Prefrontal Cortical Dysfunction in Depression Determined by Wisconsin Card Sorting Test Performance
Abstract
OBJECTIVE: Neuroimaging studies have demonstrated reduced prefrontal cortical blood flow and metabolism in depression, but the neurobehavioral significance of these observations is not yet established. METHOD: The Wisconsin Card Sorting Test, a widely used neuropsychological index of prefrontal cortical function, was administered to 79 patients with major depression who had been unmedicated for at least 28 days, to 47 patients with schizophrenia who had never received antipsychotic medication, and to 61 healthy comparison subjects. RESULTS: Depressed patients demonstrated significant deficits on multiple Wisconsin Card Sorting Test measures compared with healthy individuals. These deficits were correlated with the severity of depression and were less severe than those demonstrated by patients with schizophrenia. CONCLUSIONS: These results provide neuropsychological evidence for significant prefrontal cortical dysfunction in depression.
Neurocognitive deficits suggesting disturbances in the prefrontal cortex have been reported in both schizophrenia and major depression. These deficits include difficulty initiating voluntary responses, shifting attention and cognitive set, inhibiting context-inappropriate responses, and maintaining information in working memory (1, 2). Converging with these neuropsychological findings are results from neuroimaging studies indicating both resting-state and activation deficits of the frontal cortex in major depression (3, 4). Given this evidence, it is somewhat surprising that reports of Wisconsin Card Sorting Test performance in major depression have been discrepant—performance deficits have been reported in some (2) but not all (5, 6) studies. These differences may be due to methodological limitations of the neuropsychological studies, such as small sample sizes, concurrent administration of psychotropic medications, the inclusion of patients at different phases of their illness, and large discrepancies between control subjects and patients on important measures such as IQ and education. Additionally, few comparisons with other psychiatric disorders, such as schizophrenia, have been made to evaluate the relative severity of neuropsychological performance deficits in major depression. We conducted a study that took these limitations into account.
METHOD
Seventy-nine patients with DSM-IV nonbipolar major depression (including five with psychotic features), who had been unmedicated for at least 1 month, and 47 patients with schizophrenia or schizoaffective disorder, who had never received antipsychotic medication, were recruited and tested before treatment initiation. We used a best-estimate consensus diagnosis method to generate research diagnoses, made by two or three senior diagnosticians in a clinical case conference in which all available data, including data from the Structured Clinical Interview for DSM-IV Axis I Disorders (7), were reviewed. Sixty-one medically healthy subjects were recruited from the community. All subjects were 18–50 years of age. Written informed consent was obtained from all subjects after the details of the study had been explained.
None of the subjects had a lifetime history of ECT, neurological disorder, head injury, or substance dependence, and no subjects had a history of substance abuse within the 6 months preceding testing. Patients were assessed for depressive symptoms with the 17-item Hamilton Depression Rating Scale (8) within 1 week of testing. The Scale for the Assessment of Positive Symptoms (SAPS) (9) and the Scale for the Assessment of Negative Symptoms (SANS) (10) were completed for patients with schizophrenia. Intraclass reliability coefficients for these ratings ranged from 0.80 to 0.90.
Subjects completed the computerized version of the Wisconsin Card Sorting Test, in which they were directed to choose one of four target cards that matched a test card in shape, color, or number of stimuli. Computer feedback indicated whether the responses were correct. Once 10 consecutive correct matches were made, the sorting criteria changed without warning, and subjects were required to use a different sorting strategy.
RESULTS
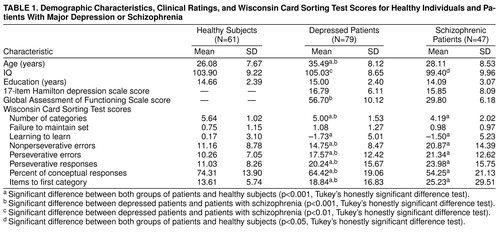
The male-female ratio for the 61 healthy comparison subjects was 35:26; for the 79 depressed patients it was 31:48; and for the 47 patients with schizophrenia it was 25:22. Further demographic information, Hamilton depression scale ratings, Global Assessment of Functioning Scale ratings, and Wisconsin Card Sorting Test scores are presented in table 1. Patients with major depression were older than both healthy subjects and patients with schizophrenia (F=26.26, df=2, 186, p<0.001), and patients with schizophrenia had somewhat lower IQs than the healthy subjects and the patients with major depression (F=5.76, df=2, 186, p<0.05). The groups had similar levels of formal education (F=1.85, df=2, 186, n.s.). Global Assessment of Functioning Scale scores revealed considerably higher functioning in the patients with major depression than in the patients with schizophrenia (t=16.08, df=111, p<0.001).
Analyses of covariance (ANCOVAs) were conducted with age and IQ as covariates (because of group differences on these measures) for each score obtained from the Wisconsin Card Sorting Test. Age was not related to Wisconsin Card Sorting Test performance in any group, but IQ was significantly and similarly related to most Wisconsin Card Sorting Test measures in all groups. Results of the ANCOVAs revealed significant group differences for all test measures except failure to maintain set (table 1). Follow-up pairwise comparisons of residual scores (after the effects of age and IQ had been partialled out) with Tukey’s honestly significant difference multiple comparison procedure revealed that both patients with major depression and patients with schizophrenia made more perseverative and nonperseverative errors, took longer to reach the first category, completed fewer categories overall, and had fewer conceptual-level responses and lower learning-to-learn scores than the healthy subjects. Patients with schizophrenia demonstrated significantly more severe deficits than depressed patients on all of these measures except for learning-to-learn.
In patients with major depression, Hamilton depression scale scores were moderately correlated with the number of categories achieved (r=–0.24, df=77, p<0.05), number of perseverative errors (r=0.27, df=77, p<0.05), number of perseverative responses (r=0.26, df=77, p<0.05), and the percentage of conceptual-level responses (r=–0.25, df=77, p<0.05). In patients with schizophrenia, depression ratings did not correlate significantly with any Wisconsin Card Sorting Test scores. However, their SAPS total scores (mean=9.20, SD=3.31) were correlated significantly with the learning-to-learn measure (r=–0.38, df=43, p<0.05) and the number of items to complete the first category (r=0.34, df=43, p<0.05). These observations suggest an association between severity of psychosis and the ability to learn how to perform the Wisconsin Card Sorting Test, but not in the ability to perform the test after the general test principle is acquired. Total SANS scores (mean=13.09, SD=3.19) correlated only with the percentage of conceptual-level responses (r=–0.31, df=43, p<0.05), suggesting a relationship between negative symptoms and impairments in conceptual thinking among the patients with schizophrenia.
DISCUSSION
Our results show that patients with major depression exhibited significant deficits when performing the Wisconsin Card Sorting Test. These deficits were less severe than those in patients with schizophrenia, as has been previously reported (11). In evaluating differences in performance deficits across these disorders, it should be noted that the schizophrenia group consisted of acutely psychotic patients requiring hospitalization and with considerably poorer global functioning than the patients with major depression, most of whom (66 of 79) were ambulatory outpatients. The greater deficits in the patients with schizophrenia might be due either to disorder-specific effects on prefrontal cortex function or to general illness severity. Our finding of a significant association between depressive symptoms and the magnitude of Wisconsin Card Sorting Test deficits in major depression suggests that there may be a relationship between cognitive impairments and state-related physiological abnormalities of prefrontal cortex function during episodes of depression (4).
Successful performance on the Wisconsin Card Sorting Test requires a complex set of cognitive functions, including the ability to think abstractly, selectively attend to a particular perceptual dimension, and shift cognitive set, and it is not entirely clear which of these functions are disturbed during episodes of depression. Furthermore, it is not yet clear whether the observed deficit reflects localized dysfunction of the prefrontal cortex or a more generalized impairment affecting multiple brain regions. Further research is needed to delineate which frontally mediated functions are disturbed in depression, to determine the specificity of these deficits to the frontal cortex, to compare patients with major depression and patients with schizophrenia who are more similar in illness severity, and to determine if impairments in major depression are state dependent and if they have prognostic significance.
Received April 14, 1998; revision received Oct. 30, 1998; accepted Nov. 10, 1998. From the Neurobehavioral Studies Program, Department of Psychiatry, University of Pittsburgh School of Medicine. Address reprint requests to Dr. Sweeney, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported by NIMH grants MH-42969, MH-45156, MH-41884, MH-01433, and MH-30915 and by the National Alliance for Research in Schizophrenia and Affective Disorders.
 |
1. Sweeney JA, Strojwas MH, Mann JJ, Thase ME: Prefrontal and cerebellar abnormalities in major depression: evidence from oculomotor studies. Biol Psychiatry 1998; 43:584–594Crossref, Medline, Google Scholar
2. Franke P, Maier W, Hardt J, Frieboes R, Lichtermann D, Hain C: Assessment of frontal lobe functioning in schizophrenia and unipolar major depression. Psychopathology 1993; 26:76–84Crossref, Medline, Google Scholar
3. George MS, Ketter TA, Post RM: Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59–72Crossref, Google Scholar
4. Bench CJ, Frackowiak RSJ, Dolan RJ: Changes in regional cerebral blood flow on recovery from depression. Psychol Med 1995; 25:247–251Crossref, Medline, Google Scholar
5. Berman KF, Doran AR, Pickar D, Weinberger DR: Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? regional cerebral blood flow during cognitive activation. Br J Psychiatry 1993; 162:183–192Crossref, Medline, Google Scholar
6. Martin DJ, Oren Z, Boone K: Major depressives’ and dysthmics’ performance on the Wisconsin Card Sorting Test. J Clin Psychol 1991; 47:684–690Crossref, Medline, Google Scholar
7. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. Washington, DC, American Psychiatric Press, 1996Google Scholar
8. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
9. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
10. Andreasen NC: Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1984Google Scholar
11. Goldberg TE, Gold JM, Greenberg R, Griffin S, Schulz SC, Pickar D, Kleinman JE, Weinberger DR: Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry 1993; 150:1355–1362Google Scholar



