Predictors of Treatment Response From a First Episode of Schizophrenia or Schizoaffective Disorder
Abstract
OBJECTIVE: This study examined the treatment response of patients with first-episode schizophrenia and schizoaffective disorder and potential predictors of response. METHOD: First-episode patients were assessed on measures of psychopathology, cognition, social functioning, and biological parameters and treated according to a standardized algorithm. RESULTS: One hundred eighteen patients (52% male, mean age 25.2 years) entered the study. The cumulative percentage of patients responding by 1 year was 87%; the median time to response was 9 weeks. The following variables were significantly associated with less likelihood of response to treatment: male sex, obstetric complications, more severe hallucinations and delusions, poorer attention at baseline, and the development of parkinsonism during antipsychotic treatment. Variables not significantly related to treatment response were diagnosis (schizophrenia versus schizoaffective disorder), premorbid functioning, duration of psychotic symptoms prior to study entry, baseline disorganization, negative and depressive symptoms, baseline motor function, akathisia and dystonia during treatment, growth hormone and homovanillic acid measures, psychotic symptom activation to methylphenidate, and magnetic resonance measures. CONCLUSIONS: Patients with first-episode schizophrenia and schizoaffective disorder have high rates of response to antipsychotic treatment; there are specific clinical and pathobiologic predictors of response.
Treatment studies of first-episode schizophrenia are of interest from both a clinical and a theoretical perspective. Most treatment study groups largely contain chronic patients who have had multiple episodes of illness; these groups may be systematically enriched in patients who are not fully responsive to treatment or not compliant with treatment (or both). First-episode study groups are not subject to these potential biases and thus may be more representative of the full spectrum of treatment response in schizophrenia. Further, because almost all patients with schizophrenia are treated with antipsychotic medications, multiepisode patients usually have taken antipsychotics for prolonged periods. This prolonged antipsychotic exposure may confound efforts to elicit associations between predictor variables and treatment response. Also, if some forms of schizophrenia involve a neurodegenerative process (1–6), treatment response may change over the course of the illness. If this is the case, studies of treatment response during the initial, middle, and later stages of the illness are needed.
We have been engaged in a study of patients with first-episode schizophrenia and schizoaffective disorder since 1986; patients in the study have extensive baseline assessments and are treated according to a standardized medication algorithm and followed for at least 5 years. In previous communications (4, 7, 8), we presented findings on treatment response for patients who initially entered the study. This communication presents the findings on acute treatment response for the entire final study group of 118 patients; data for the entire group on baseline extrapyramidal symptoms (9) and tardive dyskinesia (10) have been previously reported.
METHOD
The study methodology has been described in detail previously (7, 11). The study began in 1986; data collected up to June 1, 1996, are included in this report. All patients were recruited from Hillside Hospital, Glen Oaks, N.Y.; the study was conducted using the guidelines of the Long Island Jewish Institutional Review Board. Written informed consent for the study was obtained from subjects and, if available, from their families. Inclusion criteria were 1) a Research Diagnostic Criteria (RDC)-defined (12) diagnosis of schizophrenia or schizoaffective disorder based on a Schedule for Affective Disorders and Schizophrenia (13) interview, 2) total lifetime exposure to antipsychotic medications of 12 weeks or less, 3) current psychotic symptoms of sufficient severity to achieve a rating of 4 or more on at least one psychotic symptom item (severity of delusions, severity of hallucinations, impaired understandability, derailment, illogical thinking, and bizarre behavior) on the Schedule for Affective Disorders and Schizophrenia—Change Version With Psychosis and Disorganization Items rating scale (SADS-C+PDI) (14), 4) no medical contraindications to treatment with antipsychotic medications, and 5) no prior serious neurological or endocrine disorder or any neuromedical illness that could influence the diagnosis or the assessment of the biological variables in the study.
The assessment instruments used and their frequency of administration were as follows.
Psychopathology.
The SADS-C+PDI and the Clinical Global Impression (CGI) (15) were completed at baseline and every 2 weeks during acute treatment and every 4 weeks at other times; the Scale for the Assessment of Negative Symptoms (SANS) (16) was completed at baseline and every 4 weeks.
Side effects/motor symptoms.
The Simpson-Angus Rating Scale (17) was administered at baseline and every 2 weeks during acute treatment and every 4 weeks at other times; a modified Tardive Dyskinesia Rating Scale (18) was completed at baseline and every 8 weeks.
Premorbid social adjustment.
The Premorbid Adjustment Scale (19) was completed at baseline by using information both from patients and family members. “Premorbid” was defined as the period ending 6 months prior to the first psychiatric contact, hospitalization, or any florid psychotic symptoms. The Premorbid Adjustment Scale has sections covering childhood, early adolescence, late adolescence, and adulthood; the scores on items for each section were averaged to provide a mean score for each age category.
Obstetric histories.
These were obtained from mothers of patients by questionnaire and interview and from birth records (when available) and scored using the McNeil-Sjöström scale (20).
Neuropsychological assessments.
Measures of baseline attention and motor functions were completed before patients began taking antipsychotics; raw scores were converted to factor scores on the basis of principal components analysis.
Magnetic resonance brain scans were obtained during the index episode by using a 1.0-T whole-body magnetic resonance imaging (MRI) system (Magnetom, Siemens, Erlangen, Germany). Images were acquired using a three-dimensional gradient echo sequence, fast low-angle shots (FLASH) (coronal acquisition, 3.1-mm-thick contiguous slices, with 256×256 matrix in a 24-cm field of view; number of excitations [NEX]=1; TR=40 msec, TE=15 msec, flip angle=50°) (21). Whole brain, ventricular, caudate, superior temporal gyrus, and hippocampal volumes were assessed by using a semiautomated mensuration system; the methods for these assessments have been previously described (7, 22–24). Presence of the septum pellucidum abnormality was rated as absent, questionably present, or present by using previously reported methods (25).
Subsets of patients had biological assessments during different phases of the study. Prior to starting antipsychotics, patients were rated for psychotic symptom activation in response to intravenous methylphenidate (7). Homovanillic acid (HVA) (8) and baseline and apomorphine-stimulated growth hormone (GH) (7) levels were also obtained by indwelling catheter serial sample collection.
Patients were treated according to a standard algorithm under open conditions, progressing from one phase of the algorithm to the next until they responded. The sequence of medication trials was as follows. Patients initially received fluphenazine, up to 20 mg/day; those who did not respond after 6 weeks had their dose increased up to 40 mg/day for 4 additional weeks. Patients who did not respond to fluphenazine were switched to a regimen of haloperidol, 20 mg/day, for 6 weeks, which was raised to 40 mg/day for 4 additional weeks if needed. If patients were still unresponsive, they were given lithium with haloperidol followed, if needed, by a trial of a third neuroleptic from a different biochemical class—either molindone, up to 300 mg/day, or loxapine, up to 150 mg/day. (Because of a protocol modification during the course of the study, not all eligible patients received lithium augmentation.) Patients who were still treatment resistant were given clozapine, up to 900 mg/day. Benztropine, 2–6 mg/day, was given only if extrapyramidal symptoms developed. Lorazepam, 1–3 mg/day, or propranolol, 10–60 mg/day, was given for akathisia. Adjuvant medications for mood stabilization were used as clinically warranted. Patients who did not respond to the treatment algorithm were given additional medications as deemed clinically indicated by the research team.
There was no limitation on length of participation when the study began. Later, the maximum length of study participation was fixed at 5 years, and patients who had been in the protocol longer than this were terminated. (The longest period a patient was followed without responding from an initial episode was 76 months.)
Treatment response was operationally defined as a CGI rating of “much” or “very much” improved and a rating of 3 (mild) or less on all of the following SADS-C+PDI items: severity of delusions, severity of hallucinations, impaired understandability, derailment, illogical thinking, and bizarre behavior. To be classified as responders, patients had to sustain this level of improvement for 8 consecutive weeks; treatment response was dated from the time response criteria were first met, i.e., the beginning of this 8-week period.
For analyses of predictors of treatment response, the following definitions of risk factors were used. Obstetric complications were present if the patient had one or more level 5 “potentially greatly harmful/relevant” events on the McNeil-Sjöström scale. Severity of baseline hallucinations and delusions was measured by the mean of the ratings for the severity of delusions and severity of hallucinations items of the SADS-C+PDI. Severity of baseline disorganization was defined as the mean of the SADS-C+PDI ratings for bizarre behavior, inappropriate affect, and a composite measure of thought disorder consisting of the mean of the impaired understandability, derailment, and illogical thinking items. Severity of baseline negative symptoms was measured by the mean of the global ratings for affective flattening, alogia, avolition-apathy, and anhedonia-asociality of the SANS. Severity of baseline depressive symptoms was measured by a Hamilton Depression Rating Scale score extracted from the SADS-C+PDI (14). Baseline extrapyramidal signs were present if patients had a score of 1 (mild) or more on the items of rigidity, cogwheel rigidity, akinesia, or bradykinesia on the Simpson-Angus Rating Scale. Parkinsonism was present if patients had a rating of 3 (marked) or more on the rigidity item or a rating of 2 (moderate) on rigidity and 2 (definitely present) on the cogwheeling items of the Simpson-Angus Rating Scale. Akathisia was present if patients had a rating of 2 (moderate) or greater on the akathisia Simpson-Angus Rating Scale item.
Survival analytic techniques were used to estimate the cumulative rate of treatment response and to estimate the effects of potential predictors of treatment response. These techniques are appropriate for our data because of the different lengths of follow-up for study patients. In addition, not all patients responded to treatment during the follow-up, leading to what is termed “right-censored data.” The ultimate time to treatment response is unknown for patients who did not respond during the study but is at least as long as the follow-up period. Survival analysis adjusts for right-censored data. Survival curves and the cumulative rate of treatment response were estimated using life table analysis, with 95% confidence limits to indicate the precision of these treatment response estimates.
Analyses of the effects of single and multiple potential predictors were done by using Cox proportional hazards regression. To ensure that the proportional hazards assumption of Cox regression was not violated, additional Cox regression models that incorporated interaction terms of the predictors with time were run. Because of the number of predictors included in the Cox regression analysis, we defined statistical significance as p<0.01. We thus used 99% confidence intervals (CI) to indicate the precision of the hazard ratios from these analyses.
RESULTS
Six hundred thirty-six patients were screened for study eligibility. One hundred fifty patients entered the study; of these, 32 were later withdrawn from the study (because of a change in diagnosis based on additional information [N=15], study refusal after initial consent [N=9], discovery of severe substance abuse history [N=4], MRI findings indicative of neurologic illness [N=3], and discovery of prior antipsychotic treatment [N=1]), leaving a final study group of 118 patients. The group was 52% male (N=61), had a mean age of 25.2 years (SD=6.6), was 41% white (N=48), 37% black (N=44), 12% Hispanic (N=14), 7% Asian (N=8), and 3% mixed racial or ethnic background (N=4). There was a range of socioeconomic classes and educational levels but a predominance of the middle and lower classes (mean socioeconomic status on the Hollingshead scale [26] was 3.4, SD=1.3). Patients had been ill for an extended period, a mean of 143 weeks (SD=205) since the onset of first behavioral changes related to the illness and 71 weeks (SD=150) since the onset of first psychotic symptoms. At study entry, they were severely ill, with a mean CGI score of 5.5 (SD=1.0) and a Global Assessment Scale score of 27.1 (SD=9.0). Diagnoses, based on the RDC, were schizophrenia, N=83 (70%; 53% paranoid, 11% undifferentiated, 4% disorganized, 2% catatonic subtypes), and schizoaffective disorder, N=35. Seventy-three percent had taken no antipsychotics prior to admission; 16% had less than 2 weeks of medication; and 11% had between 2 and 12 weeks of exposure to medication.
Fourteen patients received some treatment that did not conform to the standard medication algorithm. Eleven started treatment with a typical antipsychotic other than fluphenazine in pilot versions of the protocol. Three patients began treatment with fluphenazine but subsequently received antipsychotics not specified in the algorithm for a variety of clinical reasons.
Magnetic resonance, neuropsychological, and obstetric measures were not obtained on all subjects because of clinical condition or patient refusal.
The cumulative response rate is presented in figure 1. The cumulative percentage of patients responding by 1 year was 87% (95% CI=80.4–93.1); the median time to response was 9 weeks (95% CI=8–12).
The mean neuroleptic dose in fluphenazine equivalents while the patients were in the first acute treatment period was 18.9 mg/day (SD=8.7). Antipsychotic drug dose did not predict treatment outcome in Cox analyses in which the dose was a time-varying covariate (χ2=0.11, df=1, p=0.75). This analysis was constrained by the fact that patients were treated with a standardized protocol that allowed little variability in dose.
To obtain a more qualitative view of patients whose illness was refractory to treatment from the first episode, we reviewed the records of the 10 patients who did not meet response criteria despite being treated for 1 year or longer. Only two were female, all were diagnosed with schizophrenia (three paranoid, four undifferentiated, one disorganized, and one catatonic subtype). They were followed in the study for 12 to 76 months and had persistent, severe, positive symptoms throughout. Seven of the 10 had a trial of clozapine. Clozapine was discontinued in three patients because of nonresponse and continued in four patients long-term; these four patients were better able to remain in the community despite continued positive symptoms. Two patients also received ECT, and two were eventually placed in state hospital facilities.
Analyses of potential predictors of initial treatment response are presented in table 1.. Women were more likely to respond than were men; all of the remaining analyses of predictors reported in table 1 therefore controlled for sex. Among premorbid variables, patients with a history of serious obstetric complications were almost four times less likely to respond than were patients without such a history. At initial episode presentation, patients with more severe hallucinations and delusions and those with poorer attention were less likely to respond. During treatment of the initial episode, patients who developed parkinsonism during antipsychotic drug treatment were approximately half as likely to respond as patients without these signs. Variables not significantly related to treatment response were diagnosis (schizophrenia versus schizoaffective disorder), premorbid functioning, baseline disorganization, negative and depressive symptoms, akathisia and dystonia during the first 16 weeks of treatment, HVA and GH measures, psychotic symptom activation to methylphenidate, baseline motor function on neuropsychological testing, and magnetic resonance measures.
To examine whether our predictor variables shared common mechanisms, we performed a multivariate analysis using sex, baseline hallucinations and delusions, and parkinsonism as predictors of treatment response. (Obstetric complications and baseline attention could not be included as predictors because data on all relevant predictors were available on too few patients.) The multivariate analysis was run by using data from 88 patients who had complete information on all three predictors. The hazard ratios were 1.79 (99% CI=0.99–3.25) for sex, 0.65 (99% CI=0.47–0.89) for baseline hallucinations and delusions, and 0.54 (99% CI=0.22–1.36) for parkinsonism. These estimates were basically unchanged from those in our earlier analyses, indicating that these effects were statistically independent.
DISCUSSION
Our patients were very responsive to treatment of their initial episode, achieving a cumulative response rate of 87% by 1 year. This degree of response is remarkable in comparison with the response rates often reported for multiepisode patients, especially given that our response criteria were more stringent than that often used in treatment studies. Published first-episode trials (27–29) that lasted several weeks have reported high response rates averaging around 60%. Our data suggest that these may underestimate the ultimate degree of responsiveness of first-episode patients because the median time to response of our patients was 9 weeks.
Our study was not placebo controlled; thus, we cannot definitively state what proportion of our response rate consists of response to medication and what proportion represents spontaneous remissions. However, we suspect that spontaneous remissions were few on these bases: 1) our patients were ill for long periods without remission before the study, 2) the extended time to remission does not suggest a placebo effect, and 3) as presented in a separate manuscript, relapse rates were extremely high for patients who stopped maintenance antipsychotic medication during longitudinal follow-up.
Although our overall response rate was very large, 10 of our patients were unresponsive from their first episode to intensive treatment efforts; other first-episode studies have found similar percentages of patients who did not respond (30). Studies of novel treatments for these patients are clearly needed.
Several significant relationships between baseline demographic and clinical variables and treatment response emerged from analyses with our complete study group. As has been found in multiepisode patients (31–34), first-episode women in our study were more likely to respond than were men. Regarding premorbid characteristics, our finding that patients with a history of serious obstetric complications were almost four times less likely to respond than were patients without such a history is consistent with an earlier study (35). That study found that perinatal insult was more common in chronic as compared with good prognosis patients with schizophrenia. Poorer response of patients with prolonged psychotic symptoms prior to study entry has been found in other first-episode patients (28, 36); in our complete study group, such patients were less likely to respond, but the results did not reach the 0.01 threshold of statistical significance (χ2=5.04, df=1, p=0.03).
At study entry, patients with more severe positive symptoms and worse attention were less likely to respond. Cornblatt et al. (37) found that attention as measured by the Continuous Performance Test—Identical Pairs did not improve with conventional antipsychotics in a first-episode study group and that attention was independent of positive and negative symptoms. Although attention may not change with conventional antipsychotic treatment, our data suggest that baseline attention (assessed in a different manner) predicts positive symptom improvement in first-episode patients. We are not aware of other first-episode studies testing the association of attention and treatment response. However, nonresponders in the Scottish First Episode study (28) had a nonsignificantly greater vocabulary discrepancy at baseline; this suggests that some neurocognitive deficits may predict treatment response in first-episode patients. An association between negative symptoms and lack of response has been found in another first-episode study group (28); our data are consistent with this, although our results did not reach statistical significance (χ2=5.81, df=1, p=0.02).
Our data suggest that patients with motor vulnerabilities are less likely to respond to treatment. We have reported previously that patients with spontaneous extrapyramidal symptoms prior to antipsychotic exposure (9) and patients who later develop tardive dyskinesia (10) are less likely to respond to treatment. Our current data extend these findings to show that patients who develop parkinsonism during acute antipsychotic treatment are also less likely to respond. Patients with spontaneous extrapyramidal symptoms were more likely to develop parkinsonism during acute treatment in our study group; however, there was no relationship between spontaneous extrapyramidal symptoms or parkinsonism during acute treatment and the likelihood of developing tardive dyskinesia. Comparable data from other first-episode studies are lacking, but the Scottish First Episode study (28) found that patients with neurological signs were (nonsignificantly) less likely to respond.
Our treatment algorithm, although very successful in treating our first-episode group and representing current practice when the study began, used higher medication doses than are often now used in first-episode cases. Our algorithm also emphasized conventional agents, with clozapine for patients resistant to conventional agents. This medication strategy probably produced more motor side effects than a low-dose conventional or first-line, novel-agent strategy would have. Thus, our finding that parkinsonism during initial treatment predicts ultimate treatment response may not apply for newer treatment regimens.
Our response criteria were based on positive psychotic symptom reduction and a measure of global clinical improvement. Outcome is multidimensional; data on other outcome variables will be presented in separate publications. Our response data considered in isolation may present the impression that almost all of our patients become asymptomatic; this is not the case for some outcomes such as cognitive and social functioning, where many of our patients had persistent deficits.
Given the responsiveness of first-episode patients to treatment, an obvious question is, why do multiepisode patients in most studies respond so poorly? One possibility is that first-episode patients become more treatment resistant over time; another is that good-prognosis, first-episode patients do well and do not enter typical treatment studies. Longitudinal follow-up of first-episode patients is of great interest in addressing this question.
Received June 25, 1998; revision received Oct. 30, 1998; accepted Nov. 10, 1998. From the Department of Psychiatry, Hillside Hospital, Long Island Jewish Medical Center, and the Albert Einstein College of Medicine, Bronx, N.Y. Address reprint requests to Dr. Robinson, Hillside Hospital Research Department, 75-59 263rd St., Glen Oaks, NY 11004. Supported by NIMH grants MH-41646, MH-00537, and MH-41960 (Hillside Mental Health Clinical Research Center for the Study of Schizophrenia). Magnetic resonance image analysis was performed in association with the Brain Morphometry and Image Analysis Center of the Long Island Jewish Medical Center, New Hyde Park, N.Y., supported by a grant from the Helen and Irving Schneider family.
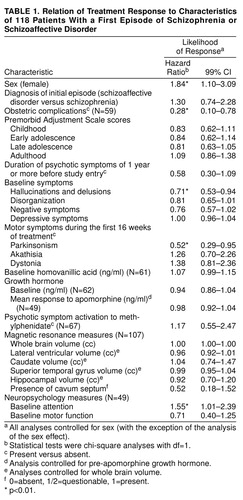 |
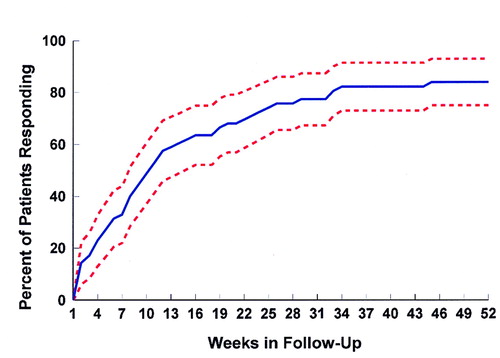
FIGURE 1. Percent of Patients With Schizophrenia or Schizoaffective Disorder Who Responded to Antipsychotic Treatmenta
aDotted lines represent 95% confidence intervals for percent of patients responding.
1. Lieberman JA, Kinon BJ, Loebel AD: Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull 1990; 16:97–110Crossref, Medline, Google Scholar
2. Wyatt RJ: Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351Crossref, Medline, Google Scholar
3. Longitudinal perspectives on the pathophysiology of schizophrenia: examining the neurodevelopmental versus neurodegenerative hypotheses. Schizophr Res 1991; 5:183–210Crossref, Medline, Google Scholar
4. Loebel AD, Lieberman JA, Alvir JMJ, Mayerhoff DI, Geisler SH, Szymanski SR: Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992; 149:1183–1188Link, Google Scholar
5. Olney JW, Farber NB: Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52:998–1007Crossref, Medline, Google Scholar
6. Lieberman JA, Sheitman BB, Kinon BJ: Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 1997; 17:205–229Crossref, Medline, Google Scholar
7. Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, Woerner M, Borenstein M: Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 1993; 50:369–376Crossref, Medline, Google Scholar
8. Koreen AR, Lieberman J, Alvir J, Mayerhoff D, Loebel A, Chakos M, Amin F, Cooper T: Plasma homovanillic acid levels in first-episode schizophrenia: psychopathology and treatment response. Arch Gen Psychiatry 1994; 51:132–138Crossref, Medline, Google Scholar
9. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar
10. Chakos MH, Alvir JM, Woerner MG, Koreen A, Geisler S, Mayerhoff D, Sobel S, Kane JM, Borenstein M, Lieberman JA: Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry 1996; 53:313–319Crossref, Medline, Google Scholar
11. Lieberman JA, Alvir JM, Woerner M, Degreef G, Bilder R, Ashtari M, Bogerts B, Mayerhoff DI, Loebel A, Levy D, Hinrichsen G, Szymanski S, Chakos M, Borenstein M, Kane JM: Prospective study of psychobiology in first-episode schizophrenia at Hillside Hospital. Schizophr Bull 1992; 18:351–371Crossref, Medline, Google Scholar
12. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1977Google Scholar
13. Endicott J, Spitzer RL: A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
14. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia—Change Version, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
15. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Rockville, Md, US Department of Health, Education, and Welfare, 1976, pp 217–222Google Scholar
16. Andreasen NC, Olsen S: Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789–794Crossref, Medline, Google Scholar
17. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand (Suppl) 1970; 212:11–19Crossref, Medline, Google Scholar
18. Simpson GM, Lee JH, Zoubok B, Gardos G: A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979; 64:171–179Crossref, Medline, Google Scholar
19. Cannon-Spoor HE, Potkin SG, Wyatt RJ: Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 1982; 8:470–484Crossref, Medline, Google Scholar
20. McNeil TF, Cantor-Graae E, Sjöström K: Obstetric complications as antecedents of schizophrenia: empirical effects of using different obstetric complication scales. J Psychiatr Res 1994; 28:519–530Crossref, Medline, Google Scholar
21. Ashtari M, Zito JL, Gold BI, Lieberman JA, Borenstein MT, Herman PG: Computerized volume measurement of brain structure. Invest Radiol 1990; 25:798–805Crossref, Medline, Google Scholar
22. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA: Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47–58Crossref, Medline, Google Scholar
23. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JMJ, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531–537Crossref, Medline, Google Scholar
24. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430–1436Link, Google Scholar
25. Degreef G, Lantos G, Bogerts B, Ashtari M, Lieberman J: Abnormalities of the septum pellucidum on MR scans in first-episode schizophrenic patients. AJNR Am J Neuroradiol 1992; 13:835–840Medline, Google Scholar
26. Hollingshead AB: Two-Factor Index of Social Position. New Haven, Conn, Yale University, 1965Google Scholar
27. Kopala LC, Fredrikson D, Good KP, Honer WG: Symptoms in neuroleptic-naive, first-episode schizophrenia: response to risperidone. Biol Psychiatry 1996; 39:296–298Crossref, Medline, Google Scholar
28. Scottish Schizophrenia Research Group: The Scottish First Episode Schizophrenia study, II: treatment: pimozide versus flupenthixol. Br J Psychiatry 1987; 150:334–338Crossref, Medline, Google Scholar
29. McCreadie RG: Managing the first episode of schizophrenia: the role of new therapies. Eur Neuropsychopharmacol 1996; 6(2):S3–S5Google Scholar
30. Macmillan JF, Crow TJ, Johnson AL, Johnstone EC: Short-term outcome in trial entrants and trial eligible patients. Br J Psychiatry 1986; 148:128–133Crossref, Medline, Google Scholar
31. Seeman MV: Current outcome in schizophrenia: women vs men. Acta Psychiatr Scand 1986; 73:609–617Crossref, Medline, Google Scholar
32. Seeman MV: Gender differences in schizophrenia. Can J Psychiatry 1982; 27:107–112Crossref, Medline, Google Scholar
33. Goldstein JM: Gender differences in the course of schizophrenia. Am J Psychiatry 1988; 145:684–689Link, Google Scholar
34. Angermeyer MC, Kuhn L: Gender differences in age at onset of schizophrenia: an overview. Eur Arch Psychiatry Neurol Sci 1988; 237:351–364Crossref, Medline, Google Scholar
35. Wilcox JA, Nasrallah HA: Perinatal distress and prognosis of psychotic illness. Biol Psychiatry 1987; 17:173–175Google Scholar
36. Szymanski SR, Cannon TD, Gallacher F, Erwin RJ, Gur RE: Course of treatment response in first-episode and chronic schizophrenia. Am J Psychiatry 1996; 153:519–525Link, Google Scholar
37. Cornblatt B, Obuchowski M, Schnur DB, O’Brien JD: Attention and clinical symptoms in schizophrenia. Psychiatr Q 1997; 68:343–359Crossref, Medline, Google Scholar



