Cognitive Deficit Induced by Acute Tryptophan Depletion in Patients With Alzheimer’s Disease
Abstract
OBJECTIVE: The study assessed the effects on global cognitive function and mood of a reduction of brain serotonin by means of acute tryptophan depletion in 16 patients with dementia of the Alzheimer type and in 16 cognitively intact comparison subjects. METHOD: In a double-blind, crossover design, subjects received a tryptophan-free amino acid drink to induce acute tryptophan depletion and, on a separate occasion, a placebo drink containing a balanced mixture of amino acids. On each occasion, ratings of depressed mood were made at baseline and 4 and 7 hours later, and the Modified Mini-Mental State was administered at baseline and 4 hours later. RESULTS: Patients with dementia of the Alzheimer type had a significantly lower mean score on the Modified Mini-Mental State after acute tryptophan depletion than after receiving placebo. The comparison group showed no difference in mean score on the Modified Mini-Mental State after acute tryptophan depletion and after receiving placebo. No significant changes in mood were found in either group. CONCLUSIONS: Acute tryptophan depletion significantly impaired cognitive function in patients with dementia of the Alzheimer type. Compromised serotonergic function, in combination with cholinergic deficit, may make an important contribution to cognitive decline in dementia of the Alzheimer type.
Dementia of the Alzheimer type is associated with cholinergic loss, but levels of other neurotransmitters, particularly serotonin (5-HT), are abnormal in dementia of the Alzheimer type and may contribute to both cognitive and noncognitive features of the disorder.
Acute tryptophan depletion by dietary means reduces central 5-HT levels and 5-HT function (1). In young, healthy volunteers acute tryptophan depletion has been shown to cause impairment in learning (2).
We examined the effect of 5-HT depletion induced by acute tryptophan depletion in patients with dementia of the Alzheimer type and in healthy comparison subjects. We hypothesized that acute tryptophan depletion would cause impairment in cognitive function and that the impairment would be potentiated in dementia of the Alzheimer type by the preexisting cholinergic deficit. We also measured the effect of 5-HT depletion on mood to determine whether changes in cognitive function could be secondary to mood changes induced by acute tryptophan depletion.
METHOD
Subjects were receiving no medication known to affect the 5-HT system, had no personal or family history of depression, and had no history of serious coexistent physical illness. Subjects with dementia of the Alzheimer type were recruited from hospital outpatient clinics and day hospitals. They had a computerized tomography scan and full laboratory tests to screen for problems that could cause dementia and met criteria for probable Alzheimer’s disease outlined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (3). Comparison subjects were matched for age, sex, and years of education, were cognitively intact, and had scored 90 or more on the Cambridge Mental Disorders of the Elderly Examination (4). They were recruited from among spouses of the patients. After subjects and their caretakers were given a complete description of the study, their written informed consent was obtained. Subjects were warned of the possibility of a transient lowering of mood and worsening of performance on tests of learning and memory. The study was approved by the Newcastle and North Tyneside Health Authority Joint Ethics Committee.
Subjects were tested twice, at least 1 week apart, in a double-blind, placebo-controlled, balanced-order, crossover design. Subjects fasted overnight, and at 9:00 a.m., they completed baseline measures and then received either a placebo drink consisting of a nutritionally balanced mixture of 52 g of amino acids (2) or a drink that was identical except that it contained no tryptophan. Subjects remained at rest in the laboratory for 7 hours. Blood was taken at baseline and after 4 and 7 hours; the Montgomery-ijberg Depression Rating Scale (5) and Geriatric Depression Scale (6) were administered at the same three time points. At 4 hours, the Modified Mini-Mental State was administered (7).
Plasma and an ultrafiltrate were stored at –20˚C. Free plasma tryptophan and total plasma tryptophan were measured using high-performance liquid chromatography (8). Intra- and interassay coefficients of variation were 3.4% and 4.4%, respectively, for free tryptophan and 3.3% and 4.4%, respectively, for total tryptophan.
Subjects’ scores on the Modified Mini-Mental State were analyzed by repeated measures analysis of variance (ANOVA) with treatment (depletion or placebo) as a within-subject variable and order of the study conditions (placebo first or acute trytophan depletion first) and study group as between-subject variables. For analysis of the data from the mood rating scales and of the data on free and total tryptophan, time was added as a within-subject variable. The reported p values of all ANOVAs used the Huynh-Feldt correction factor.
Scores on the Modified Mini-Mental State and mood rating scales at 4 hours were also analyzed for each group separately using post hoc t tests for paired samples (two-tailed). Where a significant group effect was found, post hoc t tests for independent samples were used to compare the groups at equivalent time points, under equivalent experimental conditions. These data are reported as means with standard deviations and 95% confidence intervals (CIs).
RESULTS
Sixteen subjects with dementia of the Alzheimer type and 16 comparison subjects completed the study. The subjects with dementia consisted of 10 women and six men with a mean age of 75.0 years (SD=5.7) and a mean score of 60.3 (SD=9.8) on the Cambridge Mental Disorders of the Elderly Examination. The comparison subjects consisted of eight women and eight men with a mean age of 73.1 years (SD=4.9) and a mean score of 99.4 (SD=3.7) on the Cambridge Mental Disorders of the Elderly Examination. An additional female comparison subject withdrew after the first visit because of side effects (nausea). There were no significant differences between the groups in age, sex, or years of education.
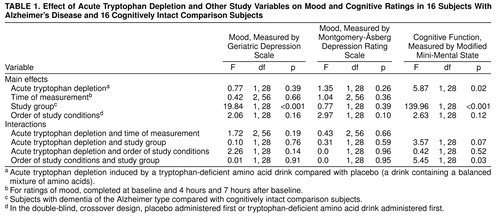
A considerable depletion of free plasma tryptophan was recorded at 4 and 7 hours after subjects ingested the tryptophan-deficient drink. Free plasma tryptophan decreased by an average of 73% and 62%, at 4 and 7 hours, respectively, in the comparison group and by an average of 69% and 62%, respectively, in the group with dementia of the Alzheimer type. Total tryptophan levels followed a similar pattern. Repeated measures ANOVA showed a significant effect of tryptophan depletion on mean score on the Modified Mini-Mental State (Table 1) and a significant effect of group on mean score on the Modified Mini-Mental State but no significant interaction of tryptophan depletion and group. In the group with dementia of the Alzheimer type, mean score on the Modified Mini-Mental State was significantly lower after tryptophan depletion (mean=55.7, SD=14.8) than after ingestion of placebo (mean=60.3, SD=13.8) (t=2.38, df=15, p=0.03; 95% CI=0.48–8.64). In the comparison group, there was no significant difference between mean score on the Modified Mini-Mental State after tryptophan depletion (mean=96.0, SD=4.3) and after ingestion of placebo (mean=96.6, SD=3.1) (t=0.74, df=15, p=0.47; 95% CI=–2.19 to 1.07). In both the placebo and depletion conditions, mean scores on the Modified Mini-Mental State were, as expected, significantly lower in the group with Alzheimer’s disease (data not given). There was a significant interaction between order of the study conditions and study group (Table 1), which probably reflected a learning effect in the group with Alzheimer’s disease. Scores on the mood rating scales showed no significant effects of tryptophan depletion, time, or order of the study conditions and no significant interactions between tryptophan depletion and time (Table 1). There was a significant effect of group on score on the Geriatric Depression Scale (Table 1). Post hoc paired t tests showed significantly lower scores on the Geriatric Depression Scale in the comparison group (t=3.27, df=30, p=0.003).
DISCUSSION
Acute tryptophan depletion produced a robust reduction in both free and total plasma tryptophan consistent with that previously described in the literature (2). Reduction of plasma tryptophan gave rise to a significant reduction in score on the Modified Mini-Mental State in subjects with dementia of the Alzheimer type, but not in the cognitively intact comparison group. The mean difference in score on the Modified Mini-Mental State after acute tryptophan depletion compared with placebo was 4.6 points, or 8% of the placebo score, which is clinically significant. We found no change in mood after tryptophan depletion or after placebo in either group, suggesting that vulnerability to depressive illness in patients with dementia of the Alzheimer type may not be solely secondary to reduced 5-HT function.
l-Tryptophan is also metabolized to kynurenic acid and quinolinic acid, which influence neural transmission mediated by l-glutamate (9). However, if this process was a significant factor in the results reported here, it is more likely that the low-tryptophan state would improve rather than worsen cognitive function. Rosse et al. (10) have demonstrated an improvement in cognitive function after diet-induced tryptophan depletion in schizophrenia. It is notable that the study by Rosse et al., like our study, demonstrated changes in cognitive function in the absence of mood changes.
Cholinergic neurotransmission has been clearly linked to cognitive processes, and animal evidence suggests an additive effect on certain mnemonic processes of combined cholinergic and serotonergic lesions (11). We propose that, in subjects with dementia of the Alzheimer type but not in comparison subjects, the induced reduction in 5-HT function potentiated impairment caused by a preexisting cholinergic deficit.
Studies of supplementation with l-tryptophan in dementia of the Alzheimer type have failed to demonstrate any consistent improvement in cognitive function (12), and large studies of treatment with selective serotonin reuptake inhibitors (SSRIs) in dementia of the Alzheimer type have failed to show clear improvement in cognitive function (13). Serotonergic strategies may fail to improve cognitive function because they are ineffective in the presence of impaired cholinergic function. Although it is likely that some patients have received a combination of cholinesterase inhibitors and SSRIs, we know of no systematic evaluation of such a strategy. Our data suggest that such a combined replacement strategy may be useful.
Received Jan. 27, 1999; revisions received July 15 and Aug. 30, 1999; accepted Sept. 28, 1999. From the Academic Department of Psychiatry, University of Newcastle upon Tyne; Institute for the Health of the Elderly, Newcastle General Hospital, Newcastle upon Tyne. Address reprint requests to Dr. O’Brien, Academic Department of Psychiatry, University of Newcastle upon Tyne, Royal Victoria Infirmary, Queen Victoria Road, Newcastle upon Tyne, England NE1 4LP; j.t.o’[email protected] (e-mail). This study was supported by an award from Lilly Education. The authors thank Mel Leitch for measurement of plasma tryptophan and Alastair Gray for assistance in data collection.
 |
1. Nishizawa S, Benkelfat C, Young SN, Leyton N, Mzengeza S, de Montigny C, Blier P, Diksic M: Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA 1997; 94:5308–5313Google Scholar
2. Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, Cowen PJ: Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 1994; 33:575–588Crossref, Medline, Google Scholar
3. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
4. Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, Goddard R: CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986; 149:698–709Crossref, Medline, Google Scholar
5. Montgomery SA, ijberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
6. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49Crossref, Medline, Google Scholar
7. Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48:314–318Medline, Google Scholar
8. Marshall EF, Kennedy WN, Eccleston D: Whole blood serotonin and plasma tryptophan using high-pressure liquid chromatography with electrochemical detection. Biochem Med Metab Biol 1987; 37:81–86Crossref, Medline, Google Scholar
9. Freese AKJ, Schwartz KJ, During MJ, Martin JB: Kynurenine metabolites of tryptophan: implications for neurologic diseases. Neurology 1990; 40:691–695Crossref, Medline, Google Scholar
10. Rosse RB, Swartz BL, Zlotolow S, Banay-Schwartz M, Trinidad AC, Peace TD, Deutsch SI: Effect of a low-tryptophan diet as an adjuvant to conventional neuroleptic therapy in schizophrenia. Clin Neuropharmacol 1992; 15:129–141Crossref, Medline, Google Scholar
11. Steckler T, Sahgal A: The role of serotonergic-cholinergic interactions in the mediation of cognitive behaviour. Behav Brain Res 1995; 67:165–199Crossref, Medline, Google Scholar
12. Whitford GM: Alzheimer’s disease and serotonin: a review. Neuropsychobiology 1986; 15:133–142Crossref, Medline, Google Scholar
13. Gottfries CG, Nyth AL: Effect of citalopram, a selective 5-HT reuptake blocker, in emotionally disturbed patients with dementia. Ann NY Acad Sci 1991; 640:276–279Crossref, Medline, Google Scholar



