was significantly higher in the patients with OCD than in the healthy subjects. The immunolabeling of the catalytic subunit was significantly lower in patients with OCD than in the healthy subjects. Immunostaining for actin showed no difference in the levels of this cytoskeletal protein between the two groups (table 1).
To investigate whether the altered levels of protein kinase A could reflect functional changes, the activity of protein kinase A was measured in the whole platelets of both groups. Although the basal activity of the enzyme did not significantly differ between groups (t=1.19, df=22, p=0.25), the cAMP-stimulated activity was significantly diminished in patients with OCD compared with healthy subjects (table 1).
DISCUSSION
Our data show that patients with OCD have significantly higher levels of platelet regulatory subunits type I and II and lower levels of the catalytic subunit than healthy subjects. Moreover, cAMP-stimulated activity was significantly diminished in patients with OCD with respect to healthy subjects. It has been reported (3) that altered protein kinase A levels are generally accompanied by modifications in its activity. Given that all of our assays were carried out in the same patients, the abnormal protein kinase A activity seems to be caused by the altered levels of this enzyme. These findings suggest that protein kinase A is affected in patients with OCD. To our knowledge, this is the first time that such a connection has been demonstrated between OCD and proteins involved in cAMP signaling.
We ruled out the possibility that the observed alterations could be the consequence of pharmacological treatments because all of the patients and healthy subjects had been medication free for at least 1 month. However, given early data reporting that protein kinase A is altered by SSRIs (4–6) and the well-documented efficacy of these medications in the treatment of OCD (11), prospective studies in this area will be of interest in testing whether SSRIs could elicit normalization of protein kinase A in these patients. Such an investigation might suggest a possible explanation for the observation that the duration, dose, and response rate of SSRIs differ when used to treat patients with OCD and those with depression (11).
The mechanism(s) of our results and whether they reflect a primary condition or a secondary response to neurohormonal perturbations remain unclear. It is unlikely, however, that the altered protein kinase A levels are caused simply by unspecific variables or effects on platelet proteins because the levels of actin, a cytoskeletal protein used as an internal control, did not differ between groups.
Although the involvement of protein kinase A in the pathophysiology of OCD seems potentially clear, we are aware that considerable caution is necessary when extrapolating from data obtained in peripheral cells to the brain. The main limitation of peripheral models is that blood cells are exposed to a different environment, including circulating catecholamines and hormones. Taking this limitation into account, we think that our findings are in line with those of previous studies showing abnormalities of cAMP signaling in patients with affective disorders. Nevertheless, it should be emphasized that the components of such systems seem to be altered in different ways in patients with bipolar disorder (10), major depression (8), or OCD.
In conclusion, these findings suggest that protein kinase A is altered in patients with OCD. Further replication in a larger group of subjects will help to clarify the relevance of these preliminary data in the pathophysiology of OCD.
Received Dec. 23, 1998; revision received July 15, 1999; accepted Sept. 2, 1999. From the Istituto Scientifico H. San Raffaele, Department of Neuropsychiatric Sciences, Milan; the Center of Neuropharmacology, Institute of Pharmacological Sciences, University of Milan; Istituto di Ricovero e Cura a Carretere Scientifico (IRCCS) Centro San Giovanni di Dio-Fatebenefratelli, Ospedale Sacro Cuore, Brescia; and the Department of Neuroscience, University of Turin, Italy. Address reprint requests to Dr. Perez, Istituto Scientifico H. San Raffaele, Department of Neuropsychiatric Sciences, Via Luigi Prinetti, 29, 20127 Milan, Italy; [email protected] (e-mail). Supported by grant M2511 from the Istituto Scientifico H. San Raffaele, Milan; Dr. Tardito was a fellow of the IRCCS Centro San Giovanni di Dio-Fatebenefratelli. The authors thank Dr. Marco Battaglia for statistical advice and Dr. Marco Falzolgher for skillful technical assistance.
 |
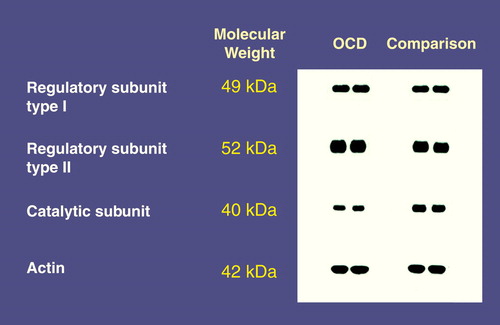
FIGURE 1. Representative Immunoblots of Protein Kinase A Subunits and Actin From Two Patients With OCD and Two Healthy Comparison Subjects
1. Warsh JJ, Li PP: Second messenger system and mood disorders. Current Opinion in Psychiatry 1996; 9:23–29Crossref, Google Scholar
2. Duman RS, Heninger GR, Nestler EJ: A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54:597–606Crossref, Medline, Google Scholar
3. Spaulding SW: The ways in which hormones change cyclic adenosine 3",5"-monophosphate-dependent protein kinase subunits, and how such changes affect cell behavior. Endocrinol Rev 1993; 14:632–650Medline, Google Scholar
4. Perez J, Tinelli D, Bianchi E, Brunello N, Racagni G: cAMP binding proteins in the rat cerebral cortex after administration of selective 5-HT and NE reuptake blockers with antidepressant activity. Neuropsychopharmacology 1991; 4:57–64Medline, Google Scholar
5. Mori S, Garbini S, Caivano M, Perez J, Racagni G: Time-course changes in rat cerebral cortex subcellular distribution of the cyclic-AMP binding after treatment with selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol 1998; 1:3–10Crossref, Medline, Google Scholar
6. Duman RS: Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry 1998; 44:324–335Crossref, Medline, Google Scholar
7. Perez J, Zanardi R, Mori S, Gasperini M, Smeraldi E, Racagni G: Abnormalities of cAMP-dependent endogenous phosphorylation in platelets from patients with bipolar disorder. Am J Psychiatry 1995; 152:1204–1206Google Scholar
8. Shelton RC, Manier DH, Sulser F: cAMP-dependent protein kinase activity in major depression. Am J Psychiatry 1996; 153:1037–1042Google Scholar
9. Rahman S, Li PP, Young LT, Kofman O, Kish SJ, Warsh JJ: Reduced [3H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem 1997; 68:297–304Crossref, Medline, Google Scholar
10. Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R: Abnormalities of cyclic adenosine monophosphate signaling in platelets from untreated patients with bipolar disorder. Arch Gen Psychiatry 1999; 56:248–253Crossref, Medline, Google Scholar
11. Goodman WK, McDougle CJ, Price LH: Pharmacotherapy of obsessive compulsive disorder. J Clin Psychiatry 1992; 53(4 suppl):29–37Google Scholar



