Age-Related Cognitive Deficits Mediated by Changes in the Striatal Dopamine System
Abstract
OBJECTIVE: The study examined the influence of losses in dopaminergic function on age-related cognitive deficits. METHOD: Eleven healthy subjects (21–68 years of age) completed a set of cognitive tasks used to assess perceptual speed and episodic memory. D2 receptor binding was measured in the caudate and the putamen by using positron emission tomography. RESULTS: A gradual age-related deterioration was found for all cognitive tasks and for D2 binding in both striatal structures. Statistical control of D2 binding eliminated the age-related cognitive variation, whereas residual effects of D2 binding were seen after the analysis controlled for age. CONCLUSIONS: D2 receptor binding is a more important factor than chronological age in accounting for variation in cognitive performance across the adult lifespan. Changes in dopaminergic neurotransmission play an important role in aging-related cognitive decline.
Several cognitive functions decline in aging, including speed of processing (1) and episodic memory (2). The striatum is a particularly interesting brain region in which to examine biological correlates of age-related cognitive changes. From early to late adulthood, decreases in striatal dopaminergic function average 6%–10% per decade, as indicated by various postmortem indices (3) and positron emission tomography (PET) markers. The D2 receptor subtype has been most extensively investigated in studies of age-related changes in the dopamine system (4).
Volkow and colleagues (5) demonstrated parallel age-related reductions for D2 receptor binding in the caudate and the putamen and for tasks assessing speed and executive functions. Further, a relationship between age-related loss of D1 receptors and motor deficits has been demonstrated (6). In the study reported here we conducted further examination of the extent to which PET-based measures of D2 binding in the striatum account for age-related cognitive deficits. We were particularly interested in determining the relative importance of age and D2 binding to performance in tasks assessing perceptual speed and episodic memory.
METHOD
Six men and five women (21–68 years of age) participated in the study. Written informed consent was obtained from the subjects after the nature and possible risks of the study were fully explained. Results of medical histories, physical examinations, and laboratory screening indicated that all subjects were healthy. The screening procedures have been described in more detail elsewhere (7). The study was approved by the ethics committee of the Karolinska hospital.
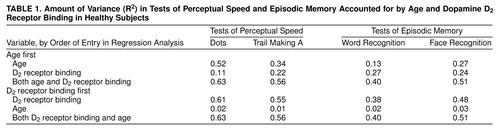
Dots (8) and Trail Making Test A (9) were used to assess perceptual speed. The Dots test consisted of six trials, each of which lasted 60 seconds. During each trial, subjects were presented with 342 configurations of dots in which the number of dots varied from three to five. Subjects were asked to cross off the configurations that included four dots. The total number of correctly marked configurations in all six trials was scored. In Trail Making Test A, the task was to connect serially 25 circles containing numbers 1–25 that were distributed on a sheet of paper. Completion time was registered. Tests of word and face recognition were used to assess episodic memory (10). In these tests, subjects made yes-no recognition judgments for items presented in the experimental session, and d′ served as the outcome measure.
The brain imaging procedure has been described in detail elsewhere (7). Regions of interest were delineated on T2-weighted magnetic resonance imaging images (GE Signa, 1.5 tesla) for volumetric measurements. To calculate regional brain radioactivity, the regions of interest for the dorsal caudate, the dorsal putamen, and the cerebellum were transformed to the corresponding PET images (ECAT EXACT HR47, Siemens CTI) obtained after intravenous injection of the radioligand [11C]raclopride with high specific radioactivity (>35 GBq/mol). The binding potential was calculated using the transient equilibrium analysis with the cerebellum as reference region.
RESULTS
For all cognitive tasks, the means and indicators of variability were highly representative of normal adult samples (1, 2). Increasing age was associated with decreasing performance in all tasks. Although the age-cognition correlations were generally high, not all were statistically significant at the level of p<0.10 (for Dots, r=–0.72, df=9, p<0.02; for Trail Making Test A, r=0.58, df=9, p<0.10; for face recognition, r=–0.52, df=9, p<0.10; for word recognition, r=–0.36, df=9, p>0.10). These results likely reflect low statistical power, although other explanations are possible.
There were systematic age-related reductions of D2 binding in the caudate (r=–0.75, df=9, p<0.01) and putamen (r=–0.87, df=9, p<0.001). By contrast, age was weakly related to caudate (r=–0.09) and putamen (r=–0.12) volumes. Because the D2 data for the caudate and the putamen were strongly correlated (r=0.98, df=9, p<0.001), a composite D2 score was used in subsequent analyses.
Regression analyses were performed in which age and the D2 composite were included as predictors of cognitive performance (Table 1). Although age contributed strongly to performance, D2 added substantial variance when entered second. Most interestingly, initial entry of D2 effectively eliminated the influence of age on performance.
DISCUSSION
This study extends earlier observations of a relationship between age-related reductions in dopamine activity and cognitive decline (5). Most importantly, although the four cognitive tasks were selected because of their age sensitivity (1, 2), D2 emerged as the stronger predictor of performance across all tasks. Thus, age-related changes in the striatal dopamine system serve as a powerful mediator of the cognitive losses that occur during the normal aging process.
Although the age-related cognitive variation was absorbed after controlling for D2 binding, the reverse was not true. Residual D2-related cognitive variation after partialing out age has been reported previously (5). This finding suggests that the variability in D2 demonstrated in age-homogeneous samples (11) may be related to cognitive performance.
Age-related reductions have also been documented for the D1 receptor (6) and the dopamine transporter (12). Thus the relationship between age, dopamine, and cognition observed in this study may generalize across multiple pre- and postsynaptic markers of the dopamine system.
The striatum has been implicated in perceptual and cognitive speed (13). However, its role in episodic memory is less well established. Evidence indicates that the prefrontal cortex is critically involved in episodic memory (14). Thus our finding that episodic memory was strongly related to D2 binding may indicate a functional influence of dopaminergic neurotransmission through the parallel cortical-striatal-pallidal-thalamic circuits that form a basis for information processing in the brain (15). However, patients with focal striatal lesions show deficits in tasks (e.g., executive and memory tasks) that are traditionally thought to draw on the integrity of the prefrontal cortex (16). Thus the strong relationship observed between D2 binding and memory performance may reflect a direct influence of striatal function on higher cognitive abilities.
Received April 12, 1999; revisions received Aug. 11, Oct. 12, and Dec. 15, 1999; accepted Dec. 22, 1999. From the Department of Psychology, Uppsala University; Stockholm Gerontology Research Center and the Department of Clinical Neuroscience and Family Medicine, Geriatrics and Psychiatry Sections, Karolinska Institute, Stockholm, Sweden; and the Department of Psychology, University of Victoria, Victoria, Canada. Address reprint requests to Dr. B㢫man, Department of Psychology, Uppsala University, Box 1225, SE-751 42 Uppsala, Sweden; [email protected] (e-mail). Supported by grants from the Swedish Council for Research in the Humanities and the Social Sciences (Dr. B㢫man) and from NIMH and the Swedish Medical Research Council (Dr. Farde). The assistance of the PET center at the Karolinska hospital is gratefully acknowledged.
 |
1. Salthouse TA: The processing-speed theory of adult age differences in cognition. Psychol Rev 1996; 103:403–428Crossref, Medline, Google Scholar
2. Kausler DH: Learning and Memory in Normal Aging. San Diego, Academic Press, 1994Google Scholar
3. Scherman D, Desnos C, Darchen F, Pollack P, Javoy-Agid F, Agid Y: Striatal dopamine deficiency in Parkinson’s disease: role of aging. Ann Neurol 1989; 26:551–557Crossref, Medline, Google Scholar
4. Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E: Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab 1993; 13:310–314Crossref, Medline, Google Scholar
5. Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J: Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155:344–349Link, Google Scholar
6. Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ: Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse 1998; 30:56–61Crossref, Medline, Google Scholar
7. Ito H, Hietala J, Blomqvist G, Halldin C, Farde L: Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab 1998; 18:941–950Crossref, Medline, Google Scholar
8. Ekberg K, Hane M: Test battery for investigating functional disorders—the TUFF battery. Scand J Work Environ Health 1984; 10:14–17Medline, Google Scholar
9. Reitan RM, Davison LA: Clinical Neuropsychology: Current Status and Applications. Washington, DC, VH Winston & Sons, 1984Google Scholar
10. B㢫man L, Forsell Y: Episodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive support. J Abnorm Psychol 1994; 103:361–370Crossref, Medline, Google Scholar
11. Farde L, Hall H, Pauli S, Halldin C: Variability in D2 dopamine receptor density and affinity: a PET study with [11C]raclopride in man. Synapse 1995; 20:200–208Crossref, Medline, Google Scholar
12. van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Charney DS, Hoffer PB: Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. J Nucl Med 1995; 36:1175–1181Google Scholar
13. Crosson BA: Subcortical Functions in Language and Memory. New York, Guilford, 1992Google Scholar
14. Wheeler MA, Stuss DT, Tulving E: Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc 1995; 1:525–536Crossref, Medline, Google Scholar
15. Gerfen CR: The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science 1989; 246:385–388Crossref, Medline, Google Scholar
16. Weniger G, Markowitsch HJ, Irle E: Anterograde and retrograde mnemonic deficits after unilateral damage of neostriatal, ventral striatal, and basal forebrain structures. Neurocase 1995; 1:231–238Crossref, Google Scholar



