Meta-Analysis of Regional Brain Volumes in Schizophrenia
Abstract
OBJECTIVE: The authors’ goal was to determine whether patients with schizophrenia differ from comparison subjects in regional brain volumes and whether these differences are similar in male and female subjects. METHOD: They conducted a systematic search for structural magnetic resonance imaging (MRI) studies of patients with schizophrenia that reported volume measurements of selected cortical, subcortical, and ventricular regions in relation to comparison groups. They carried out a meta-analysis of the volumes of these regions in the patients with schizophrenia and the comparison subjects using a random effects model; they also used random effects regression analysis to examine the influence of gender on effect sizes. RESULTS: Fifty-eight studies were identified as suitable for analysis; these studies included 1,588 independent patients with schizophrenia. Assuming a volume of 100% in the comparison group, they found that the mean cerebral volume of the subjects with schizophrenia was smaller (98%), but the mean total ventricular volume of the subjects with schizophrenia was greater (126%). Relative to the cerebral volume differences, the regional volumes of the subjects with schizophrenia were 94% in the left and right amygdala, 94% in the left and 95% in the right hippocampus/amygdala, and 93% in the left and 95% in the right parahippocampus. Relative to the global ventricular system differences, the largest differences in ventricular subdivisions were in the right and left body of the lateral ventricle, where the volumes of schizophrenic subjects were 116% and 116%, respectively. For most regions, effect size was not significantly related to gender. CONCLUSIONS: Regional structural differences in patients with schizophrenia include bilaterally reduced volume of medial temporal lobe structures. There is a need for greater integration of results from structural MRI studies to avoid redundant research activity.
Magnetic resonance imaging (MRI) has provided an important method for investigating structural brain changes in schizophrenia. However, there are several difficulties in interpreting the published literature. First, sample sizes have often been small, and, because the magnitudes of brain differences are also small, the results of individual studies may be unduly influenced by sampling variation. Second, the results of the studies have generally been reported with an emphasis on tests of significance rather than confidence intervals, and this may give the misleading impression of a large number of inconsistent positive or negative findings. Third, many different brain regions have been examined, but only a few of these have been measured in each individual study. Finally, the results of some studies have been reported in multiple publications.
Meta-analysis provides a method for integrating quantitative data from multiple studies. It has the advantages that 1) the results from individual studies are combined, improving the estimation of the overall effect size, 2) statistical power is increased, 3) confidence intervals for the overall effect size can be calculated, and 4) factors causing heterogeneity in the results reported by individual studies can be investigated (1).
Previous meta-analyses of structural imaging studies of schizophrenia have found that patients have higher ventricle-to-brain ratios (2), greater volumes of the lateral ventricles (3), reduced brain size (3, 4), reduced cross-sectional area of the corpus callosum (5), and reduced volumes of the bilateral temporal lobe (3), hippocampus (6), and amygdala-hippocampal complex (3, 6).
Our meta-analysis goes beyond the previous literature by 1) including new studies published up to August 1998, 2) including a wider range of regional structures in order to delineate the pattern of volume changes throughout the brain, and 3) using random effects models for the meta-analyses (7).
We have separated regional and global volume differences. In particular, we have addressed the following questions: 1) Are regional volume differences in cortical/subcortical structures in excess of the overall difference in cerebral volume? and 2) Are regional volume differences in ventricular subdivisions in excess of the overall difference in ventricular volume?
We have investigated two possible explanations for heterogeneity (8) in study results: gender and MRI study slice thickness. Finally, we have investigated the studies included in this meta-analysis using a statistical method developed to test for evidence of potential publication bias (9, 10).
METHOD
Study Ascertainment
Studies were considered for inclusion if 1) they were published before September 1998 as an article (rather than a letter or an abstract), 2) they compared a group of subjects with schizophrenia and a normal comparison group (either related or unrelated to the subjects; comparison groups consisting of patients with minor nonpsychiatric illnesses were also included), and 3) they reported volumetric brain MRI measurements of any structure considered in this meta-analysis (selected on the basis of a previous systematic review [3]). Since studies applied different criteria for measuring these structures, we followed operational definitions for including measurements within a particular category of structure; in some cases we also estimated cerebral volume and total ventricular volume measures from other volume measures (full details of the rules for data extraction and estimation are available on request).
If measurements were reported for both an unrelated and a related comparison group, then the former measurements were used. Studies reporting results on a patient group in which the majority of patients had a diagnosis of schizophrenia but some patients had related diagnoses (e.g., schizoaffective disorder) were also included. Since we were interested in gender-related effects, studies that reported separate results for male and female subjects were entered as separate studies.
Structure measurements were excluded if 1) insufficient data were reported to extract the number of subjects in each group or the mean or the standard deviation of the structure volumes, 2) the structure measurement was an area (from a single slice) rather than a volume (i.e., from multiple slices), 3) the measurement was on less than the complete or near-complete volume for the whole brain or brain lobes, 4) there were fewer than six subjects in either the schizophrenia group or the comparison group, 5) the measurements contributed to another publication (in which case the publication with the largest group size for the structure was selected), or 6) the mean age in either the schizophrenia or the comparison group was greater than 40 years. Studies of early-onset schizophrenia were included.
A systematic search strategy was used to identify relevant studies. First, we carried out computer searches of the databases MEDLINE, PSYCHLIT, and EMBASE. We searched the databases for papers published in the years 1980–1998 using the following Medical Subject Heading categories: magnetic resonance imaging or MRI and schizophrenia. A manual search was also conducted of the titles of published papers in the following psychiatric journals for the period January 1998 to August 1998: TheAmerican Journal of Psychiatry,Archives of General Psychiatry,Biological Psychiatry,British Journal of Psychiatry,Psychiatric Research,Psychological Medicine,Schizophrenia Bulletin, and Schizophrenia Research. Finally, we searched the reference lists of the studies identified for inclusion.
Statistical Analysis
Meta-analyses were performed in Microsoft Excel 7.0 and meta-regression analyses in STATA 5.0 (Stata Corp., College Station, Tex.).
As a measure of effect size, we used the ratio of the mean volume in the schizophrenia group divided by the mean volume in the comparison group. We chose this measure of effect size because it is more readily interpretable, in this context, than effect size measures based on the difference in means divided by a standard deviation (e.g., Cohen’s d).
We carried out a meta-analysis of the volume ratios of all the measured structures in the subjects with schizophrenia and the comparison subjects (absolute effect sizes) using a random effects model (7, 11). A random effects model assumes that the “true effect size” estimated by different studies differs between studies because of differences in samples or paradigms and that these true effect sizes have a normal distribution, i.e., that heterogeneity exists. In our analyses, we used the natural logarithms of brain ratios and converted the final results back to ratios. Thus, for a region R in study i (i=1, 2, 3,…k), we used the absolute effect size (ϕi) (where p refers to the patient group, c to the comparison group, m to the group regional volume mean, s to the group regional volume standard deviation, n to the group sample number, var to the variance, and nl to the natural logarithm):
The following equation assumes independence between mp,i and mc,i and applies the delta method (12):
For each region, we calculated Q, a homogeneity test statistic (7). In general, if Q exceeds the upper-tail critical value of chi-square on k–1 degrees of freedom, the observed variance in study effect sizes is significantly greater than expected under the null hypothesis that the two groups share a common population effect size. For comparison of our results with other meta-analyses, we also calculated an overall effect size using Cohen’s d for each region.
We also investigated whether regional volume differences exceeded global volume differences in the brain or ventricles (global-corrected effect sizes). For each region, we selected studies in which both regional data and global data (cerebral volume for cerebral regions and total ventricular volume for ventricular subdivisions) were available. We calculated the global-corrected effect size for a region as the overall effect size for the ratio of regional volume means (patient group volumes divided by comparison group volumes) divided by the overall effect size for the ratio of global volume means. Although we obtained point estimates for global-corrected effect sizes by this method, we were unable to calculate confidence intervals for these estimates because the covariances between regional and global volumes in each study were generally not reported. Thus for a region R inside a global structure G, the global-corrected effect size for R (ϕRcorr) was calculated as:
Sources of Effect Size Heterogeneity
For regions on which there were 10 or more contributory studies (and therefore reasonable power to detect an effect), we investigated gender and MRI slice thickness as potential sources of heterogeneity in study findings. We regressed absolute effect sizes against the percentage of female subjects with schizophrenia in each primary study and the MRI slice thickness used to measure the region. We used random effects regression analysis (8) (analogous to the random effects meta-analysis) implemented in STATA and adopted a significance level of p<0.05.
Statistical Evidence for Potential Publication Bias
To investigate the possibility of publication bias in study findings, we used the Egger test (9) implemented in STATA to test for the presence of an excess of low-precision studies giving effect sizes of magnitude greater than the average. We used this technique to investigate the absolute effect sizes when there were five or more contributory studies (we used a lower study number threshold than for the previous analyses because we did not know whether publication bias would be more important when the number of published studies was small). We adopted a significance level of p<0.05.
RESULTS
The articles identified for inclusion are given in table 1; full details of the data are given in a Microsoft Excel 7.0 file, available from a Web site (http://www.iop.kcl.ac.uk/home/depts/psychmed/wright/) or on request.
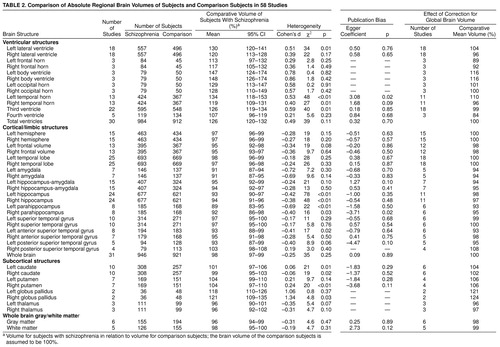
Absolute total ventricular volume was greater in the patients with schizophrenia than in the comparison subjects (assuming a comparison group volume of 100%, we found that the mean for patients with schizophrenia was 126% with a 95% confidence interval (CI) of 120%–132%). Absolute volumes of all ventricular subdivisions were also greater in patients with schizophrenia (table 2).
Relative to the global difference in total ventricular volume, there was a slightly greater enlargement of the left lateral ventricle (104%) than the right lateral ventricle (96%). Some ventricular subdivisions showed increases greater than the global difference: the body of the left (116%) and right (116%) lateral ventricle and the left temporal horn (110%). The relative difference in third ventricular volume (99%) was approximately in line with the global difference, and the relative difference in the fourth ventricle was less than the total difference (84%). The frontal horns also showed lower increases relative to the global difference (left 89%, right 92%).
Absolute whole brain volume was lower in the patients than the comparison subjects (98%) (table 2). Relative volumes of whole brain gray matter and white matter were approximately in line with the global difference: 98% for gray matter and 99% for white matter.
Relative to the global reduction of cerebral volume, there were no hemispheric differences (left 100%, right 100%). The frontal lobes were relatively small (left 98%, right 98%), but the temporal lobe volume differences were in line with the whole brain differences (left 100%, right 100%). Medial temporal lobe structures were relatively small: amygdala (left 94%; right 94%), hippocampus/amygdala (left 94%; right 95%), hippocampus (left 98%; right 97%), and parahippocampus (left 93%; right 95%). The left anterior superior temporal gyrus was also relatively small (93%).
The relative volumes of basal ganglia structures were higher in the subjects with schizophrenia: left caudate was 104%, right caudate was 102%, left putamen was 106%, right putamen was 106%, left globus pallidus was 121%, and right globus pallidus was 124%. Relative volumes of the thalamus were reduced: left thalamus was 96%, and right thalamus was 97%.
For the majority of structures, there was little evidence for a major gender effect modulating the volume differences in schizophrenia. Global ventricular enlargement was nonsignificantly greater in studies with a higher percentage of male patients (z=–1.93, N=30, p=0.05). We therefore calculated the absolute total ventricular volume difference in studies with only male patients or only female patients. In the 11 studies with only male patients, total ventricular volume was greater in the patients than in the comparison subjects by 130% (95% CI=118%–144%); in the five studies with only female patients it was greater by 116% (95% CI=105%–128%).
The only significant coefficients for regression of study effect size versus MRI slice thickness were for the left hemisphere (z=2.99, N=15, p=0.003), right hemisphere (z=3.27, N=15, p=0.001), and left temporal horn (z=1.99, N=13, p=0.05). Positive coefficients in the cases of the left and right hemispheres indicated that with more modern “thin-slice” MRI studies, a larger reduction in volume of these structures has been found in patients with schizophrenia than in comparison subjects; however, this finding depended on the outlying results of one study (49) and was not evident for cerebral volume (z=0.84, N=31, p=0.40). Therefore, it was likely to be due to chance. A positive coefficient in the case of the left temporal horn may indicate that older MRI studies using thicker slices overestimated the true difference in volume of this region in patients with schizophrenia and comparison subjects.
Table 2 lists the coefficient for the Egger publication bias test for each region. There were significant coefficients (in a direction indicating potential publication bias) at the p<0.05 level for the left temporal horn (t=2.61, df=11, p=0.02) and the right parahippocampus (t=–3.01, df=6, p=0.02). This indicated possible publication bias operating for these structures.
DISCUSSION
This meta-analysis demonstrates the enormous research effort that has been directed toward the investigation of structural brain changes in schizophrenia during the last decade. Some regions have been measured in almost 1,000 patients.
The results confirm the presence of global brain volume differences in schizophrenia: cerebral volume was lower (98%) and total ventricular volume was higher (126%) in patients with schizophrenia than in comparison subjects.
Relative to the global differences, the cerebral regions with the lowest volumes in the patients with schizophrenia were the left amygdala (overall mean global-corrected effect size=94%), right amygdala (94%), left hippocampus/amygdala (94%), right hippocampus/amygdala (95%), left parahippocampus (93%), right parahippocampus (95%), and left anterior superior temporal gyrus (93%). Intriguingly, the distribution of ventricular volume changes (maximal increases in the body of the lateral ventricle bilaterally) appeared to differ from the pattern of cerebral changes (maximal reductions in frontal and medial temporal regions bilaterally).
In this analysis, effect size was not significantly related to gender. The pattern of structural change in schizophrenia appears fairly similar in male and female patients and does not support a different pathological process in the two genders. For most regions there was little evidence that effect sizes were related to MRI slice thickness, providing reassurance that the pattern of regional volume changes detected has been independent of advances in MRI technology.
The findings also suggest several implications for the methodology of structural neuroimaging studies. Historically, the majority of neuroimaging studies have used relatively small samples to detect small effects (table 1). It is therefore important that the results of all studies conducted be made available in a form that permits their combination. At increasing levels of complexity, this may be achieved by 1) publishing summary results in a form that permits meta-analysis with explicit cross-referencing or exclusion of overlapping studies, 2) registration of study results in an international electronic database with a cumulative meta-analysis (71) of regional effect sizes, 3) provision of individual (anonymous) brain volume measurements for different regions to an electronic database—this would allow examination of effects such as laterality differences that are difficult to extract from summary statistics, 4) provision of the actual images to an electronic database—this would both facilitate the comparison of image analytic techniques and reduce “brain data wastage.”
Although the results of this meta-analysis indicated that regional volume reductions were present in the medial temporal lobes, there was also evidence for global volume changes in the ventricular system and cerebrum and for regional volume decreases in the left superior temporal gyrus, bilateral frontal lobes, and thalamus. These findings are consistent with a pathological process in schizophrenia involving distributed volume changes within the brain. However, studies employing region of interest measurements usually measure only a small number of selected brain regions because the measurement process is very labor-intensive; table 2 shows that most brain regions have been measured in only a fraction of the 1,588 patients included in this meta-analysis. Furthermore, region of interest studies are limited in their treatment of neocortical morphology because of the inherent difficulties in defining structurally complex and variable regions of cortex. This may give a distorted picture of the pattern of brain changes. Automated “brain averaging” (72, 73) or voxel-based methods (74) provide a complementary technique for investigating the “broader gestalt of morphologic deviation” (73). For example, these methods have indicated structural change in schizophrenia in the insula (74), which is a region not easily measured by region of interest studies.
It is important to consider several potential limitations of this study. As with all meta-analyses, the results are dependent on the quality of the primary studies. In addition, various types of publication bias may arise during publication of the primary studies (75). However, we did not detect evidence for publication bias for most brain regions, and we excluded duplicated results when there was evidence for previous publication. We did not test for laterality differences in brain volume measures because the statistical test requires extra information regarding the correlation between left and right regional volumes (76), and these data were not generally provided by the original studies. Finally, we cannot exclude the possibility that some of these differences were caused by confounding factors rather than the pathology of schizophrenia (e.g., antipsychotic medication causing basal ganglia volume increases [77]).
In summary, this meta-analysis demonstrated global structural differences between patients with schizophrenia and nonschizophrenic comparison subjects: cerebral volume was smaller and total ventricular volume was greater. Regional volume reductions in excess of these global differences were particularly marked in the bilateral medial temporal lobe regions. A general theory of the structural pathology of schizophrenia will need to explain both a complex pattern of cerebral changes and ventricular changes with a different spatial distribution. In the future, the collection of structural neuroimaging data in an electronic database may be valuable both in providing a powerful resource for testing hypotheses regarding the pathology of schizophrenia and in facilitating new structural analyses in large samples of patients and comparison subjects.
Received Feb. 24, 1999; revisions received July 6 and July 30, 1999; accepted Aug. 4, 1999. From the Department of Psychological Medicine, Institute of Psychiatry; the School of Psychiatry and Behavioural Sciences, University of Manchester, Manchester, U.K.; and the Department of Psychiatry, University of Cambridge, Cambridge, U.K. Address reprint requests to Dr. Wright, Department of Psychological Medicine, Institute of Psychiatry, De Crespigny Park, Denmark Hill, London, SE5 8AF, U.K. Dr. Wright and Dr. Bullmore were supported by the Wellcome Trust. The authors thank Dr. Z. Ellison and Dr. N. Takei for advice and Dr. R. Fukuda for translation of articles from Japanese.
 |
 |
1. Thompson SG, Smith TC, Sharp SJ: Investigating underlying risk as a source of heterogeneity in meta-analysis. Stat Med 1997; 16:2741–2758Google Scholar
2. Van Horn JD, McManus IC: Ventricular enlargement in schizophrenia: a meta-analysis of studies of the ventricle:brain ratio (VBR). Br J Psychiatry 1992; 160:687–697Crossref, Medline, Google Scholar
3. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998; 172:110–120Crossref, Medline, Google Scholar
4. Ward KE, Friedman L, Wise A, Schulz SC: Meta-analysis of brain and cranial size in schizophrenia. Schizophr Res 1996; 22:197–213Crossref, Medline, Google Scholar
5. Woodruff PW, McManus IC, David AS: Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 1995; 58:457–461Crossref, Medline, Google Scholar
6. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 1998; 55:433–440Crossref, Medline, Google Scholar
7. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188Crossref, Medline, Google Scholar
8. Berkey CS, Hoaglin DC, Mosteller F, Colditz GA: A random-effects regression model for meta-analysis. Stat Med 1995; 14:395–411Crossref, Medline, Google Scholar
9. Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629–634Crossref, Medline, Google Scholar
10. Galbraith R: A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988; 7:889–894Crossref, Medline, Google Scholar
11. Shadish WR, Haddock CK: Combining estimates of effect size, in The Handbook of Research Synthesis. Edited by Cooper H, Hedges LV. New York, Russell Sage Foundation, 1994, pp 265–275Google Scholar
12. Dunn G: Design and Analysis of Reliability Studies: The Statistical Evaluation of Measurement Errors. London, Edward Arnold, 1989Google Scholar
13. Kelsoe JR Jr, Cadet JL, Pickar D, Weinberger DR: Quantitative neuroanatomy in schizophrenia: a controlled magnetic resonance imaging study. Arch Gen Psychiatry 1988; 45:533–541Crossref, Medline, Google Scholar
14. Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
15. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
16. Becker T, Elmer K, Mechela B, Schneider F, Taubert S, Schroth G, Grodd W, Bartels M, Beckmann H: MRI findings in medial temporal lobe structures in schizophrenia. Eur Neuropsychopharmacol 1990; 1:83–86Crossref, Medline, Google Scholar
17. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
18. Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
19. Swayze VW II, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT: Structural brain abnormalities in bipolar affective disorder: ventricular enlargement and focal signal hyperintensities. Arch Gen Psychiatry 1990; 47:1054–1059Google Scholar
20. DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta SM, Henn FA, Anand AK: Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry 1991; 29:159–175Crossref, Medline, Google Scholar
21. Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL: Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry 1991; 48:881–890Crossref, Medline, Google Scholar
22. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
23. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JM, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531–537Crossref, Medline, Google Scholar
24. DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A: The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 1992; 31:241–254Crossref, Medline, Google Scholar
25. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
26. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
27. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195–205Crossref, Medline, Google Scholar
28. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236–246Crossref, Medline, Google Scholar
29. Kawasaki Y, Maeda Y, Urata K, Higashima M, Yamaguchi N, Suzuki M, Takashima T, Ide Y: A quantitative magnetic resonance imaging study of patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 1993; 242:268–272Crossref, Medline, Google Scholar
30. McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA: Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 1993; 50:190–197Crossref, Medline, Google Scholar
31. Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JMJ, Snyder PJ, Lieberman JA: Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry 1994; 151:1437–1447Google Scholar
32. DeLisi LE, Hoff AL, Neale C, Kushner M: Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res 1994; 12:19–28Crossref, Medline, Google Scholar
33. Gur RE, Mozley PD, Shtasel DL, Cannon TD, Gallacher F, Turetsky B, Grossman R, Gur RC: Clinical subtypes of schizophrenia: differences in brain and CSF volume. Am J Psychiatry 1994; 151:343–350Link, Google Scholar
34. Marsh L, Suddath RL, Higgins N, Weinberger DR: Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res 1994; 11:225–238Crossref, Medline, Google Scholar
35. Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, Di Michele V, Casacchia M: Magnetic resonance imaging findings of amygdala-anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Res 1994; 52:43–53Crossref, Medline, Google Scholar
36. Waldo MC, Cawthra E, Adler LE, Dubester S, Staunton M, Nagamoto H, Baker N, Madison A, Simon J, Scherzinger A, Drebing C, Gerhardt G, Freedman R: Auditory sensory gating, hippocampal volume, and catecholamine metabolism in schizophrenics and their siblings. Schizophr Res 1994; 12:93–106Crossref, Medline, Google Scholar
37. Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501–516Crossref, Medline, Google Scholar
38. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
39. Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O’Donnell BF, Jolesz FA, McCarley RW: Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61:209–229Crossref, Medline, Google Scholar
40. Vita A, Dieci M, Giobbio GM, Caputo A, Ghiringhelli L, Comazzi M, Garbarini M, Mendini AP, Morganti C, Tenconi F, Cesana B, Invernizzi G: Language and thought disorder in schizophrenia: brain morphological correlates. Schizophr Res 1995; 15:243–251Crossref, Medline, Google Scholar
41. Becker T, Elmer K, Schneider F, Schneider M, Grodd W, Bartels M, Heckers S, Beckmann H: Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res 1996; 67:135–143Crossref, Medline, Google Scholar
42. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554–1563Google Scholar
43. Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE: Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am J Psychiatry 1996; 153:799–805Link, Google Scholar
44. Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL: Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry 1996; 153:564–566Link, Google Scholar
45. Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL: Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry 1996; 153:355–361; correction, 153:851Link, Google Scholar
46. Kulynych JJ, Vladar K, Jones DW, Weinberger DR: Superior temporal gyrus volume in schizophrenia: a study using MRI morphometry assisted by surface rendering. Am J Psychiatry 1996; 153:50–56Link, Google Scholar
47. Lim KO, Tew W, Kushner M, Chow K, Matsumoto B, DeLisi LE: Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatry 1996; 153:1548–1553Google Scholar
48. Lim KO, Sullivan EV, Zipursky RB, Pfefferbaum A: Cortical gray matter volume deficits in schizophrenia: a replication. Schizophr Res 1996; 20:157–164Crossref, Medline, Google Scholar
49. Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR: Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res 1996; 22:27–40Crossref, Medline, Google Scholar
50. Woods BT, Yurgelun Todd D, Goldstein JM, Seidman LJ, Tsuang MT: MRI brain abnormalities in chronic schizophrenia: one process or more? Biol Psychiatry 1996; 40:585–596Google Scholar
51. Barr WB, Ashtari M, Bilder RM, Degreef G, Lieberman JA: Brain morphometric comparison of first-episode schizophrenia and temporal lobe epilepsy. Br J Psychiatry 1997; 170:515–519Crossref, Medline, Google Scholar
52. Fukuzako H, Yamada K, Kodama S, Yonezawa T, Fukuzako T, Takenouchi K, Kajiya Y, Nakajo M, Takigawa M: Hippocampal volume asymmetry and age at illness onset in males with schizophrenia. Eur Arch Psychiatry Clin Neurosci 1997; 247:248–251Crossref, Medline, Google Scholar
53. Hajek M, Huonker R, Boehle C, Volz HP, Nowak H, Sauer H: Abnormalities of auditory evoked magnetic fields and structural changes in the left hemisphere of male schizophrenics—a magnetoencephalographic-magnetic resonance imaging study. Biol Psychiatry 1997; 42:609–616Crossref, Medline, Google Scholar
54. Hinsberger AD, Williamson PC, Carr TJ, Stanley JA, Drost DJ, Densmore M, MacFabe GC, Montemurro DG: Magnetic resonance imaging volumetric and phosphorus 31 magnetic resonance spectroscopy measurements in schizophrenia. J Psychiatry Neurosci 1997; 22:111–117Medline, Google Scholar
55. Jacobsen LK, Giedd JN, Berquin PC, Krain AL, Hamburger SD, Kumra S, Rapoport JL: Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry 1997; 154:1663–1669Google Scholar
56. Lauriello J, Hoff A, Wieneke MH, Blankfeld H, Faustman WO, Rosenbloom M, DeMent S, Sullivan EV, Lim KO, Pfefferbaum A: Similar extent of brain dysmorphology in severely ill women and men with schizophrenia. Am J Psychiatry 1997; 154:819–825Link, Google Scholar
57. Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, Csernansky JG, DeMent S, Faustman WO, Sullivan EV, Pfefferbaum A: Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Arch Gen Psychiatry 1997; 54:1104–1112Google Scholar
58. Nopoulos P, Flaum M, Andreasen NC: Sex differences in brain morphology in schizophrenia Am J Psychiatry 1997; 154:1648–1654Google Scholar
59. Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H: A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin Neurosci 1997; 51:9–15Crossref, Medline, Google Scholar
60. Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY: Ziskind-Somerfeld Research Award 1996: medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry 1997; 41:1–14Crossref, Medline, Google Scholar
61. Torres IJ, Flashman LA, O’Leary DS, Swayze V II, Andreasen NC: Lack of an association between delayed memory and hippocampal and temporal lobe size in patients with schizophrenia and healthy controls. Biol Psychiatry 1997; 42:1087–1096Google Scholar
62. Woodruff PW, Wright IC, Shuriquie N, Russouw H, Rushe T, Howard RJ, Graves M, Bullmore ET, Murray RM: Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med 1997; 27:1257–1266Google Scholar
63. Yeo RA, Hodde Vargas J, Hendren RL, Vargas LA, Brooks WM, Ford CC, Gangestad SW, Hart BL: Brain abnormalities in schizophrenia-spectrum children: implications for a neurodevelopmental perspective. Psychiatry Res 1997; 76:1–13Crossref, Medline, Google Scholar
64. Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R: Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophr Res 1997; 26:85–92Crossref, Medline, Google Scholar
65. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049–1055Google Scholar
66. Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW: Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 1998; 155:774–778Abstract, Google Scholar
67. Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, Kikinis R, Donnino R, Jolesz FA, McCarley RW: Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 1998; 43:649–659Crossref, Medline, Google Scholar
68. Sharma T, Lancaster E, Lee D, Lewis S, Sigmundsson T, Takei N, Gurling H, Barta P, Pearlson G, Murray R: The Maudsley Study, V: brain changes in familial schizophrenics and their relatives: a volumetric MRI study. Br J Psychiatry 1998; 173:132–138Crossref, Medline, Google Scholar
69. Whitworth AB, Honeder M, Kremser C, Kemmler G, Felber S, Hausmann A, Wanko C, Wechdorn H, Aichner F, Stuppaeck CH, Fleischhacker WW: Hippocampal volume reduction in male schizophrenic patients. Schizophr Res 1998; 31:73–81Crossref, Medline, Google Scholar
70. Zipursky RB, Lambe EK, Kapur S, Mikulis DJ: Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry 1998; 55:540–546Crossref, Medline, Google Scholar
71. Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC: Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 1992; 327:248–254Crossref, Medline, Google Scholar
72. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
73. Wolkin A, Rusinek H, Vaid G, Arena L, Lafargue T, Sanfilipo M, Loneragan C, Lautin A, Rotrosen J: Structural magnetic resonance image averaging in schizophrenia. Am J Psychiatry 1998; 155:1064–1073Google Scholar
74. Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray R, McGuire PK: Mapping of grey matter changes in schizophrenia. Schizophr Res 1999; 35:1–14Crossref, Medline, Google Scholar
75. Naylor CD: Meta-analysis and the meta-epidemiology of clinical research. Br Med J 1997; 315:617–619Crossref, Medline, Google Scholar
76. Dunn OJ, Clark V: Correlation coefficients measured on the same individuals. J Am Statistical Association 1969; 64:366–377Crossref, Google Scholar
77. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Google Scholar