Biology and the Future of Psychoanalysis: A New Intellectual Framework for Psychiatry Revisited
Abstract
The American Journal of Psychiatry has received a number of letters in response to my earlier “Framework” article (1). Some of these are reprinted elsewhere in this issue, and I have answered them briefly there. However, one issue raised by some letters deserves a more detailed answer, and that relates to whether biology is at all relevant to psychoanalysis. To my mind, this issue is so central to the future of psychoanalysis that it cannot be addressed with a brief comment. I therefore have written this article in an attempt to outline the importance of biology for the future of psychoanalysis.
We must recollect that all of our provisional ideas in psychology will presumably one day be based on an organic substructure.
— Sigmund Freud, “On Narcissism” (2)
The deficiencies in our description would probably vanish if we were already in a position to replace the psychological terms with physiological or chemical ones.…We may expect [physiology and chemistry] to give the most surprising information and we cannot guess what answers it will return in a few dozen years of questions we have put to it. They may be of a kind that will blow away the whole of our artificial structure of hypothesis.
— Sigmund Freud, “Beyond the Pleasure Principle” (3)
During the first half of the twentieth century, psychoanalysis revolutionized our understanding of mental life. It provided a remarkable set of new insights about unconscious mental processes, psychic determinism, infantile sexuality, and, perhaps most important of all, about the irrationality of human motivation. In contrast to these advances, the achievements of psychoanalysis during the second half of this century have been less impressive. Although psychoanalytic thinking has continued to progress, there have been relatively few brilliant new insights, with the possible exception of certain advances in child development (for a review of recent progress, see references 4–7). Most important, and most disappointing, psychoanalysis has not evolved scientifically. Specifically, it has not developed objective methods for testing the exciting ideas it had formulated earlier. As a result, psychoanalysis enters the twenty-first century with its influence in decline.
This decline is regrettable, since psychoanalysis still represents the most coherent and intellectually satisfying view of the mind. If psychoanalysis is to regain its intellectual power and influence, it will need more than the stimulus that comes from responding to its hostile critics. It will need to be engaged constructively by those who care for it and who care for a sophisticated and realistic theory of human motivation. My purpose in this article is to suggest one way that psychoanalysis might re-energize itself, and that is by developing a closer relationship with biology in general and with cognitive neuroscience in particular.
A closer relationship between psychoanalysis and cognitive neuroscience would accomplish two goals for psychoanalysis, one conceptual and the other experimental. From a conceptual point of view, cognitive neuroscience could provide a new foundation for the future growth of psychoanalysis, a foundation that is perhaps more satisfactory than metapsychology. David Olds has referred to this potential contribution of biology as “rewriting metapsychology on a scientific foundation.” From an experimental point of view, biological insights could serve as a stimulus for research, for testing specific ideas about how the mind works.
Others have argued that psychoanalysis should be satisfied with more modest goals; it should be satisfied to strive for a closer interaction with cognitive psychology, a discipline that is more immediately related to psychoanalysis and more directly relevant to clinical practice. I have no quarrel with this argument. It seems to me, however, that what is most exciting in cognitive psychology today and what will be even more exciting tomorrow is the merger of cognitive psychology and neuroscience into one unified discipline, which we now call cognitive neuroscience (for one example of this merger see reference 8). It is my hope that by joining with cognitive neuroscience in developing a new and compelling perspective on the mind and its disorders, psychoanalysis will regain its intellectual energy.
Meaningful scientific interaction between psychoanalysis and cognitive neuroscience of the sort that I outline here will require new directions for psychoanalysis and new institutional structures for carrying them out. My purpose in this article, therefore, is to describe points of intersection between psychoanalysis and biology and to outline how those intersections might be investigated fruitfully.
THE PSYCHOANALYTIC METHOD AND THE PSYCHOANALYTIC VIEW OF THE MIND
Before I outline the points of congruence between psychoanalysis and biology, it is useful to review some of the factors that have led to the current crisis in psychoanalysis, a crisis that has resulted in good part from a restricted methodology. Three points are relevant here.
First, at the beginning of the twentieth century, psychoanalysis introduced a new method of psychological investigation, a method based on free association and interpretation. Freud taught us to listen carefully to patients and in new ways, ways that no one had used before. Freud also outlined a provisional schema for interpretation, for making sense out of what otherwise seemed to be unrelated and incoherent associations of patients. This approach was so novel and powerful that for many years, not only Freud but also other intelligent and creative psychoanalysts could argue that psychotherapeutic encounters between patient and analyst provided the best context for scientific inquiry. In fact, in the early years, psychoanalysts could and did make many useful and original contributions to our understanding of the mind simply by listening to patients, or by testing ideas from the analytic situation in observational studies, a method that has proved particularly useful for studying child development. This approach may still be useful clinically because, as Anton Kris has emphasized, one listens differently now. Nevertheless, it is clear that as a research tool this particular method has exhausted much of its novel investigative power. One hundred years after its introduction, there is little new in the way of theory that can be learned by merely listening carefully to individual patients. We must, at last, acknowledge that at this point in the modern study of mind, clinical observation of individual patients, in a context like the psychoanalytic situation that is so susceptible to observer bias, is not a sufficient basis for a science of mind.
This view is shared even by senior people within the psychoanalytic community. Thus, Kurt Eissler (9) wrote, “The decrease in momentum of psychoanalytic research is due not to subjective factors among the analysts, but rather to historical facts of wider significance: the psychoanalytic situation has already given forth everything it contains. It is depleted with regard to research possibilities, at least as far as the possibility of new paradigms is concerned.”
Second, as these arguments make clear, although psychoanalysis has historically been scientific in its aim, it has rarely been scientific in its methods; it has failed over the years to submit its assumptions to testable experimentation. Indeed, psychoanalysis has traditionally been far better at generating ideas than at testing them. As a result of this failure, it has not been able to progress as have other areas of psychology and medicine.
The concerns of modern behavioral science for controlling experimenter bias by means of blind experiments has largely escaped the concern of psychoanalysts (for important exceptions, see references 10–12). With rare exception, the data gathered in psychoanalytic sessions are private: the patient’s comments, associations, silences, postures, movements, and other behaviors are privileged. In fact, the privacy of communication is central to the basic trust engendered by the psychoanalytic situation. Here is the rub. In almost all cases, we have only the analysts’ subjective accounts of what they believe has happened. As the research psychoanalyst Hartvig Dahl (11) has long argued, hearsay evidence of this sort is not accepted as data in most scientific contexts. Psychoanalysts, however, are rarely concerned that their account of what happened in a therapy session is bound to be subjective and biased.
As a result, what Boring (13) wrote, nearly 50 years ago, still stands: “We can say, without any lack of appreciation for what has been accomplished, that psychoanalysis has been prescientific. It has lacked experiments, having developed no techniques for control. In the refinement of description without control it is impossible to distinguish semantic specification from fact.”
Thus, in the future, psychoanalytic institutes should strive to have at least a fraction of all supervised analyses be accessible to this sort of scrutiny. This is important not only for the psychoanalytic situation but also for other areas of investigation. Insights gained in therapy sessions have importantly inspired other modes of investigation outside the psychoanalytic situation. A successful example is the direct observation of children and the experimental analysis of attachment and parent-child interaction. Basing future experimental analyses on insights gained from the psychoanalytic situation makes it all the more important that the scientific reliability of these situations be optimized.
Third, unlike other areas of academic medicine, psychoanalysis has a serious institutional problem. The autonomous psychoanalytic institutes that have persisted and proliferated over the last century have developed their own unique approaches to research and training, approaches that have become insulated from other forms of research. With some notable exceptions, the psychoanalytic institutes have not provided their students or faculty with appropriately academic settings for questioning scholarship and empirical research.
To survive as an intellectual force in medicine and in cognitive neuroscience, and indeed in society as a whole, psychoanalysis will need to adopt new intellectual resources, new methodologies, and new institutional arrangements for carrying out its research. Several medical disciplines have grown by incorporating the methodologies and concepts of other disciplines. By and large, psychoanalysis has failed to do so. Because psychoanalysis has not yet recognized itself as a branch of biology, it has not incorporated into the psychoanalytic view of the mind the rich harvest of knowledge about the biology of the brain and its control of behavior that has emerged in the last 50 years. This, of course, raises the question, Why has psychoanalysis not been more welcoming of biology?
THE CURRENT GENERATION OF PSYCHOANALYSTS HAVE RAISED ARGUMENTS FOR AND AGAINST A BIOLOGY OF MIND
In 1894 Freud argued that biology had not advanced enough to be helpful to psychoanalysis. It was premature, he thought, to bring the two together. One century later, a number of psychoanalysts have a far more radical view. Biology, they argue, is irrelevant to psychoanalysis. To give an example, Marshall Edelson (14) in his book Hypothesis and Evidence in Psychoanalysis, wrote:
Efforts to tie psychoanalytic theory to a neurobiological foundation, or to mix hypotheses about mind and hypotheses about brain in one theory, should be resisted as expressions of logical confusion.
I see no reason to abandon the position Reiser takes despite his avowed belief in the “functional unity” of mind and body, when he considers the mind-body relation:
“The science of the mind and the science of the body utilize different languages, different concepts (with differing levels of abstraction and complexity), and different sets of tools and techniques. Simultaneous and parallel psychological and physiological study of a patient in an intense anxiety state produces of necessity two separate and distinct sets of descriptive data, measurements, and formulations. There is no way to unify the two by translation into a common language, or by reference to a shared conceptual framework, nor are there as yet bridging concepts that could serve…as intermediate templates, isomorphic with both realms. For all practical purposes, then, we deal with mind and body as separate realms; virtually, all of our psychophysiological and psychosomatic data consist in essence of covariance data, demonstrating coincidence of events occurring in the two realms within specified time intervals at a frequency beyond chance.” [15, p. 479]
I think it is at least possible that scientists may eventually conclude that what Reiser describes does not simply reflect the current state of the art, methodologically, or the inadequacy of our thought but represents, rather, something that is logically or conceptually necessary, something that no practical or conceptual developments will ever be able to mitigate.
In my own numerous interactions with Reiser I have never sensed him to have difficulty relating brain to mind. Nevertheless, I have quoted Edelson at length because his view is representative of that shared by a surprisingly large number of psychoanalysts, and even by Freud in some of his later writings. This view, often referred to as the hermeneutic as opposed to the scientific view of psychoanalysis, reflects a position that has hindered psychoanalysis from continuing to grow intellectually (16, 17).
Now, psychoanalysis could, if it wanted to do so, easily rest on its hermeneutic laurels. It could continue to expound on the remarkable contributions of Freud and his students, on the insights into the unconscious mental processes and motivations that make us the complex, psychologically nuanced individuals we are (18–26). Indeed, in the context of these contributions, few would challenge Freud’s position as the great modern thinker on human motivation or would deny that our century has been permanently marked by Freud’s deep understanding of the psychological issues that historically have occupied the Western mind from Sophocles to Schnitzer.
But if psychoanalysis is to rest on its past accomplishments, it must remain, as Jonathan Lear (27) and others have argued, a philosophy of mind, and the psychoanalytic literature—from Freud to Hartmann to Erickson to Winnicott—must be read as a modern philosophical or poetic text alongside Plato, Shakespeare, Kant, Schopenhauer, Nietzsche, and Proust. On the other hand, if the field aspires, as I believe most psychoanalysts do aspire, to be an evolving, active contributor to an emerging science of the mind, then psychoanalysis is falling behind.
I therefore agree with the sentiment expressed by Lear (27): “Freud is dead. He died in 1939, after an extraordinary productive and creative life…it is important not to get stuck on him, like some rigid symptom, either to idolize him or to denigrate him.”
BIOLOGY IN THE SERVICE OF PSYCHOANALYSIS
My focus in this article is on ways that biology might reinvigorate the psychoanalytic exploration of mind. I should say at the outset that although we have the outlines of what could evolve into a meaningful biological foundation for psychoanalysis, we are very much at the beginning. We do not yet have an intellectually satisfactory biological understanding of any complex mental processes. Nevertheless, biology has made remarkable progress in the last 50 years, and the pace is not slacking. As biologists come to focus more of their efforts on the brain-mind, most of them have become convinced that the mind will be to the biology of the twenty-first century what the gene has been to the biology of the twentieth century. Thus, Francois Jacob (28) writes, “The century that is ending has been preoccupied with nucleic acids and proteins. The next one will concentrate on memory and desire. Will it be able to answer the questions they pose?”
My key argument is that the biology of the next century is, in fact, in a good position to answer some of the questions about memory and desire, that these answers will be all the richer and more meaningful if they are forged by a synergistic effort of biology and psychoanalysis. In turn, answers to these questions, and the very effort of providing them in conjunction with biology, will provide a more scientific foundation for psychoanalysis.
In the next century, biology is likely to make deep contributions to the understanding of mental processes by delineating the biological basis for the various unconscious mental processes, for psychic determinism, for the role of unconscious mental processes in psychopathology, and for the therapeutic effect of psychoanalysis. Now, biology will not immediately enlighten these deep mysteries at their core. These issues represent, together with the nature of consciousness, the most difficult problems confronting all of biology—in fact, all of science. Nevertheless, one can begin to outline how biology might at least clarify some central psychoanalytic issues, at least at their margins. Here I outline eight areas in which biology could join with psychoanalysis to make important contributions: 1) the nature of unconscious mental processes, 2) the nature of psychological causality, 3) psychological causality and psychopathology, 4) early experience and the predisposition to mental illness, 5) the preconscious, the unconscious, and the prefrontal cortex, 6) sexual orientation, 7) psychotherapy and structural changes in the brain, and 8) psychopharmacology as an adjunct to psychoanalysis.
1. Unconscious Mental Processes
Central to psychoanalysis is the idea that we are unaware of much of our mental life. A great deal of what we experience—what we perceive, think, dream, fantasize—cannot be directly accessed by conscious thought. Nor can we explain what often motivates our actions. The idea of unconscious mental processes is not only important in its own right, but it is critical for understanding the nature of psychic determinism. Given the centrality of unconscious psychic processes, what can biology teach us about them?
In 1954 Brenda Milner (29) made the remarkable discovery, based on studies of the amnestic patient H.M., that the medial temporal lobe and the hippocampus mediate what we now call declarative (explicit) memory storage, a conscious memory for people, objects, and places. In 1962 she made the further discovery that even though H.M. had no conscious recall of new memories about people, places, and objects, he was nonetheless fully capable of learning new perceptual and motor skills (for a recent review see reference 8). These memories—what we now call procedural or implicit memory—are completely unconscious and are evident only in performance rather than in conscious recall.
Using the two memory systems together is the rule rather than the exception. These two memory systems overlap and are commonly used together so that many learning experiences recruit both of them. Indeed, constant repetition can transform declarative memory into a procedural type. For example, learning to drive an automobile at first involves conscious recollection, but eventually driving becomes an automatic and nonconscious motor activity. Procedural memory is itself a collection of processes involving several different brain systems: priming, or recognition of recently encountered stimuli, is a function of sensory cortices; the acquisition of various cued feeling states involves the amygdala; formation of new motor (and perhaps cognitive) habits requires the neostriatum; learning new motor behavior or coordinated activities depends on the cerebellum. Different situations and learning experiences recruit different subsets of these and other procedural memory systems, in variable combination with the explicit memory system of the hippocampus and related structures (30, 31) (figure 1).
In procedural memory, then, we have a biological example of one component of unconscious mental life. How does this biologically delineated unconscious relate to Freud’s unconscious? In his later writings Freud used the concept of the unconscious in three different ways (for a review of Freud’s ideas on consciousness see reference 32). First, he used the term in a strict or structural way to refer to the repressed or dynamic unconscious. This unconscious is what the classical psychoanalytic literature refers to as the unconscious. It includes not only the id but also that part of the ego which contains unconscious impulses, defenses, and conflicts and therefore is similar to the dynamic unconscious of the id. In this dynamic unconscious, information about conflict and drive is prevented from reaching consciousness by powerful defensive mechanisms such as repression.
Second, in addition to the repressed parts of the ego, Freud proposed that still another part of the ego is unconscious. Unlike the unconscious parts of the ego that are repressed and therefore resemble the dynamic unconscious, the unconscious part of the ego that is not repressed is not concerned with unconscious drives or conflicts. Moreover, unlike the preconscious unconscious, this unconscious part of the ego is never accessible to consciousness even though it is not repressed. Since this unconscious is concerned with habits and perceptual and motor skills, it maps onto procedural memory. I shall therefore refer to it as the procedural unconscious.
Finally, Freud used the term descriptively, in a broader sense—the preconscious unconscious—to refer to almost all mental activities, to most thoughts and all memories that enter consciousness. According to Freud, an individual is not aware of almost all of the mental processing events themselves yet can have ready conscious access to many of them by an effort of attention. From this perspective, most of mental life is unconscious much of the time and becomes conscious only as sensory percepts: as words and images.
Of these three unconscious mental processes, only the procedural unconscious, the unconscious part of the ego that is not conflicted or repressed, appears to map onto what neuroscientists call procedural memory (for a similar argument see also reference 33). This important correspondence between cognitive neuroscience and psychoanalysis was first recognized in a thoughtful article by Robert Clyman (34), who considered procedural memory in the context of emotion and its relevance for transference and for treatment. This idea has been developed further by Louis Sanders, Daniel Stern, and their colleagues in the Boston Process of Change Study Group (35), who have emphasized that many of the changes that advance the therapeutic process during an analysis are not in the domain of conscious insight but rather in the domain of unconscious procedural (nonverbal) knowledge and behavior. To encompass this idea, Sanders (36), Stern (37), and their colleagues have developed the idea that there are moments of meaning—moments in the interaction between patient and therapist—which represent the achievement of a new set of implicit memories that permits the therapeutic relationship to progress to a new level. This progression does not depend on conscious insights; it does not require, so to speak, the unconscious becoming conscious. Rather, moments of meaning are thought to lead to changes in behavior that increase the patient’s range of procedural strategies for doing and being. Growth in these categories of knowledge leads to strategies for action that are reflected in the ways in one person interacts with another, including ways that contribute to transference.
Marianne Goldberger (38) has extended this line of thought by emphasizing that moral development also is advanced by procedural means. She points out that people do not generally remember, in any conscious way, the circumstances under which they assimilated the moral rules that govern their behavior; these rules are acquired almost automatically, like the rules of grammar that govern our native language.
I illustrate this distinction between procedural and declarative memory that comes from cognitive neuroscience to emphasize the utility for psychoanalytic thought of a fundamentally neurobiological insight. But in addition, I would suggest that as applied to psychoanalysis, these biological ideas are still only ideas. What biology offers is the opportunity to carry these ideas one important step further. We now know a fair bit about the biology of this procedural knowledge, including some of its molecular underpinnings (8).
The interesting convergence of psychoanalysis and biology on the problem of procedural memory confronts us with the task of testing these ideas in a systematic way. We will need to examine, from both a psychoanalytic and a biological perspective, the range of phenomena we have subsumed under the term “procedural memory” and see how they map onto different neural systems. In so doing we will want to examine, in behavioral, observational, and imaging studies, to what degree different components of a given moment of meaning or different moments of this sort recruit one or another anatomical subsystem of procedural memory.
As these arguments make clear, one of the earlier limitations to the study of unconscious psychic processes was that no method existed for directly observing them. All methods for studying unconscious processes were indirect. Thus, a key contribution that biology can now make—with its ability to image mental processes and its ability to study patients with lesions in different components of procedural memory—is to change the basis of the study of unconscious mental processes from indirect inference to direct observation. By these means we might be able to determine which aspects of psychoanalytically relevant procedural memory are mediated by which of the subcortical systems concerned. In addition, imaging methods may also allow us to discern which brain systems mediate the two other forms of unconscious memory, the dynamic unconscious and the preconscious unconscious.
Before I turn to the preconscious unconscious and its possible relation to the prefrontal cortex, I first want to consider three other features related to the procedural unconscious: its relation to psychic determinism, to conscious mental processes, and to early experience.
2. The Nature of Psychological Determinacy: How Do Two Events Become Associated in the Mind?
In Freud’s mind, unconscious mental processes provided an explanatory mechanism for psychic determinism. The fundamental idea of psychic determinism is that little, if anything, in one’s psychic life occurs by chance. Every psychic event, whether procedural or declarative, is determined by an event that precedes it. Slips of the tongue, apparently unrelated thoughts, jokes, dreams, and all images within each dream are related to preceding psychological events and have a coherent and meaningful relationship to the rest of one’s psychic life. Psychological determinacy is similarly important in psychopathology. Every neurotic symptom, no matter how strange it may seem to the patient, is not strange in the unconscious mind but is related to preceding mental processes. The connections between symptoms and causative mental processes or between the images of a dream and their preceding psychically related events are obscured by the operation of ubiquitous and dynamic unconscious processes.
The development of many ideas within psychoanalytic thought and its core methodology, free association, derives from the concept of psychic determinism (39). The purpose of free association is to have the patient report to the psychoanalyst all thoughts that come to mind and to refrain from exercising over them any degree of censorship or direction (39, 40). The key idea of psychic determinism is that any mental event is causally related to its preceding mental event. Thus, Brenner (40) wrote, “In the mind, as in physical nature about us, nothing happens by chance, or in a random way. Each psychic event is determined by the ones which precede it.”
Although we do not have a rich biological model of psychic declarative explicit knowledge, we have in biology a good beginning of an understanding of how associations develop in procedural memory (for a review see reference 31). Insofar as aspects of procedural knowledge are relevant to moments of meaning, these biological insights should prove useful for understanding the procedural unconscious.
In the last decade of the nineteenth century, at the time that Freud was working on his theory of psychological determinacy, Ivan Pavlov was developing an empirical approach to a particular instance of psychic determinism at the level of what we now call procedural knowledge: learning by association. Pavlov sought to elucidate an essential feature of learning that had been known since antiquity. Western thinkers since Aristotle had appreciated that memory storage requires the temporal association of contiguous thoughts, a concept later developed systematically by John Locke and the British empiricist philosophers. Pavlov’s brilliant achievement was to develop an animal model of learning by association that could be studied rigorously in the laboratory. By changing the timing of two sensory stimuli and observing changes in simple reflex behavior, Pavlov (41) established a procedure from which reasonable inferences could be made about how changes in the association between two stimuli could lead to changes in behavior—to learning (for more recent reviews see references 31 and 42–44). Pavlov thus developed powerful paradigms for associative learning that led to a permanent shift in the study of behavior, moving it from an emphasis on introspection to an objective analysis of stimuli and responses. This is exactly the sort of shift we are looking for in psychoanalytic investigations of psychic determinism.
I have described this familiar paradigm because I want to emphasize three points relevant to psychoanalytic thought. First, in learning to associate two stimuli, a subject does not simply learn that one stimulus precedes the other. Instead, in learning to associate two stimuli, a subject learns that one stimulus comes to predict the other (for a discussion of this point, see references 44 and 45). Second, as we shall see below, classical conditioning is a superb paradigm for analyzing how knowledge can move from being unconscious to entering consciousness (46). Finally, classical conditioning can be used to acquire not only appetitive responses but also aversive ones and thus can give us insight into the emergence of psychopathology. I now turn to each of these points.
The psychic determinism of classical conditioning is probabilistic
For many years psychologists thought that classical conditioning followed rules of psychic determinism similar to those outlined by Freud. They thought that classical conditioning depended only on contiguity, on a critical minimum interval between the conditioned and the unconditioned stimulus, so that the two were experienced as connected. According to this view, each time a conditioned stimulus is followed by a reinforcing or unconditioned stimulus, a neural connection is strengthened between the stimulus and the response or between one stimulus and another, until eventually the bond becomes strong enough to change behavior. The only relevant variable determining the strength of conditioning was thought to be the number of pairings of the conditioned stimulus and unconditioned stimulus. In 1969 Leon Kamin (47) made what now is generally considered the most significant empirical discovery in conditioning since Pavlov’s initial findings at the turn of the century. Kamin found that animals learn more than contiguity; they learn contingencies. They do not simply learn that the conditioned stimulus precedes the unconditioned stimulus but rather that the conditioned stimulus predicts the unconditioned stimulus. Thus, associative learning does not depend on a critical number of pairings of conditioned stimulus and unconditioned stimulus but on the power of the conditioned stimulus to predict a biologically significant unconditioned stimulus (44).
These considerations suggest why animals and people acquire classical conditioning so readily. Classical conditioning, and perhaps all forms of associative learning, likely evolved to enable animals to learn to distinguish events that regularly occur together from those that are only randomly associated. In other words, the brain seems to have evolved a simple mechanism that “makes sense” out of events in the environment by assigning a predictive function to some events. What environmental conditions might have shaped or maintained a common learning mechanism in a wide variety of species? All animals must be able to recognize and avoid danger; they must search out rewards such as food that is nutritious and avoid food that is spoiled or poisoned. An effective way to achieve this knowledge is to be able to detect regular relationships between stimuli or between behavior and stimuli. It is possible that by examining this relationship in cell biological terms, we may well be looking at the elementary mechanism of psychic determinism.
Classical conditioning and the relationship of conscious procedural to unconscious declarative mental processes
Conventional classical conditioning is usually carried out in a form called delay conditioning, in which the onset of the conditioned stimulus typically precedes the onset of the unconditioned stimulus by about 500 msec, and both the conditioned stimulus and the unconditioned stimulus terminate together (figure 2). This form of conditioning is prototypically procedural (31, 48). When a normal human subject learns an eyeblink response to a weak tactile stimulus on his brow, that subject is unaware that he or she is being conditioned. Patients with damage to the hippocampus and the medial temporal neocortex, who therefore lack explicit (declarative) memory altogether, can be conditioned like normal subjects in a delay conditioning paradigm.
A slight variation, trace conditioning, converts implicit conditioning into explicit memory. With trace conditioning the conditioned stimulus terminates before the unconditioned stimulus occurs, so that the conditioned stimulus is brief, and there is a 500-msec gap between the termination of the conditioned stimulus and the onset of the unconditioned stimulus (figure 2). Richard Thompson and his colleagues (49, 50) found that trace conditioning depends on the hippocampus and is eliminated in experimental animals with lesions of the hippocampus. Clark and Squire (48) extended these experiments to humans and found that trace conditioning requires conscious recall. In the course of trace conditioning, normal subjects usually become consciously aware of the temporal gap in the relationship between the conditioned stimulus and unconditioned stimulus. Those subjects who do not become aware of this gap do not acquire trace conditioning. Moreover, this task cannot be mastered by people who suffer from amnesia—from a defect in declarative memory—as a result of lesions to the medial temporal lobe.
Thus, a small shift in temporal sequence changes an instance of psychic determinism from being unconscious to being conscious! This is consistent with the idea that the two memory systems, procedural and declarative, are often jointly recruited by a common task and encode different aspects of the sensory pattern of stimuli (or of the external world) present to the subject. Where in the medial temporal lobe is this shift from one type of memory storage to the other occurring? Eichenbaum (51) has argued that the hippocampus functions to associate noncontiguous events over space and time. We in fact now know that trace conditioning recruits the hippocampus and the circuitry of the medial temporal lobe. Which parts of the hippocampal circuitry are key for trace conditioning? Do other regions become involved? Does the prefrontal cortex (which we shall consider below)—an area concerned with working memory that is thought to represent an aspect of the preconscious unconscious—mediate associations between unconscious and conscious memories that are the subject of analysis?
3. Psychological Causality and Psychopathology
We have seen that one point of convergence between biology and psychoanalysis is the relevance of procedural memory for early moral development, for aspects of transference, and for moments of meaning in psychoanalytic therapy. We have considered a second point of convergence in examining the relationship between the associative characteristic of classical conditioning and psychological determinacy. Here, I want to illustrate a third point of convergence: that between Pavlovian fear conditioning, a form of procedural memory mediated by the amygdala, signal anxiety, and posttraumatic stress syndromes in humans.
Early in his work on classical conditioning, Pavlov appreciated that conditioning is appetitive when the unconditioned stimulus is rewarding, but the same procedure will produce defensive conditioning when the unconditioned stimulus is aversive. Pavlov next found that defensive conditioning provides a particularly good experimental model of signal anxiety, a form of learned fear that can be advantageous.
It is pretty evident that under natural conditions the normal animal must respond not only to stimuli which themselves bring immediate benefit or harm, but also to other physical or chemical agencies…which in themselves only signal the approach of these stimuli; though it is not the sight or the sound of the beast of prey which is itself harmful to smaller animals, but its teeth and claws. (41, p. 14)
A similar proposal was made independently by Freud. Because painful stimuli are often associated with neutral stimuli, symbolic or real, Freud postulated that repeated pairing of neutral and noxious stimuli can cause the neutral stimulus to be perceived as dangerous and to elicit anxiety. Placing this argument in a biological context, Freud wrote:
The individual will have made an important advance in his capacity for self-preservation if he can foresee and expect a traumatic situation of this kind which entails helplessness, instead of simply waiting for it to happen. Let us call a situation which contains the determinant for such expectation a danger situation. It is in this situation that the signal of anxiety is given. (52, p. 166; italics added)
Thus, both Pavlov and Freud appreciated that it is biologically adaptive to have the ability to respond defensively to danger signals before the real danger is present. Signal or anticipatory anxiety prepares the individual for fight or flight if the signal is from the environment. Freud suggested that mental defenses substitute for actual flight or withdrawal in response to internal danger. Signal anxiety therefore provides an opportunity for studying how mental defenses are recruited: how psychic determinism gives rise to psychopathology.
We know that the amygdala is important for emotionally charged memory, as in classical conditioning of fear by pairing a neutral tone with a shock (53). The amygdala coordinates the flow of information between the areas of the thalamus and the cerebral cortex that process the sensory cues and areas that process the expression of fear: the hypothalamus, which regulates the autonomic response to fear, and the limbic neocortical association areas, the cingulate cortex and prefrontal cortex, which are thought to be involved in evaluating the conscious evaluation of emotion. LeDoux has argued that in anxiety, the patient experiences the autonomic arousal as something threatening happening, an arousal mediated by the amygdala. LeDoux attributes the absence of awareness to a shutting down of the hippocampus by stress, a mechanism considered below. We now have excellent methods for imaging these structures in both experimental animals and humans in order to address the question of how these linkages are established and, once established, how they are maintained (53–55).
4. Early Experience and Predisposition to Psychopathology
Signal anxiety represents a simple example of an acquired psychopathology. But, as is the case with all things acquired, some people have a greater constitutional disposition than others to acquire neurotic anxiety. What factors predispose an individual to associate a variety of neutral stimuli with threatening ones?
In Mourning and Melancholia and in his other writings, Freud emphasized two components in the etiology of acquired psychopathology: constitutional (including genetic) predispositions and early experiential factors, especially loss. Indeed, there is evidence in the development of many forms of mental illness for both genetic components and experiential factors (both early developmental factors and later acute precipitating factors). As one example, while there is a clear genetic contribution to susceptibility to depression, many patients with major depression have experienced stressful life events during childhood, including abuse or neglect, and these stressors are important predictors of depression (56–61). The case is most clear for posttraumatic stress disorder (PTSD), which requires for its diagnosis the presence of stressful experience so severe as to be outside the range of usual human experience. About 30% of individuals traumatized in this way subsequently develop the full syndrome of PTSD (57, 58). This incomplete penetrance raises the question, What (besides genes) predisposes people to developing PTSD and other stress-related disorders?
The component of the early environment thought to be most important for humans, and in fact for all mammals, is the infant’s major caretaker, usually the mother. Psychoanalysis has long argued that the manner in which a mother and her infant interact creates within the child’s mind the first internal representation not only of another person but of an interaction, of a relationship. This initial representation of people and of relationships is thought to be critical for the subsequent psychological development of the child. The interaction goes both ways. The way the infant behaves toward the mother exerts a considerable influence on the mother’s behavior. Secure attachment of mother and infant is thought to foster in the infant comfort with itself and basic trust in others, whereas insecure attachment is thought to foster anxiety.
One of the key initial ideas to emerge from both cognitive and neurobiological study of development is that the development of these internal representations can only be induced during certain early and critical periods in the infant’s life. During these critical periods, and only during these periods, the infant (and its developing brain) must interact with a responsive environment (an “average expectable” environment, to use Heinz Hartmann’s term) if the development of the brain and of the personality is to proceed satisfactorily.
The first compelling evidence for the importance of early relationships between parents and offspring came from Anna Freud’s studies on the traumatic effects of family disruption during World War II (62). The importance of family disruption was further developed by René Spitz (21), who compared two groups of infants separated from their mothers. One group was raised in a foundling home where the infants were cared for by nurses, each of whom was responsible for seven infants; the other group was in a nursing home attached to a women’s prison, where the infants were cared for daily by their mothers. By the end of the first year, the motor and intellectual performance of the children in the orphanage had fallen far below that of the children in the nursing home; those children were withdrawn and showed little curiosity or gaiety.
Harry Harlow extended this work one important step further by developing an animal model of infant development (63, 64). He found that when newborn monkeys were isolated for 6 months to 1 year and then returned to the company of other monkeys, they were physically healthy but behaviorally devastated. These monkeys crouched in a corner of their cages and rocked back and forth like severely disturbed or autistic children. They did not interact with other monkeys, nor did they fight, play, or show any sexual interest. Isolation of an older animal for a comparable period was innocuous. Thus, in monkeys, as in humans, there is a critical period for social development. Harlow next found that the syndrome could be partially reversed by giving the isolated monkey a surrogate mother, a cloth-covered wooden dummy. This surrogate elicited clinging behavior in the isolated monkey but was insufficient for the development of fully normal social behavior. Normal social development could only be rescued if, in addition to a surrogate mother, the isolated animal had contact for a few hours each day with a normal infant monkey who spent the rest of the day in the monkey colony.
The work of Anna Freud, Spitz, and Harlow was importantly extended by John Bowlby, who began to think about the interaction of the infant and its caregiver in biological terms. Bowlby (23, 65) formulated the idea that the defenseless infant maintains a closeness to its caretaker by means of a system of emotive and behavioral response patterns that he called the attachment system. Bowlby conceived of the attachment system as an inborn instinctual or motivational system, much like hunger or thirst, that organizes the memory processes of the infant and directs it to seek proximity to and communication with the mother. From an evolutionary point of view, the attachment system clearly enhances the infant’s chances for survival by allowing the immature brain to use the parents’ mature functions to organize its own life processes. The infant’s attachment mechanism is mirrored in the parents’ emotionally sensitive responses to the infant’s signals. Parental responses serve both to amplify and reinforce the infant’s positive emotional state and attenuate the infant’s negative emotional states by giving the infant secure protection when upset. These repeated experiences become encoded in procedural memory as expectations that help the infant feel secure.
It should be noted that during the first 2–3 years of life, when an infant’s interaction with its mother is particularly important, the infant relies primarily on its procedural memory systems. Both in humans and in experimental animals, declarative memory develops later. Thus, infantile amnesia, which results in the fact that very few memories from early childhood are accessible to later recall, is evident not only in humans but also in other mammals, including rodents. This amnesia presumably occurs not because of the powerful repression of memories during resolution of the oedipal complex, but because of slow development of the declarative memory system (34).
Bowlby described the response to separation as occurring in two phases: protest and despair. Events that disturb the proximity of the infant to the attachment object elicit protest: clinging, following, searching, crying, and acute physiological arousal lasting minutes to hours. These behaviors serve to restore proximity. When contact is regained, these clinging behaviors are shut off, according to Bowlby, by a feedback mechanism, and alternative behavioral systems, most notably exploratory behavior, become activated. If separation is prolonged, despair gradually replaces the early responses as the infant recognizes that separation may be prolonged or permanent and shifts from anxiety and anger to sadness and despair. Whereas protest is thought to be adaptive by increasing the likelihood that the parent and infant find each other again, despair is thought to prepare the infant for prolonged passive survival achieved by conserving energy and withdrawing from danger.
We owe to Levine and colleagues (66–68), Ader and Grota (69), and Hofer (70, 71) the discovery that a similar attachment system exists in rodents. The extension of this research to a rodent model system, which is much simpler, but still mammalian, holds great power. For example, in mice individual genes can be expressed or ablated, which allows a powerful approach for relating individual genes to behavior. Levine found that rat pups show an immediate protest to separation consisting of repeated high-intensity vocalization, agitated searching, and high levels of self-grooming. If the mother fails to return and the separation continues, the protest behaviors wane over a period of hours and are replaced by a number of slower-developing behaviors—akin to despair—as the pups become progressively less alert and responsive, and their body temperature and heart rate drop. Much as Harlow was able to dissect the components of the caregiver that were essential for normal character development, so Hofer was able to show that three different aspects of pups’ protest-despair responses were triggered by three different hidden regulators within the mother-infant interaction: loss of warmth, loss of food, and loss of tactile stimulation.
Levine and his colleagues (68) were the first to carry the analysis to a molecular level by studying how varying degrees of infant attachment affected the animals’ subsequent ability to respond to stress. Hans Selye (72) had pointed out as early as 1936 that humans and experimental animals respond to stressful experiences by activating their hypothalamic-pituitary-adrenal (HPA) axis. The end product of the HPA system is the release of glucocorticoid hormones by the adrenal gland. These hormones serve as major regulators of homeostasis—of intermediary metabolism, muscle tone, and cardiovascular function. Together with catecholamines released by the autonomic nervous system and by the adrenal medulla, the secretion of glucocorticoids is essential for survival in the face of stress.
Levine therefore asked the question, Can the long-term response of the HPA system to stress be modulated by experience? If so, is it particularly sensitive to early experience? Levine discovered that when, during the first 2 weeks of life, pups were removed from their mothers for only a few minutes, the pups showed increased vocalization, which elicited increased maternal care. The mothers responded by licking, grooming, and carrying these pups around more often than if they had not been removed. This increase in the mother’s attachment behavior reduced, for the rest of the animal’s life, the pup’s HPA response—its plasma levels of glucocorticoid—to a variety of stressors! Concomitantly, it reduced the pup’s fearfulness and vulnerability to stress-related disease (73, 74). By contrast, when, during the same 2-week period of life, pups were separated from their mothers for prolonged periods of time (3–6 hours per day for 2 weeks), the opposite reaction ensued. Now the mothers ignored the pups, and the pups showed an increase in plasma ACTH and glucocorticoid responses to stress as adults. Thus, differences in an infant’s interactions with its mother—differences that fall in the range of naturally occurring individual differences in maternal care—are crucial risk factors for an individual’s future response to stress. Here we have a remarkable example of how early experience alters the set point for a biological response to stress.
Studies by Charles Nemeroff and Paul Plotsky have found that these early adverse life experiences result in increased gene expression for corticotropin-releasing factor (CRF), the hormone released from the hypothalamus to initiate the HPA response. Daily maternal separation during the first 2 weeks is associated in the rat with profound and persistent increases in the expression of the messenger RNA for CRF, not only in the hypothalamus but also in limbic areas including the amygdala and the bed nucleus of the stria terminalis (74–76).
However, the biological insights into attachment theory do not stop here. Bruce McEwen (77), Robert Sapolsky (78), and their colleagues have discovered that the increases in glucocorticoids which follow prolonged separation have adverse effects on the hippocampus. There are two types of receptors for glucocorticoids: type 1 (the mineralocorticoid receptors) and type 2 (the glucocorticoid receptors). The hippocampus is one of the few sites in the body that has both! Thus, repeated stress (or exposure to elevated glucocorticoids over a number of weeks) causes atrophy of neurons of the hippocampus, which is reversible when the stress or glucocorticoid exposure is discontinued. However, when stress or elevated glucocorticoid exposure is prolonged over many months or even years, permanent damage occurs, and there is a loss of hippocampal neurons. As we might predict from the key role of the hippocampus in declarative memory, both reversible atrophy and permanent damage result in significant impairment of memory. This deficit in memory is detectable at the cellular level; it is evident in a weakening of a process called long-term potentiation, an intrinsic mechanism that is thought to be critical for learning-related strengthening of synaptic connections (31, 77) (figure 3). Thus, what may initially appear as repression may actually prove to be a true amnesia: damage to the medial temporal lobe system of the brain.
This set of experiments has deep significance for the relationship of early unconscious mental processes to later conscious mental processes. Stress early in life produced by separation of the infant from its mother produces a reaction in the infant that is stored primarily by the procedural memory system, the only well-differentiated memory system that the infant has early in its life, but this action of the procedural memory system leads to a cycle of changes that ultimately damages the hippocampus and thereby results in a persistent change in declarative memory.
This rodent model has direct clinical relevance. Patients with Cushing’s syndrome overproduce glucocorticoids as a result of having a tumor in the adrenal gland, the pituitary gland, or the part of the hypothalamus that controls the pituitary. Starkman and her colleagues (79) have studied these patients and found that those who have had the disease for over 1 year have selective atrophy of the hippocampus and concomitant memory loss. Similar atrophy and memory loss are thought to occur with posttraumatic stress. Bremner and his colleagues (56, 80) have found that patients with combat-related PTSD have deficits in declarative memory as well as an 8% reduction in the volume of the right hippocampus (figure 3). Here, however, the atrophy and memory loss are not secondary to increased glucocorticoids but are due to some other mechanisms, since in these patients the glucocorticoid levels are lower than normal.
In the 1970s, Sachar (81) first showed that similar events occur in the hypothalamic-pituitary axis of patients with depression. Over 50% of depressed patients have sustained levels of glucocorticoids. Subsequent studies showed that elevated glucocorticoids are associated with a decrease in the number of glucocorticoid receptors and with resistance to cortisol suppression by dexamethasone. Consistent with the data from rodents, patients with depression have a significant reduction in the volume of the hippocampus and an elevated loss of declarative memory.
Nemeroff and his colleagues (reviewed in reference 82) have found that in depressed patients, the secretion of CRF is markedly increased. This has suggested the interesting idea that in depressed patients, the neurons in the brain that secrete CRF are hyperactive. Consistent with this idea, when CRF is injected directly into the central nervous system of mammals, it produces many of the signs and symptoms of depression, including decreased appetite, altered autonomic nervous system activity, decreased libido, and disrupted sleep. In view of the evidence that early untoward life experience increases the likelihood in adulthood of suffering from depression or certain anxiety disorders, Nemeroff has suggested that this vulnerability is probably mediated by the hypersecretion of CRF.
These insights are likely to have several applications. First is the development of progressively more refined animal models for the factors that predispose to stress and depression, models that may allow one to identify—in experimental animals and perhaps later in humans—the genes that are activated by CRF and that predispose to anxiety. Second, drugs that block the actions of CRF on its receptors in target tissue may prove useful for certain types of depression. Finally, with increased resolution, one might conceivably be able to follow the therapeutic responses of patients by imaging the hippocampus and seeing to what degree anatomical changes are halted, or even reversed, and by seeing how responses to psychotherapy correlate with levels of CRF and glucocorticoids.
5. The Preconscious Unconscious and the Prefrontal Cortex
We have so far only considered the implicit unconscious. What about the preconscious unconscious concerned with all memories and thought capable of reading consciousness and the repressed or unconscious? We have reasons to believe that aspects of the preconscious unconscious may be mediated by the prefrontal cortex. Perhaps the strongest argument is that the prefrontal cortex is involved in bringing a variety of explicit knowledge to conscious awareness. The prefrontal association cortex has two major functions: it integrates sensory information, and it links it to planned movement. Because the prefrontal cortex mediates these two functions, it is thought to be one of the anatomical substrates of goal-directed action in long-term planning and judgment. Patients with damaged prefrontal association areas have difficulty in achieving realistic goals. As a result, they often achieve little in life, and their behavior suggests that their ability to plan and organize everyday activities is diminished (83, 84).
Over the last two decades, it has become clear that the prefrontal cortex subserves as one component of a system that serves as a critical short-term holding function for information, including information that is stored in or recalled from declarative memory stores. This idea emerged from the discovery that lesions in the prefrontal cortex produce a specific deficit in a short-term component of explicit memory called working memory. The cognitive psychologist Alan Baddeley, who developed the idea of working memory (85), suggested that this type of memory integrates moment-to-moment perceptions across time, rehearses them, and combines them with stored information about past experience, actions, or knowledge. This memory mechanism is crucial for many apparently simple aspects of everyday life: carrying on a conversation, adding a list of numbers, driving a car. Baddeley’s idea was further developed in neurobiological experiments by Joaquin Fuster (86) and Patricia Goldman-Rakic (87), who first suggested that some aspects of working memory are represented in the prefrontal association cortex and that the recall of any explicit information from memory—the recall from preconscious to conscious—requires working memory. A prediction of this finding is that in trace conditioning, the unconditioned stimulus might activate the working memory system of the dorsolateral prefrontal cortex, and thereby it acts, often together with the hippocampus, to render into consciousness the otherwise procedural associative process. Clinical studies of patients with lesions suggest that the prefrontal cortex also seems to represent some aspects of moral judgments; it governs our ability to plan intelligently and responsibly (83). This raises the interesting possibility that the recall of explicit knowledge may depend on an adaptive and realistic evaluation of the information to be recalled. In this sense the prefrontal cortex may, as suggested by Solms (88), be involved in coordinating functions psychoanalysts attribute to the executive functions of the ego on the one hand and the superego on the other.
6. Sexual Orientation and the Biology of Drives
Freud conceived of drives as the energetic components of mind. A drive, he argued, leads to a state of tension or excitation, a state that cognitive psychologists now call the motivational state. Motivational states impel actions with the goal of reducing tension.
Early in his career, perhaps influenced by Havelock Ellis (89), Magnus Hirschfeld (90), and Richard Krafft-Ebing (91), Freud believed that a person’s sexual orientation was significantly influenced by innate developmental processes and that all humans were constitutionally bisexual. This constitutional bisexuality was a key factor in both male and female homosexuality. Later, however, he came to think of sexual orientation as an acquired characteristic. Freud (92) specifically thought of male homosexuality as representing a failure of normal sexual development, a failure of the developing male child to separate himself adequately from an intense sexual bond with his mother. As a result, the grown boy identifies with his mother and seeks to play her role in an attempt to reenact the relationship that existed between them. Freud proposed that the boy’s failure to separate from his mother might be the result of several factors, including a close, binding relationship to a possessive mother and a weak, hostile, or absent father. In terms of his three phases of psychosexual development, Freud saw male homosexuality, with its emphasis on anal intercourse, as a failure to progress normally from the anal to the genital phase. Female homosexuality was defined less clearly in Freud’s mind, but he thought of it as the mirror image of the process he outlined for men. Freud also saw a latent homosexual component in the development of paranoia, alcoholism, and drug addiction.
Freud’s views on sexuality are now at least 50 years old, and in some cases 90 years old. Some have understandably been abandoned by modern psychoanalytic thought, and all have been modified. But I recount them not to hold Freud or the psychoanalytic community responsible for outdated ideas, but to illustrate that any psychological or clinical insight into sexuality, no matter how modern, will almost certainly be clarified by a better biological understanding of gender identification and sexual orientation, even though at the moment we know little. As homosexuality has become more openly accepted by society at large, there has been active discussion within the homosexual community, the psychoanalytic community, and society about the degree to which sexual orientation is inborn or acquired. The observation by Freud and other analysts that some gay men tend to recollect their fathers as hostile or distant and their mothers as unusually close has more recent corroboration (93). However, other studies suggest a genetic contribution to sexual orientation.
This is a complex area, because genotypic gender, phenotypic gender, gender identification, and sexual orientation are distinct from one another but interrelated. Indeed, the recognition of this complexity can render standard terms such as male, female, masculine, and feminine imprecise and in need of qualification (94).
Genotypic gender is determined by the genes, whereas phenotypic gender is defined by the development of the internal and external genitalia (94–96). Gender identification is more subtle and complex and refers to the subjective perception of one’s sex. Finally, sexual orientation refers to the preference for sexual partners. The factors that contribute to the various aspects of gender are not fully understood, but I discuss them because historically this is an area that is central to psychoanalysis; and since the nurture–nature dichotomy is one that biology has repeatedly confronted and sometimes enlightened, this is an area in which biology could make a distinctive contribution. Although gender identification and sexual orientation are complex and have features that are distinctively human and may well not be amenable to study in experimental animals, many other aspects of sexual behavior are much like feeding and drinking behavior—so essential to survival that they are extremely conserved among mammals, involving common brain and hormonal systems and even aspects of stereotypic behavior. As a result, we have learned a good deal about the neural control of sex hormones and behavior from experimental animals such as rats and mice.
Early embryonic development of the gonad is identical in males and females. Genotypic gender is determined by an individual’s complement of sex chromosomes: females have two X chromosomes, whereas males have one X and one Y. Male phenotypic gender is determined by a single gene, called testis determining factor, on the Y chromosome. This gene initiates the development of the bisexual early gonad into a testis, which produces testosterone; in the absence of testis determining factor, the gonad develops into an ovary and produces estrogen. All of the other phenotypic sexual characteristics result from the effects of gonadal hormones on other tissues. Of particular interest both to biologists and to psychoanalysts is that sexual dimorphism extends to the brain and thereby to behavior.
The behavior of males and females differs, even before puberty. Since many aspects of sexuality are conserved among all mammals, sexual behavior relevant to human sexuality can be studied in primates and even in rodents. Young male monkeys participate in more rough-and-tumble play than do female monkeys, a difference related to testosterone levels. Human girls who have been exposed prenatally to unusually high levels of androgens as a result of congenital adrenohyperplasia prefer the same play as boys (95, 97, 98). It seems likely that sex differences in the play behavior of children are influenced at least in part by the organizational effects of the level of prenatal androgens.
The level of testosterone has other dramatic effects on behavior (97, 99–101). Male rats castrated at or prior to birth fail as adults to show the mounting behavior typical of males in the presence of receptive females, even if they are given testosterone. Furthermore, if these rats are given estrogen and progesterone in adulthood, mimicking the hormonal milieu of the adult female rats, they display the same sexually receptive posture typical of females in heat. If castration is performed a few days after birth, neither of these effects occurs. Thus, like perceptual skills and motor coordination, sex-typical behavior is organized during a critical period, around the time of birth, even though the behavior itself is not seen until much, much later.
Sex differences in behavior, to the extent that they manifest differences in brain function, must at least partly result from sex differences in the structure of the central nervous system. One possible anatomical site for these differences is the hypothalamus, which is concerned with sexual behavior as well as a variety of other homeostatic drives (for a review see reference 101). Electrical stimulation of the hypothalamus in intact, awake rhesus monkeys and rats generates sex-typical sexual behavior (102). Biologists have found a striking sexually dimorphic difference in the medial preoptic area of the hypothalamus in rodents (103, 104). Here there are four functional groups of neurons—of unknown function so far —called the interstitial nuclei of the anterior hypothalamus (INAH-1 to INAH-4). One of these nuclei, INAH-3, is five times larger in the male rat than in the female. Many cells in this nucleus die during female development; these cells are rescued in male pups by circulating testosterone and can be rescued in females by testosterone injections during a critical developmental window (105, 106).
There are also sexual dimorphisms in the thickness of various regions of the cerebral cortex in the rat. For example, there is greater asymmetry in the male: the thickness of the left side of a male rat cortex is greater than the right. Perhaps as a consequence, the splenium of the corpus callosum contains more neurons in the female. Other brain regions also show sexual dimorphisms, and doubtless there are more to be found.
The finding of a biological basis for gender genotype and phenotype raises the question, What is the biological basis for sexual orientation? To begin with, it is obvious that as the development of gender is multifactorial, so the etiology of sexual orientation must also be multifactorial; presumably, it is determined by hormones, genes, and environmental factors. A behavioral trait such as sexual orientation almost certainly is not caused by a single gene, a single alteration in a hormone or in brain structure, or a single life experience. The continuing progress in studies of sexually dimorphic characteristics will no doubt help psychoanalysts better understand gender identity and sexual orientation.
Anatomical studies on sexual orientation are just beginning, and we will need much more information before we can have confidence in the published findings on anatomical differences. At the moment they should rather be considered as interesting possibilities. Simon LeVay (93, 107) obtained brains of gay men and presumed heterosexual men, all of whom died of AIDS, and the brains of women. INAH3, the most prominent of the sexually dimorphic nuclei in the rat hypothalamus, was on average two to three times bigger in the presumed heterosexual men than in the women. However, in the gay men INAH3 was on average the same size as in the women. None of the other three INAH nuclei showed any difference between the groups. In addition to potential problems with the sample under study, it is not possible on the basis of LeVay’s observations to say whether the structural differences are present at birth, whether they influence men to become gay or straight, or whether the dimorphism is a result of differences in sexual behavior. But with better sampling and improvements in brain scan imaging techniques, it may be possible to answer these questions.
Allen and Gorski (104) described still another difference between gay and straight men in the anterior commissure, a pathway between the left and right sides of the brain that is generally larger in women than in men. Allen and Gorski found that the anterior commissure is on average larger in gay men than in straight men. In fact, it is larger in gay men than in women (see also reference 108).
Another question that is now being addressed is whether sexual orientation is inherited or acquired (109–115). Sexual orientation seems to be influenced by genes, and this influence is, as one would expect, complex. Sexual orientation runs in families. If a person is gay, the chances of a twin brother being gay increase substantially. In the case of monozygotic twins, individuals who share the same genes, the concordance rate is 50%. For dizygotic twins, the concordance rate is about 25%. By contrast, in the general population, the incidence of male homosexuality is less than 10%. For female homosexuality, the genetic relationship is weaker—about 30% of monozygotic twins and about 15% of dizygotic twins. These numbers seem roughly similar to those of other complex traits, indicating that both genetic and important nongenetic factors operate.
These are all early findings, and their consistency over groups of people, both heterosexual and homosexual, is still being questioned. But the methods are at hand for establishing whether there are reliable anatomical differences between people with different sexual orientations. As I suggested above, either outcome should greatly influence psychoanalytic thinking about the dynamics of sexual orientation.
7. Outcome of Therapy and Structural Changes in the Brain
Recent work in experimental animals indicates that long-term memory leads to alterations in gene expression and to subsequent anatomical changes in the brain. Anatomical changes in the brain occur throughout life and are likely to shape the skills and character of an individual. The representation of body parts in the sensory and motor areas of the cerebral cortex depends on their use and, thus, on the particular experience of the individual. Edward Taub and his colleagues scanned the brains of string instrument players. During performance string players are continuously engaged in skillful hand movement. The second to fifth fingers of the left hand, which contact the strings, are manipulated individually, while the fingers of the right hand, which move the bow, do not express as much patterned, differentiated movement. Brain images of these musicians revealed that their brains were different from the brains of nonmusicians. Specifically, the cortical representation of the fingers of the left hand, but not of the right, was larger in the musicians (figure 4) (for review see references 31 and 116).
Such structural changes are more readily achieved in the early years of life. Thus, Johann Sebastian Bach was Bach not simply because he had the right genes but probably also because he began practicing musical skills at a time when his brain was most sensitive to being modified by experience. Taub and his colleagues (116) found that musicians who learned to play their instruments by the age of 12 years had a larger representation of the fingers of the left hand, their important playing hand, than did those who started later in life (figure 4).
These considerations raise a question central to psychoanalysis: Does therapy work in this way? If so, where do these psychotherapeutically induced changes occur? Do the therapeutically induced structural changes occur at the same sites altered by the mental disorder itself, or are the therapeutically induced changes independent compensatory changes that occur at other related sites?
Long-lasting changes in mental functions involve alteration in gene expression (31, 116). Thus, in studying the specific changes that underlie persistent mental states, normal as well as disturbed, we should also look for altered gene expression. How does altered gene expression lead to long-lasting alteration of a mental process? Animal studies of alterations in gene expression associated with learning indicate that such alterations are followed by changes in the pattern of connections between nerve cells, in some cases the growth and retraction of synaptic connections.
It is intriguing to think that insofar as psychoanalysis is successful in bringing about persistent changes in attitudes, habits, and conscious and unconscious behavior, it does so by producing alterations in gene expression that produce structural changes in the brain. We face the interesting possibility that as brain imaging techniques improve, these techniques might be useful not only for diagnosing various neurotic illnesses but also for monitoring the progress of psychotherapy.
8. Psychopharmacology and Psychoanalysis
As early as 1962, Mortimer Ostow, a psychoanalyst trained in neurology who had a long interest in the relationship of neurobiology to psychoanalysis (117, 118), pointed to the utility of using drugs in the course of psychoanalysis (119). He argued even then that in addition to its therapeutic value, pharmacological intervention can serve as a biological tool for investigating aspects of affective function. Ostow observed that one of the principal effects of psychopharmacological agents is on affect, which led him to argue that affect often is a more important determinant of behavior and of illness than ideation or conscious interpretation. This idea reinforces that of Sanders, Stern, and the Boston Process of Change Study Group on the relative importance of unconscious affect over conscious insight, and stresses once again the importance of changes in unconscious procedural knowledge (such as those that occur during the moments of meaning considered above) as indices of therapeutic progress, indices that the Boston group considers as important as conscious insight. Both the arguments of Ostow and those of the Boston group make clear that changes in the patient’s unconscious internal representations can be beneficial for progress even without reaching consciousness. Perhaps, in these cases, the unconscious is more important than even Freud appreciated! Thus, the theme that emerges from Ostow’s study on the actions of psychopharmacological agents on the psychoanalytic process echoes the ideas of Sanders and Stern, which stress that progress in psychotherapy has an important procedural component and that much of what happens in therapy need not be directly related to insight.
A GENUINE DIALOGUE BETWEEN BIOLOGY AND PSYCHOANALYSIS IS NECESSARY IF WE ARE TO ACHIEVE A COHERENT UNDERSTANDING OF MIND
As I have suggested earlier, most biologists believe that the mind will be to the twenty-first century what the gene was to the twentieth century. I have briefly discussed how the biological sciences in general and cognitive neuroscience in particular are likely to contribute to a deeper understanding of a number of key issues in psychoanalysis. An issue that is often raised is that a neurobiological approach to psychoanalytic issues would reduce psychoanalytic concepts to neurobiological ones. If that were so, it would deprive psychoanalysis of its essential texture and richness and change the character of therapy. Such a reduction is not simply undesirable but impossible. The agendas for psychoanalysis, cognitive psychology, and neural science overlap, but they are by no means identical. The three disciplines have different perspectives and aims and would only converge on certain critical issues.
The role of biology in this endeavor is to illuminate those directions that are most likely to provide deeper insights into specific paradigmatic processes. Biology’s strength is its rigorous way of thinking and its depth of analysis. Our understanding of heredity, gene regulation, the cell, antibody diversity, the development of the body plan and of the brain, and the generation of behavior have been profoundly expanded as biology probes progressively deeper into the molecular dynamics of life processes. The strengths of psychoanalysis are its scope and the complexity of the issues it addresses, strengths that cannot be diminished by biology. Just as medicine has time and again provided direction to biology, and psychiatry to neuroscience, so can psychoanalysis serve as a skillful and reality-oriented tutor for a sophisticated understanding of the mind-brain.
During the past half-century we have repeatedly seen successful unifications within the biological sciences without the disappearance of the core disciplines. For example, classical genetics and molecular biology have merged into a common discipline, molecular genetics. We now know that the traits that Gregor Mendel described and the genes on specific locations on chromosomes that Thomas Hunt described are stretches of double-stranded DNA. This insight has allowed us to understand how genes replicate and how they control cellular function. These insights have revolutionized biology, but this has hardly abolished the discipline of genetics. To the contrary, with the human genome expected to be completed in the year 2003, genetics is flourishing. It has used the powerful insight of molecular biology, applied it effectively to its own agenda, and moved on. So be it with psychoanalysis.
ARE WE SEEING THE BEGINNINGS OF A DIALOGUE?
As we have seen, biology could help psychoanalysis in two ways: conceptually and experimentally. We are in fact already beginning to see signs of conceptual progress. A number of psychoanalytic institutes, or at least a number of people within psychoanalysis, have struggled to make psychoanalysis more rigorous and to align it more closely with biology. Freud argued for this position at the beginning of his career. More recently, Mortimer Ostow of the Neuroscience Project of the New York Psychoanalytic Institute and David Olds and Arnold Cooper at the Columbia Institute (120), as well as others across the country, have earlier expressed ideas similar to those I outline here.
For many years both the Association for Psychoanalytic Medicine at Columbia and the New York Psychoanalytic Institute, to use but two examples, have instituted (with the help of my colleague, James H. Schwartz) neuropsychoanalytic centers that address interests common to psychoanalysis and neuroscience, including consciousness, unconscious processing, autobiographical memory, dreaming, affect, motivation, infantile mental development, psychopharmacology, and the etiology and treatment of mental illness. The prospectus of the New York Psychoanalytic Institute now reads as follows:
The explosion of new insights into numerous problems of vital interest to psychoanalysis needs to be integrated in meaningful ways with the older concepts and methods as do the burgeoning research technologies and pharmacological treatments. Similarly neuroscientists exploring the complex problems of human subjectivity for the first time have much to learn from a century of analytic inquiry.
Thus, psychoanalysts are beginning to learn about neural science and psychopharmacology, an exciting step forward, a step that should lead in the long run to the new curriculum for the analytic clinician.
As a result of these efforts, there has been a bit of progress in the second function of biology, the experimental function. Several investigators have seen the exciting possibility of merging psychoanalysis and biology experimentally. Most commendable are the important attempts by Karen Kaplan-Solms and Mark Solms (121) to delineate anatomical systems in the brain that are relevant to psychoanalysis by studying alterations in the mental functioning of patients with brain lesions. Kaplan-Solms and Solms believe that the power of psychoanalysis derives from its ability to investigate mental processes from a subjective perspective. However, as they point out, this very strength is also its greatest weakness. Subjective phenomena do not readily lend themselves to objective empirical analysis. We need to develop creative ways of studying subjective phenomena. As a result, these investigators argue that only by connecting psychoanalytic thought to objective neurobiological phenomena, as in personality changes following focal lesions of the brain, can one derive empirical correlates of the subjectively derived constructs of psychoanalysis. Similarly, there is also the important and long-standing tradition of work by Howard Shevrin, correlating the perception of subliminal and supraliminal stimuli with event-related potentials in the brain in an attempt to analyze aspects of unconscious mental processes (5, 46).
These beginnings are extremely encouraging. But for psychoanalysis to be reinvigorated, it will need to match its intellectual restructuring with institutional changes. For biology to help, two aspects of psychoanalysis require particular attention: therapeutic outcome and the role of psychoanalytic institutes.
THE EVALUATION OF PSYCHOANALYTIC OUTCOME
As a mode of therapy, psychoanalysis is no longer as widely practiced as it was 50 years ago. Jeffrey (122) claims that the number of patients seeking psychoanalysis steadily decreased by 10% a year over the last 20 years, as has the number of gifted psychiatrists seeking training in psychoanalytic institutes. This decline is disappointing, because psychoanalytic therapy seems to have become more realistically focused and therefore is more likely to be efficacious. During the last several decades, psychoanalysis has largely abandoned the unrealistic goals of the 1950s, when it attempted to treat by itself autism, schizophrenia, and severe bipolar illness, illnesses for which it had little, if anything, to offer. Nowadays, psychoanalysis is thought to be most successful for people with the nonpsychotic character disorders, people who have major deficits in working effectively or maintaining satisfactory relationships and who want to acquire better ways of managing their lives. A substantial number of these patients suffer from borderline personality disorder with concomitant disturbances of affect. In these cases, psychoanalysis and psychoanalytically oriented psychotherapy are thought to be an important adjunct to pharmacotherapy (see reference 123 for distribution of patients seen in psychoanalysis). As a result of this narrower focus on patients who are not psychotic, psychoanalysis and psychoanalytically oriented psychotherapy may in the best of hands be more effective today than ever before.
I am here reminded of Kay Jamison’s haunting discussion of her own manic-depressive illness and her effective response to combined lithium medication and psychotherapy (124):
At this point in my existence, I cannot imagine leading a normal life without both taking lithium and having had the benefits of psychotherapy. Lithium prevents my seductive but disastrous highs, diminishes my depressions, clears out the wool and webbing from my disordered thinking, slows me down, gentles me out, keeps me from ruining my career and relationships, keeps me out of a hospital, alive, and makes psychotherapy possible. But, ineffably, psychotherapy heals. It makes some sense of the confusion, reins in the terrifying thoughts and feelings, returns some control and hope and possibility of learning from it all. Pills cannot, do not, ease one back into reality; they only bring one back headlong, careening, and faster than can be endured at times. Psychotherapy is a sanctuary; it is a battleground; it is a place I have been psychotic, neurotic, elated, confused, and despairing beyond belief. But, always, it is where I have believed or have learned to believe—that I might someday be able to contend with all of this.
No pill can help me deal with the problem of not wanting to take pills; likewise, no amount of psychotherapy alone can prevent my manias and depressions. I need both. It is an odd thing, owing life to pills, one’s own quirks and tenacities, and this unique, strange, and ultimately profound relationship called psychotherapy.
Given these advances, why is the practice of psychoanalysis no longer thriving? This decline in the use of psychoanalytic therapy is mostly attributable to causes outside psychoanalysis: the proliferation of different forms of short-term psychotherapy (almost all of which are, to varying degrees, derived from psychoanalysis), the emergence of pharmacotherapy, and the economic impact of managed care. But one important cause derives from psychoanalysis itself. One full century after its founding, psychoanalysis still has not made the required effort to obtain objective evidence to convince an increasingly skeptical medical profession that it is a more effective mode of therapy than placebo. Thus, unlike various forms of cognitive therapy and other psychotherapies, for which compelling objective evidence now exists—both as therapies in their own right and as key adjuncts to pharmacotherapy—there is as yet no compelling evidence, outside subjective impressions, that psychoanalysis works better than nonanalytically oriented therapy or placebo (125–133).
The failure of psychoanalysis to provide objective evidence that it is effective as a therapy can no longer be accepted. Psychoanalysts must be persuaded by Arnold Cooper’s realistic and critical view (125):
To the extent that psychoanalysis lays claim to being a method of treatment, we are, for better or worse, drawn into the orbit of science, and we cannot then escape the obligations of empirical research. As long as we develop practitioners who are members of a profession and charge for their services, it is incumbent upon us to study what we do and how we affect our patients.
As Cooper points out, a number of the major studies initially designed to evaluate the outcome of therapy—Wallerstein’s study (134) and the studies reviewed by Kantrowitz (129) and by Bachrach (135)—have abandoned their long-term goal for a more accessible short-term aim unrelated to outcome. Despite their cost and complexity, rigorous outcome studies, with comparison to short-term nonanalytically oriented psychotherapy and placebo, need to be at the top of any list of priorities if psychoanalysis is to continue to be a well-recognized therapeutic option.
A FLEXNER REPORT FOR THE PSYCHOANALYTIC INSTITUTES?
But the much more difficult step is to go beyond an appreciation of biology and of having a tiny cadre of full-time researchers to the development within psychoanalysis of an intellectual climate that will make a significant fraction of psychoanalysts technically competent in cognitive neuroscience and eager to test their own ideas with new methods. The challenge for psychoanalysts is to become active participants in the difficult joint attempt of biology and psychology, including psychoanalysis, to understand the mind. If this transformation in the intellectual climate of psychoanalysis is to occur, as I believe it must, the psychoanalytic institutes themselves must change from being vocational schools—guilds, as it were—to being centers of research and scholarship.
At the cusp of the twenty-first century, the psychoanalytic institutes in the United States resemble the proprietary medical schools that populated this country in the early 1900s. At the turn of the last century, the United States experienced a great proliferation of medical schools—155 all told—most of which had no laboratories for teaching the basic sciences. At these schools, medical students were taught by private practitioners who often were busy with their own practices.
To examine this problem, the Carnegie Foundation commissioned Abraham Flexner to study medical education in the United States. The Flexner Report (136), which was completed in 1910, emphasized that medicine is a science-based profession and requires a structured education in both basic science and its application to clinical medicine. To promote a quality education, the Flexner Report recommended limiting the medical schools in this country to those that were integral to a university. As a consequence of this report, many inadequate schools were closed, and credentialed standards for the training and practice of medicine were established. To return to its former vigor and contribute importantly to our future understanding of mind, psychoanalysis needs to examine and restructure the intellectual context in which its scholarly work is done and to develop a more critical way of training the psychoanalysts of the future. Thus, what psychoanalysis may need, if it is to survive as an intellectual force into the twenty-first century, is something akin to a Flexner Report for the psychoanalytic institutes.
What drew so many of us to psychoanalysis in the late 1950s and early 1960s was its bold curiosity—its investigative zeal. I myself was drawn to the neurobiological study of memory because I saw memory as central to a deeper understanding of the mind, an interest first sparked by psychoanalysis. One would hope that the excitement and success of current biology would rekindle the investigative curiosities of the psychoanalytic community and that a unified discipline of neurobiology, cognitive psychology, and psychoanalysis would forge a new and deeper understanding of mind.
Received Oct. 22, 1998; revision received Feb. 16, 1999; accepted Feb. 19, 1999. From the Howard Hughes Medical Institute and Center for Neurobiology and Behavior, Departments of Psychiatry and Biochemistry and Molecular Biophysics, Columbia University College of Physicians and Surgeons. Address reprint requests to Dr. Kandel, 722 West 168th St., New York, NY 10032. In the course of working on this article, I have benefited greatly from insightful discussions with Marianne Goldberger, who also gave critical comments on earlier drafts of this manuscript. In addition, I have received helpful suggestions from Nancy Andreasen, Mark Barad, Robert Glick, Jack Gorman, Myron Hofer, Anton O. Kris, Charles Nemeroff, Russell Nicholls, David Olds, Mortimer Ostow, Chris Pittenger, Stephen Rayport, Michael Rogan, James Schwartz, Theodore Shapiro, Mark Solms, Anna Wolff, and Marc Yudkoff.
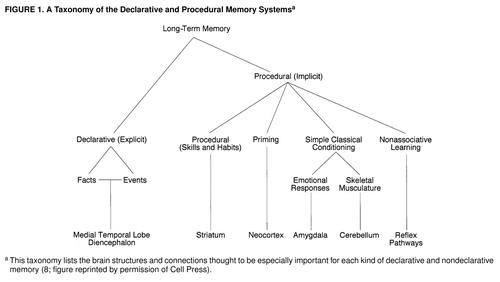
FIGURE 1. A Taxonomy of the Declarative and Procedural Memory Systems
This taxonomy lists the brain structures and connections thought to be especially important for each kind of declarative and nondeclarative memory (8; figure reprinted by permission of Cell Press).
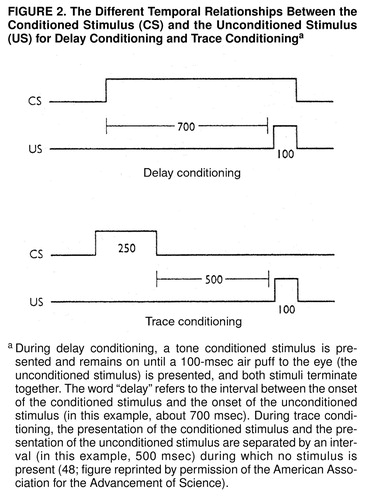
FIGURE 2. The Different Temporal Relationships Between the Conditioned Stimulus (CS) and the Unconditioned Stimulus (US) for Delay Conditioning and Trace Conditioning
During delay conditioning, a tone conditioned stimulus is presented and remains on until a 100-msec air puff to the eye (the unconditioned stimulus) is presented, and both stimuli terminate together. The word “delay” refers to the interval between the onset of the conditioned stimulus and the onset of the unconditioned stimulus (in this example, about 700 msec). During trace conditioning, the presentation of the conditioned stimulus and the presentation of the unconditioned stimulus are separated by an interval (in this example, 500 msec) during which no stimulus is present (48; figure reprinted by permission of the American Association for the Advancement of Science; http://www.sciencemag.org).
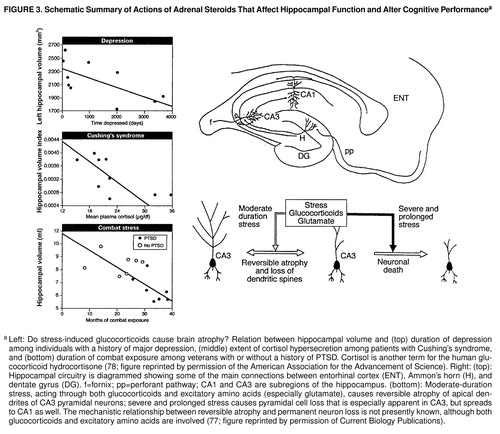
FIGURE 3. Schematic Summary of Actions of Adrenal Steroids That Affect Hippocampal Function and Alter Cognitive Performance
Left: Do stress-induced glucocorticoids cause brain atrophy? Relation between hippocampal volume and (top) duration of depression among individuals with a history of major depression, (middle) extent of cortisol hypersecretion among patients with Cushing’s syndrome, and (bottom) duration of combat exposure among veterans with or without a history of PTSD. Cortisol is another term for the human glucocorticoid hydrocortisone (78; figure reprinted by permission of the American Association for the Advancement of Science; http://www.sciencemag.org). Right: (top): Hippocampal circuitry is diagrammed showing some of the main connections between entorhinal cortex (ENT), Ammon’s horn (H), and dentate gyrus (DG). f=fornix; pp=perforant pathway; CA1 and CA3 are subregions of the hippocampus. (bottom): Moderate-duration stress, acting through both glucocorticoids and excitatory amino acids (especially glutamate), causes reversible atrophy of apical dendrites of CA3 pyramidal neurons; severe and prolonged stress causes pyramidal cell loss that is especially apparent in CA3, but spreads to CA1 as well. The mechanistic relationship between reversible atrophy and permanent neuron loss is not presently known, although both glucocorticoids and excitatory amino acids are involved (77; figure reprinted by permission of Current Biology Publications).
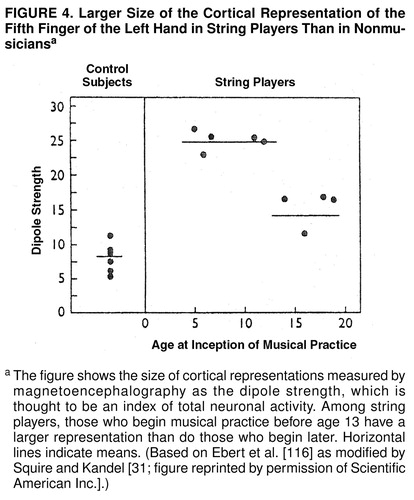
FIGURE 4. Larger Size of the Cortical Representation of the Fifth Finger of the Left Hand in String Players Than in Nonmusicians
The figure shows the size of cortical representations measured by magnetoencephalography as the dipole strength, which is thought to be an index of total neuronal activity. Among string players, those who begin musical practice before age 13 have a larger representation than do those who begin later. Horizontal lines indicate means. (Based on Ebert et al. [116] as modified by Squire and Kandel [31; figure reprinted by permission of Scientific American Inc.].)
1. Kandel ER: A new intellectual framework for psychiatry. Am J Psychiatry 1998; 155:457–469Link, Google Scholar
2. Freud S: On narcissism: an introduction (1914), in Complete Psychological Works, standard ed, vol 14. London, Hogarth Press, 1957, pp 67–102Google Scholar
3. Freud S: Beyond the pleasure principle (1920), in Complete Psychological Works, standard ed, vol 18. London, Hogarth Press, 1955, pp 7–64Google Scholar
4. Shapiro T, Emde RN (eds): Research in Psychoanalysis: Process, Development, Outcome. Madison, Conn, International Universities Press, 1995Google Scholar
5. Shevrin H: Psychoanalytic and neuroscience research. Am Psychoanalyst 1998; 32(3)Google Scholar
6. Levin FM: A brief history of analysis and cognitive neuroscience. Am Psychoanalyst 1998; 32(3)Google Scholar
7. Isenstadt L: The neurobiology of childhood emotion: anxiety. Am Psychoanalyst 1998; 32(3)Google Scholar
8. Milner B, Squire LR, Kandel ER: Cognitive neuroscience and the study of memory. Neuron Rev 1998; 20:445–468Crossref, Medline, Google Scholar
9. Eissler KR: Irreverent remarks about the present and future of psychoanalysis. Int J Psychoanal 1969; 50:461–471Google Scholar
10. Luborsky L, Luborsky E: The era of measures of transference: the CCRT and other measures, in Research in Psychoanalysis: Process, Development, Outcome. Edited by Shapiro T, Emde RN. Madison, Conn, International Universities Press, 1995, pp 329–351Google Scholar
11. Dahl H: The measurement of meaning in psychoanalysis by computer analysis of verbal contexts. J Am Psychoanal Assoc 1974; 22:37–57Crossref, Medline, Google Scholar
12. Teller V, Dahl H: What psychoanalysis needs is more empirical research, in Research in Psychoanalysis: Process, Development, Outcome. Edited by Shapiro T, Emde RN. Madison, Conn, International Universities Press, 1995, pp 31–49Google Scholar
13. Boring EG: A History of Experimental Psychology. New York, Appleton-Century, 1950, p 713Google Scholar
14. Edelson M: Hypothesis and Evidence in Psychoanalysis. Chicago, University of Chicago Press, 1984Google Scholar
15. Reiser M: Changing theoretical concepts in psychosomatic medicine, in American Handbook of Psychiatry, 2nd ed, vol IV. Edited by Reiser M; Arieti S, editor-in-chief. New York, Basic Books, 1975, pp 477–500Google Scholar
16. Shapiro T: Discussion of the structural model in relation to Solm’s neuroscience-psychoanalysis integration: the ego. J Clin Psychoanal 1996; 5:369–379Google Scholar
17. Roth MS (ed): Freud: Conflict and Culture: Essays on His Life, Work, and Legacy. New York, Alfred A Knopf, 1998Google Scholar
18. Freud S: New introductory lectures on psycho-analysis (1933 [1932]), in Complete Psychological Works, standard ed, vol 22. London, Hogarth Press, 1964, pp 1–182Google Scholar
19. Freud A: The Ego and the Mechanisms of Defense. London, Hogarth Press, 1936Google Scholar
20. Hartmann H: Ego Psychology and the Problem of Adaptation (1939). Translated by Rapaport D. New York, International Universities Press, 1958Google Scholar
21. Spitz RA: Hospitalism: an inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal Study Child 1945; 1:53–74Crossref, Medline, Google Scholar
22. Klein J: Envy and Gratitude. London, Tavistock, 1957Google Scholar
23. Bowlby J: Grief and mourning in infancy and early childhood. Psychoanal Study Child 1960; 15:9–52Crossref, Google Scholar
24. Erikson E: Childhood and Society. New York, WW Norton, 1963Google Scholar
25. Winnicott DW: The depressive position in normal emotional development (1954), in Through Paediatrics to Psycho-Analysis: Collected Papers. New York, Basic Books, 1958, pp 262–277Google Scholar
26. Kohut H: The Analysis of the Self: A Systematic Approach to the Psychoanalytic Treatment of Narcissistic Personality Disorders. New York, International Universities Press, 1971Google Scholar
27. Lear J: Open Minded, Working Out the Logic of the Soul. Cambridge, Mass, Harvard University Press, 1998Google Scholar
28. Jacob F: Of Flies, Mice and Men. Cambridge, Mass, Harvard University Press, 1998Google Scholar
29. Scoville WB, Milner B: Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957; 20:11–21Crossref, Medline, Google Scholar
30. Squire LR, Zola-Morgan S: The medial temporal lobe memory system. Science 1996; 253:1380–1386Crossref, Google Scholar
31. Squire LS, Kandel ER: Memory: From Molecules to Memory: Scientific American Library. New York, Freeman Press, 1999Google Scholar
32. Solms M: What is consciousness? Charles Fischer Memorial Lecture to the New York Psychoanalytic Society. J Am Psychoanal Assoc 1996; 45:681–778Crossref, Google Scholar
33. Lyons-Ruth K: Implicit relational knowing: its role in development and psychoanalytic treatment. Infant Ment Health J 1998; 19:282–289Crossref, Google Scholar
34. Clyman R: The procedural organization of emotion: a contribution from cognitive science to the psychoanalytic therapy of therapeutic action. J Am Psychoanal Assoc 1991; 39:349–381Google Scholar
35. Boston Process of Change Study Group: Interventions that effect change in psychotherapy: a model based on infant research. Infant Ment Health J 1998; 19:277–353Crossref, Google Scholar
36. Sanders L: Introductory comment. Infant Ment Health J 1998; 19:280–281Crossref, Google Scholar
37. Stern D: The process of therapeutic change involving implicit knowledge: some implications of developmental observations for adult psychotherapy. Infant Ment Health J 1998; 19:300–308Crossref, Google Scholar
38. Goldberger M: Daydreams: even more secret than dreams, in Symposium: The Secret of Dreams, Western New England Psychoanalytic Society. New Haven, Conn, Yale University, 1996Google Scholar
39. Kris AO: Free Association, Method and Practice. New Haven, Conn, Yale University Press, 1982Google Scholar
40. Brenner C: An Elementary Textbook of Psychoanalysis, 2nd ed. New York, International Universities Press, 1978Google Scholar
41. Pavlov I: Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Translated by Anrep GV. London, Oxford University Press, 1927Google Scholar
42. Dickinson A: Contemporary Animal Learning Theory. Cambridge, UK, Cambridge University Press, 1980Google Scholar
43. Domjan M, Burkhard B: The Principles of Learning and Behavior, 2nd ed. Monterey, Calif, Brooks/Cole, 1986Google Scholar
44. Rescorla RA: Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci 1988; 11:329–352Crossref, Medline, Google Scholar
45. Fanselow MS: Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron Minireview 1998; 20:625–627Crossref, Medline, Google Scholar
46. Shevrin H, Bond J, Brakel LAW, Hertel RK, Williams WJ: Conscious and Unconscious Processes: Psychodynamic, Cognitive and Neurophysiological Convergences. New York, Guilford Press, 1996Google Scholar
47. Kamin L: Predictability, surprise, attention, and conditioning, in Punishment and Aversive Behavior. Edited by Campbell BA, Church RM. New York, Appleton-Century Crofts, 1969, pp 279–296Google Scholar
48. Clark RE, Squire LR: Classical conditioning and brain systems: the role of awareness. Science 1998; 280:77–81Crossref, Medline, Google Scholar
49. Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ: Hippocampal and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci 1986; 100:729–744Crossref, Medline, Google Scholar
50. Kim JJ, Clark RE, Thompson RF: Hippocampectomy impairs the membrane of recently but not remotely acquired trace eye blink conditioned responses. Behav Neurosci 1995; 109:195–203Crossref, Medline, Google Scholar
51. Eichenbaum H: Amnesia, the hippocampus, and episodic memory (editorial). Hippocampus 1998; 8:197Crossref, Medline, Google Scholar
52. Freud S: Inhibitions, symptoms and anxiety (1926 [1925]), in Complete Psychological Works, standard ed, vol 20. London, Hogarth Press, 1959, pp 77–175Google Scholar
53. LeDoux J: The Emotional Brain. New York, Simon & Schuster, 1996Google Scholar
54. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RI, Strauss MM, Hyman SE, Rosen BR: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17:875–887Crossref, Medline, Google Scholar
55. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA: Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1996; 18:411–418Google Scholar
56. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Link, Google Scholar
57. Heim C, Owens MJ, Plotsky PM, Nemeroff CB: Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder, I: endocrine factors in the pathophysiology of mental disorders. Psychopharmacol Bull 1997; 33:185–192Medline, Google Scholar
58. Heim C, Owens MJ, Plotsky PM, Nemeroff CB: The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: focus on corticotropin-releasing factor. Ann NY Acad Sci 1997; 821:194–207Crossref, Medline, Google Scholar
59. Brown GW, Harris T, Copeland JR, Kendler KS: Depression and loss. Br J Psychiatry 1997; 130:1–18Crossref, Google Scholar
60. Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Trudart T, Bloch M, Heresco-Levy U, Lerer B: Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry (in press)Google Scholar
61. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: Childhood parental loss and adult psychopathology in women: a twin study perspective. Arch Gen Psychiatry 1992; 49:109–116Crossref, Medline, Google Scholar
62. Freud A, Burlingham D: Infants Without Families: Writings 3, 1944. New York, International Universities Press, 1973Google Scholar
63. Harlow H: The nature of love. Am J Psychol 1958; 13:673–686Crossref, Google Scholar
64. Harlow HF, Dodsworth RO, Harlow MK: Total social isolation in monkey. Proc Natl Acad Sci USA 1965; 54:90–97Crossref, Medline, Google Scholar
65. Bowlby J: Attachment and Loss, vols 1, 2. New York, Basic Books, 1969, 1973Google Scholar
66. Levine S: Infantile experience and resistance to physiological stress. Science 1957; 126:405–406Crossref, Medline, Google Scholar
67. Levine S: Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science 1962; 135:795–796Crossref, Medline, Google Scholar
68. Levine S, Haltmeyer GC, Kaas GG, Penenberg VH: Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2:55–63Crossref, Google Scholar
69. Ader R, Grota LJ: Effects of early experience on adrenocortical reactivity. Physiol Behav 1969; 4:303–305Crossref, Google Scholar
70. Hofer MA: The Roots of Human Behavior. New York, WH Freeman, 1981Google Scholar
71. Hofer MA: Hidden regulators in attachment, separation, and loss. Monogr Soc Res Child Dev 1994; 59:192–207Crossref, Medline, Google Scholar
72. Selye H: A syndrome produced by diverse nocuous agents. Nature 1936; 138:22–36Crossref, Google Scholar
73. Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ: Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997; 277:1659–1662Crossref, Medline, Google Scholar
74. Plotsky PM, Meaney MJ: Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 1993; 18:195–200Crossref, Medline, Google Scholar
75. Nemeroff CB: The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Molecular Psychiatry 1996; 1:326–342Google Scholar
76. Meaney MJ, Aitken DH, Sapolsky RM: Environmental regulation of the adrenocortical stress response in female rats and its implications for individual differences in aging. Neurobiol Aging 1991; 12:31–38Crossref, Medline, Google Scholar
77. McEwen BS, Sapolsky RM: Stress and cognitive function. Curr Opin Neurobiol 1995; 5:205–216Crossref, Medline, Google Scholar
78. Sapolsky RM: Why stress is bad for your brain. Science 1996; 273:749–750Crossref, Medline, Google Scholar
79. Starkman MN, Gebarski SS, Berent S, Schteingart DE: Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry 1992; 32:756–765Crossref, Medline, Google Scholar
80. Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazuro C, Capelli S, McCarthy G, Innis RB, Charney DS: Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 1997; 41:23–32Crossref, Medline, Google Scholar
81. Sachar EJ: Neuroendocrine dysfunction in depressive illness. Annu Rev Med 1976; 27:389–396Crossref, Medline, Google Scholar
82. Nemeroff CB: The neurobiology of depression. Sci Am 1998; 278:28–35Crossref, Medline, Google Scholar
83. Damasio A: The somatic marker hypothesis and the possible functions of the prefrontal cortex: review. Philos Trans R Soc London B Biol Sci 1996; 351:1413–1420Crossref, Medline, Google Scholar
84. Damasio AR: Descartes’ Error: Emotion, Reason and the Human Brain. New York, Putnam, 1994Google Scholar
85. Baddeley A: Working Memory. New York, Oxford University Press, 1986Google Scholar
86. Fuster JM: The Prefrontal Cortex: Anatomy, Physiology, and Neurophysiology of the Frontal Lobe, 3rd ed. Philadelphia, Lippincott-Raven, 1997Google Scholar
87. Goldman-Rakic PS: Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 1996; 93:13473–13480Crossref, Medline, Google Scholar
88. Solms M: Preliminaries for an integration of psychoanalysis and neuroscience. Br Psychoanal Soc Bull 1998; 34:23–37Google Scholar
89. Ellis H: The development of the sexual instinct. The Alienist and Neurologist 1901; 22:500–521; 615–623Google Scholar
90. Hirschfeld M: Die Objective Diagnose de Homosexualität. Jahrbuch f�r sexuelle Zwischenstufen, 1899Google Scholar
91. Krafft-Ebing R: Neuen Studien auf dem Gebiete der Homosexualitat. Jahrbuch fur sexuelle Zwischenstafen 1901; 3:1–36Google Scholar
92. Freud S: Three essays on the theory of sexuality (1905), in Complete Psychological Works, standard ed, vol 7. London, Hogarth Press, 1953, pp 125–243Google Scholar
93. LeVay S: The Sexual Brain. Cambridge, Mass, MIT Press, 1997Google Scholar
94. Bell AP, Weinberg MS, Hammersmith SK: Sexual Preference: Its Development in Men and Women. New York, Simon & Schuster, 1981Google Scholar
95. Gorski RA: Sexual differentiation of the nervous system, in Principles of Neural Science, 4th ed. Edited by Kandel ER, Schwartz JH, Jessell T. Stamford, Conn, Appleton & Lange (in press)Google Scholar
96. Green R: Gender identity in childhood and later sexual orientation: follow-up of 78 males. Am J Psychiatry 1985; 142:339–341Link, Google Scholar
97. Gorski RA: Gonadal hormones and the organization of brain structure and function, in Lifespan Development of Individuals: Behavioral, Neurobiological, and Psychosocial Perspectives. Edited by Masgnusson D. New York, Cambridge University Press, 1996, pp 315–340Google Scholar
98. Schiavi RC, Theilgaard A, Owen DR, White D: Sex chromosome anomalies, hormones, and sexuality. Arch Gen Psychiatry 1988; 45:19–24Crossref, Medline, Google Scholar
99. Gladue BA, Clemens LG: Androgenic influences on feminine sexual behavior in male and female rats: defeminization blocked by prenatal androgen. Endocrinology 1978; 103:1702–1709Crossref, Medline, Google Scholar
100. Imperato-McGinley J, Pichardo M, Gautier T, Voyer D, Bryden MP: Cognitive abilities in androgen-insensitive subjects: comparison with control males and females from the same kindred. Clin Endocrinol 1991; 34:341–347Crossref, Medline, Google Scholar
101. Knobil E, Neil J (eds): Physiology of Reproduction. Philadelphia, Lippincott-Raven, 1994Google Scholar
102. Perachio AA, Mar LD, Alexander M: Sexual behavior in male rhesus monkeys elicited by electrical stimulation of preoptic and hypothalamic areas. Brain Res 1979; 177:127–144Crossref, Medline, Google Scholar
103. Allen LS, Hines M, Shryne JE, Gorski RA: Two sexually dimorphic cell groups in the human brain. J Neurosci 1989; 9:497–506Crossref, Medline, Google Scholar
104. Allen LS, Gorski RA: Sexual orientation and size of the anterior commissure in the human brain. Proc Natl Acad Sci USA 1992; 89:7199–7202Crossref, Medline, Google Scholar
105. Davis EC, Popper P, Gorski RA: The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res 1996; 734:10–18Crossref, Medline, Google Scholar
106. Dodson RE, Gorski RA: Testosterone propionate administration prevents the loss of neurons within the central part of the medial preoptic nucleus. J Neurobiol 1993; 24:80–88Crossref, Medline, Google Scholar
107. LeVay S: A difference in hypothalamic structure between heterosexual and homosexual men. Science 1991; 253:1034–1037Crossref, Medline, Google Scholar
108. Zhou J, Hofman MA, Gooren LG, Swaab DR: A sex difference in the human brain and its relation to transsexuality. Nature 1995; 378:68–70Crossref, Medline, Google Scholar
109. Pillard RC, Weinrich JD: Evidence of familial nature of male homosexuality. Arch Gen Psychiatry 1986; 43:808–812Crossref, Medline, Google Scholar
110. Bailey JM, Pillard RC: A genetic study of male sexual orientation. Arch Gen Psychiatry 1991; 48:1089–1096Crossref, Medline, Google Scholar
111. Bailey JM, Pillard RC, Neale MC, Agyei Y: Heritable factors influence sexual orientation in women. Arch Gen Psychiatry 1993; 50:217–223Crossref, Medline, Google Scholar
112. Eckert ED, Bouchard TJ, Bohlen J, Heston LL: Homosexuality in monozygotic twins reared apart. Br J Psychiatry 1986; 148:421–425Crossref, Medline, Google Scholar
113. Dörner G, Poppe I, Stahl F, Kolzsch J, Uebelhack R: Gene and environment-dependent neuroendocrine etiogenesis of homosexuality and transsexualism. Exp Clin Endocrinol 1991; 98:141–150Crossref, Medline, Google Scholar
114. Hamer DH, Hu S, Magnuson VL, Hu N, Pattatucci AML: A linkage between DNA markers on the X chromosome and male sexual orientation. Science 1993; 261:321–327Crossref, Medline, Google Scholar
115. Whitman FL, Diamond M, Martin J: Homosexual orientation in twins: a report on 61 pairs and three triplet sets. Arch Sex Behav 1993; 22:187–206Crossref, Medline, Google Scholar
116. Ebert T, Panter C, Wienbruch C, Hoke M, Rockstrom B, Taub E: Increased use of the left hand in string players associated with increased cortical representation of the fingers. Science 1995; 220:21–23Google Scholar
117. Ostow M: The psychoanalytic contribution to the study of brain function, I: frontal lobes. Psychoanal Q 1954; 23:317–338Crossref, Medline, Google Scholar
118. Ostow M: The psychoanalytic contribution to the study of brain function, II: the temporal lobes; III: synthesis. Psychoanal Q 1954; 24:383–423Google Scholar
119. Ostow M: Drugs in Psychoanalysis and Psychotherapy. New York, Basic Books, 1962Google Scholar
120. Olds D, Cooper AM: Dialogues with other sciences: opportunities for mutual gain. Int J Psychoanal 1997; 78:219–225Medline, Google Scholar
121. Kaplan-Solms K, Solms M: Clinical Studies in Neuro-Psychoanalysis. New York, International Universities Press (in press)Google Scholar
122. Jeffrey DW: Lead article. Am Psychoanalyst 1998; 32(1)Google Scholar
123. Friedman RC, Bucci W, Christian C, Drucker P, Garrison WB III: Private psychotherapy patients of psychiatrist psychoanalysts. Am J Psychiatry 1998; 155:1772–1774Link, Google Scholar
124. Jamison K: An Unquiet Mind. New York, Vintage Books, 1996Google Scholar
125. Cooper A: Discussion: on empirical research, in Research in Psychoanalysis: Process, Development, Outcome. Edited by Shapiro T, Emde RN. Madison, Conn, International Universities Press, 1995, pp 381–391Google Scholar
126. Seligman MEP: The effectiveness of psychotherapy: the Consumer Reports study. Am Psychol 1995; 50:965–974Crossref, Medline, Google Scholar
127. Bachrach H, Galatzer-Levy R, Skolnikoff A, Waldron S: On the efficacy of psychoanalysis. J Am Psychoanal Assoc 1991; 39:871–916Crossref, Medline, Google Scholar
128. Doidge N: Empirical evidence for the efficacy of psychoanalytic psychotherapies and psychoanalysis: an overview. Psychoanal Inquiry Suppl 1997:102–150Google Scholar
129. Kantrowitz JL: The uniqueness of the patient-analyst pair: approaches for elucidating the analyst’s role. Int J Psychoanal 1993; 74:893–904Medline, Google Scholar
130. Weissman MM, Prusoff BA, DiMascio A, Neu C, Goklaney M, Klerman GL: The efficacy of drugs and psychotherapy in the treatment of acute depressive episodes. Am J Psychiatry 1979; 136:555–558Medline, Google Scholar
131. Weissman MM, Markowitz JC: Interpersonal psychotherapy. Arch Gen Psychiatry 1994; 51:599–606Crossref, Medline, Google Scholar
132. Roth A, Fonagy P: What Works for Whom? A Critical Review of Psychotherapy Research. New York, Guilford Press, 1996Google Scholar
133. Fonagy P (ed): An Open Door Review of Outcome Studies in Psychoanalysis. London, International Psychoanalytical Association, Research Committee, 1999Google Scholar
134. Wallerstein RS: The effectiveness of psychotherapy and psychoanalysis: conceptual issues and empirical work, in Research in Psychoanalysis: Process, Development, Outcome. Edited by Shapiro T, Emde RN. Madison, Conn, International Universities Press, 1995, pp 299–311Google Scholar
135. Bachrach HM: The Columbia Records Project and the evolution of psychoanalytic outcome research. Ibid, pp 279–297Google Scholar
136. Flexner A: Medical Education in the United States and Canada. A Report to the Carnegie Foundation for the Advancement of Teaching, Bulletin 4. Boston, Updyke, 1910Google Scholar



