Reduced Basal Ganglia Volumes After Switching to Olanzapine in Chronically Treated Patients With Schizophrenia
Abstract
OBJECTIVE: A follow-up study of patients with schizophrenia was conducted to examine change in striatal volumes and extrapyramidal symptoms after a change in medication. METHOD: Thirty-seven patients with schizophrenia and 23 healthy volunteers were examined. Patients at baseline receiving typical antipsychotics (N=10) or risperidone but exhibiting limited response (N=13) were switched to treatment with olanzapine. Patients receiving risperidone and exhibiting a good response (N=14) continued treatment with risperidone. Caudate, putamen, and pallidal volumes were assessed with magnetic resonance imaging. The Extrapyramidal Symptoms Rating Scale was used to assess clinical signs and symptoms. RESULTS: At baseline, basal ganglia volumes in patients treated with typical antipsychotics were greater than in healthy subjects (putamen: 7.0% larger; globus pallidus: 20.7% larger). After the switch to olanzapine, putamen and globus pallidus volumes decreased (9.8% and 10.7%, respectively) and did not differ from those of healthy subjects at the follow-up evaluation. Akathisia was also reduced. In the patients receiving risperidone at baseline, basal ganglia volumes did not differ between those exhibiting good and poor response, and no significant volume changes were observed in subjects with poor risperidone response after the switch to olanzapine treatment. CONCLUSIONS: Olanzapine reversed putamen and globus pallidus enlargement induced by typical antipsychotics but did not alter volumes in patients previously treated with risperidone. Changes in striatal volumes related to typical and atypical antipsychotics may represent an interactive effect between individual medications and unique patient characteristics.
Antipsychotic medications are known to alter the structure and metabolism of basal ganglia in humans and animals (1–7). In patients with schizophrenia, typical antipsychotics induce striatal enlargement, particularly in the caudate, putamen, and globus pallidus (7–9). In contrast, the majority of studies of patients switched from typical antipsychotics to clozapine report decreases in caudate volume (6, 10–13). The effects in humans of alternate atypical antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone) are less understood. In one published study, long-term administration of risperidone did not induce striatal enlargement (7). The effects seen in animal studies are inconsistent. In rats, both increases and decreases in striatal volumes occur after administration of either haloperidol or clozapine (14, 15). In comparison, long-term administration of olanzapine in rats decreases striatal volumes (15).
The effects of antipsychotic medications on basal ganglia metabolism may vary depending on medication type and patient characteristics (16, 17). Haloperidol does not reduce striatal glucose metabolism and differentially reduces cortical glucose metabolism, with nonresponsive patients experiencing decreases and responsive patients experiencing no changes in metabolic activity (18). In contrast, a study of clozapine and fluphenazine showed that both medications decreased striatal and cingulate glucose metabolism, with female subjects experiencing a greater change in glucose utilization than male subjects (19). Risperidone was also reported to decrease striatal glucose metabolism after 6 weeks of treatment (20). Parallel rat studies showed that haloperidol has little effect on neuronal activity levels in the striatum, whereas risperidone and clozapine decrease activity in the substantia nigra reticulata nucleus of the basal ganglia system in a dose-dependent manner, thus differentially affecting the output of the basal ganglia system (21). Despite their potential for differential effects, few studies have made direct drug-to-drug comparisons.
We performed two separate studies to examine in chronically treated schizophrenia patients the effects on striatal volumes of switching from treatment with either typical antipsychotics or risperidone to olanzapine. In the first study, patients were switched from typical antipsychotic medications to olanzapine and compared with healthy volunteers. Caudate, putamen, and pallidal volumes were expected to decrease after switching from typical antipsychotics to olanzapine. The severity of extrapyramidal symptoms was also expected to decrease after switching. In the second study, all subjects were taking risperidone at baseline. Subsequently, a subgroup was switched to olanzapine. The decision to switch treatments was based on clinical evaluation of the patients’ overall clinical response to risperidone. Olanzapine is pharmacologically more similar to clozapine than risperidone (22), suggesting that the effects of olanzapine on basal ganglia volumes may be similar to those of clozapine (10, 13, 23). Additionally, olanzapine is less likely to induce extrapyramidal symptoms compared with risperidone at comparable doses, as is clozapine (24–26). In the second study, basal ganglia volumes were expected to decrease after switching from risperidone to olanzapine, as were extrapyramidal symptoms.
Method
Subjects
Thirty-seven patients with DSM-IV schizophrenia and 23 healthy comparison subjects were included in this study. No subjects in this study received adjunct treatments for mood disorders during the course of treatment. The baseline scans of 15 patients were included in a previous study, and both baseline and outcome scans were reported previously for 17 of the healthy comparison subjects (7). Subjects were recruited through the Nova Scotia Early Episode Psychosis Program in Halifax. Approval was obtained from the Dalhousie University Ethics Committee. Informed written consent was obtained from all subjects. Exclusion criteria were a history of significant head injury or loss of consciousness exceeding 5 minutes, a history of facial or nasal trauma, a history of DSM-IV substance abuse, a current diagnosis of substance abuse during treatment or at follow-up, a history of seizure disorder, or a family history of psychotic disorders. Patients were reassessed after a mean interval of 45.6 weeks.
Treatment and Clinical Measures
Clinical assessments included the Positive and Negative Syndrome Scale (27) and the Extrapyramidal Symptom Rating Scale, a comprehensive rating of extrapyramidal symptoms and signs (28). Global scores on the Extrapyramidal Symptom Rating Scale subscales are reported. Interrater reliability for clinical measures based on intraclass correlations (ICCs) were high (Positive and Negative Syndrome Scale: ICC=0.85; Extrapyramidal Symptom Rating Scale: ICC=0.89). All ratings were performed by trained clinicians (L.C.K. and Heather M. Milliken, M.D., F.R.C.P.C.).
The first study investigated the effects of switching from typical antipsychotics (loxapine, trifluoperazine, chlorpromazine, fluphenazine, haloperidol) to olanzapine. Some patients were receiving additional anticholinergic medications to ameliorate extrapyramidal symptoms at baseline (Table 1). No patients required anticholinergic agents at follow-up.
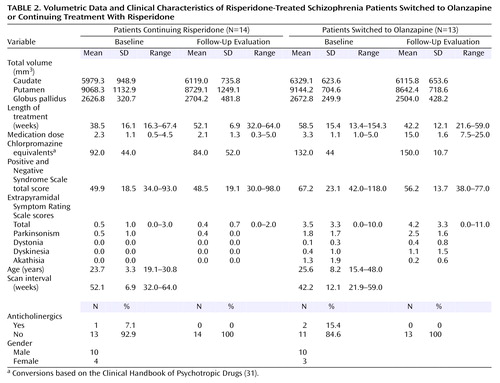
Both patient groups in the second study were being treated with low to moderate doses of risperidone at baseline (Table 2). Thirteen patients were switched to olanzapine treatment, and 14 continued receiving risperidone. Medications were switched on the basis of a clinical evaluation by a psychiatrist. Those patients switched from risperidone to olanzapine exhibited more severe extrapyramidal symptoms and more severe psychiatric symptoms at baseline compared with those who continued risperidone treatment (Table 2). Complete follow-up clinical assessments were available for 10 of the 14 patients who continued risperidone and 11 of the 13 patients switched to olanzapine.
No patients in either study required mood stabilizing or antidepressant medications.
Scanning and Measurement Protocols
Subjects were scanned with a Siemens Magnetom Vision 1.5-Tesla MRI scanner. An inversion recovery sequence in the coronal plane was obtained. The inversion recovery sequence was obtained as follows: TR/TE=2000/20 msec, field of view=200 mm, matrix=168×256 pixels. A total of 18 slices, 4 mm thick with a 1-mm interslice gap, were available for this sequence. Inversion recovery images were chosen for their superior white-gray tissue contrast. The white-to-gray pixel intensity for the images obtained with the inversion recovery sequence was 1.42, which compared favorably with three-dimensional volumetric spoiled gradient recall acquisition (pulse sequence) data from the same scanner that had a pixel intensity ratio of only 0.89. A trained rater made manual selections for all regions of interest using interactive shareware (NIH Image 1.62 pcc) (29). Selections were made based on Duvernoy’s atlas of the human brain (30). Measurements began two slices anterior to and ended two slices posterior to the anterior commissure slice to cover a total distance of 25 mm in the anterior commissure-posterior commissure plane (Figure 1). Anatomically, this protocol excluded the anterior-most portion of the head of the caudate (5-mm depth), the tail of the caudate, and the posterior-most putamen. Similarly, the posterior-most globus pallidus was excluded. The rater was blind to diagnosis, treatment, gender, and time of scan. All measures were repeated four times. Final volumes were calculated on the mean of four repetitions to reduce the possibility of rater error. Volumes were calculated on absolute slice thickness across all five slices. Total brain volumes were assessed from axial slices. Axial slices were obtained with a T2-weighted sequence; TR/TE=4000/90 msec, field of view=220 mm, and matrix=238×256 pixels. Slice thickness for all T2-weighted images was 5 mm with a 1-mm interslice gap, 22 slices were obtained in each T2-weighted plane. Digitized slices were measured using a Macintosh G4 PowerMac computer. All scans were reviewed by a neuroradiologist (J.S.L.). Intrarater reliability for all regions was greater than 0.90 (caudate: ICC=0.98; putamen: ICC=0.96; globus pallidus: ICC=0.97; total intracranial volume: ICC=0.99).
Data Analysis: Statistical Methods
Initial comparisons of left and right striatal volumes did not reveal any significant left-right asymmetries, therefore subsequent analyses were based on total (left plus right) volumes. An initial ANOVA of total brain volume did not reveal any differences between groups. Comparisons of the effects of treatment over time were made with a repeated measures analysis of variance, with time (baseline, follow-up) and region (caudate, putamen, globus pallidus) as within-subject factors, and group (schizophrenia patients, healthy subjects [first study]; continued with risperidone, switched to olanzapine [second study]) as a between-subject factor. Multivariate analyses of covariance (MANCOVAs) were performed to compare basal ganglia volumes between groups at baseline or at follow-up, using the factor group, the covariates intracranial volume and age at time of scan, and the dependent measures caudate, putamen, and globus pallidus volumes.
For analysis of changes in extrapyramidal symptoms, paired t tests were used with the Extrapyramidal Symptom Rating Scale data, with Bonferroni alpha set at p=0.01 to control for five comparisons (total score, parkinsonism, dyskinesia, dystonia, and akathisia). Additional descriptive statistics of anticholinergic usage at follow-up were tabulated.
Results
Schizophrenia Patients Versus Healthy Comparison Subjects
At baseline, patients treated with typical antipsychotic drugs had overall larger basal ganglia structures than healthy comparison subjects (Wilks’s lambda F=7.68, df=3, 27, p=0.0007). Differences were statistically significant for the putamen (7.0% larger, F=9.11, df=1, 29, p=0.005) and the globus pallidus (20.7% larger, F=24.06, df=1, 29, p=0.0001). For analysis of changes over time in patients after medication switch relative to comparison subjects, the MANCOVA indicated statistically significant effects of group (Wilks’s lambda F=5.05, df=3, 29, p=0.006), time (F=4.82, df=3, 29, p=0.008), and a group-by-time interaction (F=5.61, df=3, 29, p=0.004). As seen in Figure 2, basal ganglia volumes decreased over time in the patients switched from typical antipsychotics to olanzapine, while volumes remained steady in healthy comparison subjects. Subsequent analyses indicated volume decreases in the putamen (smaller by 9.8%) and globus pallidus (smaller by 10.7%) associated with change from typical antipsychotics to olanzapine. At follow-up, there were no statistically significant differences in basal ganglia volumes between patients and healthy comparison subjects.
At baseline, five of 10 patients being treated with typical antipsychotic agents were receiving adjunct anticholinergic medications (Table 1). While mean Extrapyramidal Symptom Rating Scale scores decreased following the switch to olanzapine, this was not statistically significant (t=1.82, df=9, p>0.10). However, at follow-up none of the 10 patients in this group were being treated with anticholinergic medications. Examination of individual Extrapyramidal Symptom Rating Scale subscores revealed a statistically significant decrease in akathisia scores (t=3.59, df=19, p=0.007) but not in parkinsonism, dystonia, or dyskinesia.
Risperidone Continuation Versus Switch to Olanzapine
As seen in Figure 3, basal ganglia volumes of risperidone-treated patients subsequently switched to olanzapine did not differ at baseline from those continuing treatment with risperidone (Wilks’s lambda F=0.55, df=3, 21, p=0.65). For analysis of changes over time, the MANCOVA indicated a significant effect of time (F=4.41, df=3, 23, p<0.02) but no statistically significant effects of group (Wilks’s lambda F=0.25, df=3, 23, p=0.86) or group-by-time interaction (F=2.87, df=3, 23, p=0.059). At follow-up, no statistically significant differences between the groups in overall basal ganglia volumes were observed (Wilks’s lambda F=1.04, df=3, 21, p=0.39).
Mean total Extrapyramidal Symptom Rating Scale scores at baseline in patients switched to olanzapine were higher than scores in patients continuing risperidone treatment (t=2.85, df=21, p=0.01) (Table 2). Extrapyramidal Symptom Rating Scale total scores at the follow-up evaluation did not significantly differ from baseline scores for either the patients continuing risperidone treatment (t=–1.00, df=9, p>0.34) or those switched to olanzapine (t=–0.37, df=10, p>0.70). Examination of the subscales of the Extrapyramidal Symptom Rating Scale did not reveal significant changes in any scores.
Discussion
As expected, treatment with typical antipsychotics was associated with larger basal ganglia volumes, and switching to olanzapine was associated with reduction in basal ganglia volumes. Specifically, the putamen and globus pallidus volumes were normalized following the switch to olanzapine. The pattern of regional changes differs somewhat from two earlier reports of the effects of switching from typical antipsychotic medications to clozapine. Following switching, Chakos et al. (3) reported a reduction in caudate volume in adult patients, and Frazier et al. (12) found reduction in both the caudate and the globus pallidus in childhood-onset schizophrenia patients. The regional inconsistencies may be a reflection of specific effects of previously administered typical antipsychotics or differences in effects of clozapine and olanzapine. The role of the putamen in both the presentation of schizophrenia-related symptoms and extrapyramidal symptoms is not fully understood. An earlier study by Stratta and colleagues (32) demonstrated a significant correlation of performance on the Wisconsin Card Sorting Test and left-sided putamen volume, suggesting a role in executive functioning, which is known to be affected in schizophrenia (33, 34).
Eight out of 10 patients receiving typical medications at baseline had movement disorders according to the Extrapyramidal Symptom Rating Scale. This scale covers a full range of potential motor abnormalities and is sensitive to subtle movement disorders (35). The mean total baseline Extrapyramidal Symptom Rating Scale scores were likely partially ameliorated by anticholinergic medication. These are most effective in treating tremors, rigidity, and bradykinesia but have low efficacy for treating antipsychotic-induced akathisia (36). The observed reduction in akathisia after the switch to olanzapine may be a true reflection of olanzapine’s low propensity to induce extrapyramidal symptoms.
In our second study, overall basal ganglia volumes did not differ between patients with good and poor responses to risperidone. Subsequent switching to olanzapine in those with a poor response to risperidone was not associated with a significant change in basal ganglia volume. This observation suggests the effects of olanzapine on basal ganglia volume in patients previously treated with typical antipsychotics represent normalization rather than atrophy.
Neither overall symptom severity nor overall extrapyramidal symptom severity changed when patients with a poor response to risperidone were switched to olanzapine. However, patients previously receiving typical antipsychotics did have moderate reductions in dyskinesia and akathisia. The extrapyramidal symptoms present in the group of patients poorly responsive to risperidone may be related to a different mechanism, perhaps intrinsic to schizophrenia, that is less responsive to switching to olanzapine than are extrapyramidal symptoms related to typical antipsychotic medications. Moreover, relative dosing with respect to D2 affinity before and after switching from risperidone to olanzapine remained relatively equal, thus there would be little expectation for a change in extrapyramidal symptom severity (37). The relationships among antipsychotic dose, striatal volume, and extrapyramidal symptom severity are not clear. Data from this study do not demonstrate any relationship of either total dose or current dose of antipsychotic medication being correlated with striatal volumes, change in striatal volumes, or severity of extrapyramidal symptoms scores (all exploratory regression analyses had r values <0.40 and corresponding p values >0.05). This suggests that striatal volume, while responsive to specific types of antipsychotic agents, does not affect the presence or severity of extrapyramidal symptoms.
Summary
The main findings of the present study were significant reductions in putamen and globus pallidus volumes in patients switched to olanzapine from typical antipsychotics. These subregional-specific results may be due to differential effects of olanzapine on striatal structures or of an unknown sampling bias of the subjects chosen for this study. As well, the results from the risperidone-to-olanzapine group may only be valid for those patients who have partial or poor risperidone response. Additional variance in the volumetric measures may have been related to MRI slice thickness and slice angulation. However, a comparison of volumes reported in other studies with thinner slices did not reveal any deviation in the volumes reported in this current study (5, 38).
Individual atypical antipsychotic agents exhibit specific neurochemical pathways of activity, and this specificity may contribute to differential volumetric changes in the striatum in response to antipsychotic challenge (15, 39). In a study of striatal volumes in rats, caudate or putamen volumes were significantly increased by chronic exposure to haloperidol and clozapine (15). In contrast, this same study found that chronic exposure to risperidone had no effect on caudate or putamen volumes whereas long-term exposure to olanzapine resulted in a significant decrease in striatal volumes (15). Additionally, a recent study employing fMRI in patients with schizophrenia demonstrated differential activation of the caudate and putamen during a cognitive challenge (40). Signal intensity was reduced in the putamen and anterior cingulate during testing in patients relative to healthy subjects, but not in the caudate. These findings are supported by differential responses to individual antipsychotic medications (41). These differences are likely related to different receptor-targeting profiles of specific antipsychotic agents (39). While the evidence for increased striatal volumes in humans or animals exposed to typical antipsychotic medications treatment is convincing (1, 3, 4, 6, 7, 10–12, 14, 15, 42), there are no data suggesting that clozapine increases striatal volumes. The majority of published reports in human subjects indicated that exposure to clozapine is associated with a reduction in striatal volumes in patients previously exposed to typical antipsychotics (12–14, 23). Studies of risperidone’s and olanzapine’s effects on striatal volumes after chronic administration are far fewer in number (7, 15). The findings from the current study in conjunction with those from animal studies suggest that closer examination in subpopulations of patients with schizophrenia is required to clarify the true physiological and clinical effects of individual antipsychotic medications in the treatment of schizophrenia.
 |
 |
Received May 30, 2003; revision received Dec. 29, 2003; accepted Jan. 9, 2004. From the Departments of Psychiatry and Radiology, University of British Columbia, Vancouver; and the Departments of Psychiatry and Radiology, Dalhousie University, Halifax, N.S., Canada; Address reprint requests to Dr. Lang, VGH Research Pavilion, Centre for Complex Disorders, West 10th Ave., Room 211–828, Vancouver, British Columbia, Canada V5Z 1L8; [email protected] (e-mail). Supported by a Canadian Institutes of Health Research Scientist Award to Dr. Honer and by a grant from the Norma Calder Foundation for Schizophrenia Research to Dr. Lang. Dr. Kopala was supported by a Clinical Scientist Award from Dalhousie University. Partial funding for MRI scanning was provided by investigator-initiated grants from Janssen-Ortho of Canada and Eli Lilly Canada. Additional funding for scanning was provided by the Queen Elizabeth-II Hospital Health Science Research Foundation and the Department of Psychiatry, Dalhousie University.The authors thank Melissa M. Butler, R.T.N.M., C.C.R.C.; Jason O. Brown, B.Sc., R.T.N.M.; Diana L. Sonnichsen, B.Sc., R.T.N.M.; Charlene A. Day, R.N., M.N.; Janet L. Gallant, R.N., B.Sc., C.N.; Heather M. Milliken, M.D., F.R.C.P.C.; and David Whitehorn, Ph.D., M.Sc.N., for their assistance with this study.
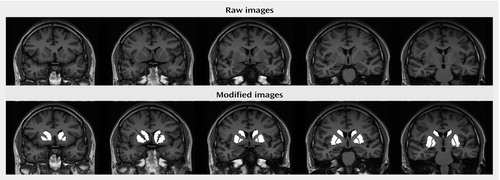
Figure 1. Sample Set of Manually Selected Striatal Regions for Volumetric Measures
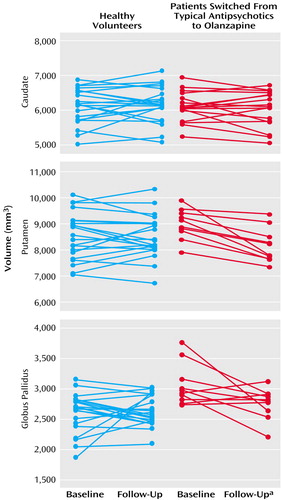
Figure 2. Baseline and Follow-Up Basal Ganglia Volumes of Healthy Volunteers and Patients With Schizophrenia Switched From Typical Antipsychotic Treatment to Olanzapine
aSignificant decrease from baseline in putamen volume (F=6.62, df=1, 31, p<0.02) and globus pallidus volume (F=6.02, df=1, 31, p=0.02).
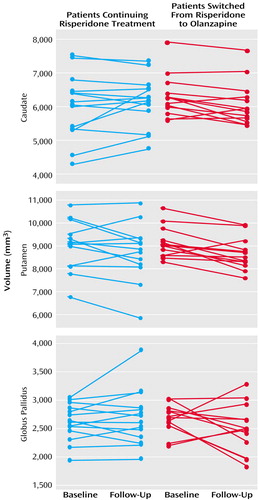
Figure 3. Baseline and Follow-Up Basal Ganglia Volumes of Risperidone-Treated Schizophrenia Patients Switched to Olanzapine or Continuing Treatment With Risperidone
1. Benes FM, Paskevich PA, Domesick VB: Haloperidol-induced plasticity of axon terminals in rat substantia nigra. Science 1983; 221:969–971Crossref, Medline, Google Scholar
2. Benes FM, Paskevich PA, Davidson J, Domesick VB: The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res 1985; 265–274Google Scholar
3. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Link, Google Scholar
4. Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA: Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry 1998; 44:675–684Crossref, Medline, Google Scholar
5. Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O’Donnell BF, Jolesz FA, McCarley RW: Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61:209–229Crossref, Medline, Google Scholar
6. Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
7. Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Honer WG: An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am J Psychiatry 2001; 158:625–631Link, Google Scholar
8. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
9. Raz S, Raz N: Structural brain abnormalities in the major psychoses: a quantitative review of the evidence from computerized imaging. Psychol Bull 1990; 108:93–108Crossref, Medline, Google Scholar
10. Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
11. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200–1204Abstract, Google Scholar
12. Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL: Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry 1996; 153:564–566Link, Google Scholar
13. Scheepers FE, de Wied CC, Pol HE, van de Flier W, van der Linden JA, Kahn RS: The effects of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology 2001; 24:47–54Crossref, Medline, Google Scholar
14. Lee H, Tarazi FI, Chakos M, Wu H, Redmond M, Alvir JM, Kinon BJ, Creese I, Lieberman JA: Effects of chronic treatment with typical and atypical antipsychotic drugs on the rat striatum. Life Sci 1999; 65:1595–1602Crossref, Google Scholar
15. Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA: Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology 2002; 27:143–151Crossref, Medline, Google Scholar
16. Colangelo V, Di Grezia R, Passarelli F, Musicco M, Pontieri FE, Orzi F: Differential effects of acute administration of clozapine or haloperidol on local cerebral glucose utilization in the rat. Brain Res 1997; 768:273–278Crossref, Medline, Google Scholar
17. Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA: Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alterations in regional brain metabolism. J Pharmacol Exp Ther 2000; 293:8–14Medline, Google Scholar
18. Bartlett EJ, Brodie JD, Simkowitz P, Schlösser R, Dewey SL, Lindenmayer J-P, Rusinek H, Wolkin A, Cancro R, Schiffer W: Effect of a haloperidol challenge on regional brain metabolism in neuroleptic-responsive and nonresponsive schizophrenic patients. Am J Psychiatry 1998; 155:337–343Link, Google Scholar
19. Cohen RM, Nordahl TE, Semple WE, Pickar D: The brain metabolic patterns of clozapine- and fluphenazine-treated female patients with schizophrenia: evidence of a sex effect. Neuropsychopharmacology 1999; 21:632–640Crossref, Medline, Google Scholar
20. Liddle PF, Lane CJ, Ngan ET: Immediate effects of risperidone on cortico-striato-thalamic loops and the hippocampus. Br J Psychiatry 2000; 177:402–407Crossref, Medline, Google Scholar
21. Bruggenman R, Westerink BHC, Timmerman W: Effects of risperidone, clozapine and haloperidol on extracellular recordings of substantia nigra reticulata neurons of the rat brain. Eur J Pharmacol 1997; 324:49–56Crossref, Medline, Google Scholar
22. Keck PE Jr, McElroy SL: Clinical pharmacodynamics and pharmacokinetics of antimanic and mood-stabilizing medications. J Clin Psychiatry 2002; 63(suppl 4):3–11Google Scholar
23. Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS: Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry 2001; 158:644–646Link, Google Scholar
24. Sacritstan JA, Gomez JC, Ferre F, Gascon J, Perez Bravo A, Olivares JM: Incidence of extrapyramidal symptoms during treatment with olanzapine, haloperidol and risperidone: results of an observational study. Actas Esp Psiquiatr 2001; 29:25–32Medline, Google Scholar
25. Tarsy D, Baldessarini RJ, Tarazi FI: Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002; 16:23–45Crossref, Medline, Google Scholar
26. Miller CH, Mohr F, Umbricht D, Woerner M, Fleishchaker WW, Lieberman JA: The prevalence of acute extrapyramidal signs and symptoms in patients treated with clozapine, risperidone, and conventional antipsychotics. J Clin Psychiatry 1998; 59:69–75Crossref, Medline, Google Scholar
27. Kay SR, Opler LA, Fiszbein A: Positive and Negative Syndrome Scale (PANSS). Toronto, Multi-Health Systems, 1987Google Scholar
28. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
29. Rasband W: NIH Image. Rockville, Md, National Institutes of Health, 1997Google Scholar
30. Duvernoy HM: The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. New York, Springer-Verlag Wien, 1991Google Scholar
31. Bezchlibnyk-Butler KZ, Jeffries JJE: Clinical Handbook of Psychotropic Drugs. Toronto, Hogrefe & Huber, 1999Google Scholar
32. Stratta P, Mancini F, Mattei P, Daneluzzo E, Casacchia M, Rossi A: Association between striatal reduction and poor Wisconsin Card Sorting Test performance in patients with schizophrenia. Biol Psychiatry 1997; 42:816–820Crossref, Medline, Google Scholar
33. Townsend LA, Malla AK, Norman RMG: Cognitive functioning in stabilized first-episode psychosis patients. Psychiatry Res 2001; 104:119–131Crossref, Medline, Google Scholar
34. Bryson G, Whelahan HA, Bell M: Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res 2001; 102:29–37Crossref, Medline, Google Scholar
35. De Deyn PP, Wirshing WC: Scales to assess the efficacy and safety of pharmacologic agents in the treatment of behavioral and psychological symptoms of dementia. J Clin Psychiatry Suppl 2001; 21:19–22Google Scholar
36. Lima AR, Weiser KVS, Bacaltchuk J, Barnes TRE: Anticholinergics for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 2004;(1):CD003727Google Scholar
37. Kapur S, Zipursky RB, Remington G: Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 1999; 156:286–293Abstract, Google Scholar
38. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Link, Google Scholar
39. Sakai K, Gao XM, Hashimoto T, Tamminga CA: Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse 2001; 39:152–160Crossref, Medline, Google Scholar
40. Rubia K, Russell T, Bullmore ET, Soni W, Brammer M, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T: An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res 2001; 52:47–55Crossref, Medline, Google Scholar
41. Miller DD, Andreasen NC, O’Leary DS, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology 1997; 17:230–240; correction, 1998; 18:323–324Google Scholar
42. Dean B, Hussain T, Scarr E, Pavey G, Copolov DL: Extended treatment with typical and atypical antipsychotic drugs differential effects on the densities of dopamine D2–like and GABAA receptors in rat striatum. Life Sci 2001; 69:1257–1268Crossref, Medline, Google Scholar



