Two-Year Outcome in First-Episode Schizophrenia: Predictive Value of Symptoms for Quality of Life
Abstract
Objective: Many studies have validated the grouping of schizophrenic symptoms into three independent dimensions: negative, psychotic, and disorganized. Negative symptoms are considered to be an important prognostic indicator, but this clinical observation requires further empirical study, especially with respect to psychosocial functioning. When present at the onset of the first episode, negative symptoms suggest that the patient will develop significant psychosocial impairment. The predictive values of the psychotic and disorganized symptom dimensions, on the other hand, have been less certain. Method: In this study of 50 first-episode schizophrenic patients, who were mostly neuroleptic-naive at intake, the authors examined the relationship between the severity of these three symptom dimensions (measured by using the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms) at index hospitalization and quality of life at 2-year follow-up. Results: Negative symptom severity was positively and significantly correlated with later occupational impairment, financial dependence on others, impaired relationships with friends, impaired ability to enjoy recreational activities, and global assessment of functioning. The magnitudes of correlation between the levels of psychotic symptoms or disorganized symptoms and 2-year quality of life measures were comparatively lower. Analyses using multivariate regression statistics also revealed similar findings.Conclusions: Severity of negative symptoms at index hospitalization may be a portent of poor outcome. In general, severity of psychotic or disorganized symptoms at intake does not appear to predict subsequent quality of life. Am J Psychiatry 1998; 155: 1196-1201
Having made the diagnosis of schizophrenia, the clinician is often asked about the patient’s prognosis. Patients and their families are most interested in the impact of the illness on subsequent quality of life. Will the patient be able to return to work or school? Will he or she relate well to relatives and friends? Lead a normal life? To the patient and the family, symptoms are usually the most prominent features of the disorder during the acute illness. Can symptoms in the acutely disturbed patient tell the clinician anything about subsequent quality of life? Do certain symptoms or groups of symptoms have better prognostic value than others?
Previous studies have examined the prognostic values of premorbid, sociodemographic, and psychopathological variables on outcome in patients with schizophrenia. Poor outcome in schizophrenia has been associated with the presence of the negative syndrome, poor premorbid adjustment, male gender, younger age at onset, insidious onset, longer interval from the onset to treatment, and the absence of any clear precipitating events (1-11
The symptoms of schizophrenia have commonly been divided into the positive and negative categories. Hughlings Jackson (12) was the first to use the terms “positive and negative symptoms” in the description of insanity (13, 14). More recently, Bilder et al. (15) proposed that there might be a third cluster of symptoms (“disorganization of thought”), independent of the positive/negative construct. Numerous studies that followed have shown that the symptoms of schizophrenia cluster into three dimensions (16-26), namely, the negative, psychotic, and disorganized dimensions. Although negative symptoms have been associated with poor outcome, the relationship between the psychotic and disorganized dimensions and subsequent outcome has been less clear. Previous studies have grouped these two dimensions under the category of “positive” symptoms.
In this study, we were interested in knowing if these three symptom dimensions, measured during the acutely disturbed state at the first psychiatric hospitalization, could predict subsequent quality of life. If so, what threshold of severity of symptoms will predict poor quality of life, and at what likelihood?
METHOD
Subjects
The subjects were evaluated in the Iowa Longitudinal Study of Recent Onset Psychoses. After complete description of the study to the subjects, written informed consent was obtained. The method for selecting the overall population has been previously described (27). This report focuses only on 50 subjects who met DSM-III-R or DSM-IV criteria for schizophrenia 2 years following the first psychiatric hospitalization for psychosis. The mean age at onset of illness was 21.4 years (SD=5.1); the mean age at first hospitalization was 23.9 years (SD=5.7). Most of the subjects were male (64%) and single (80%). The mean education was 12.5 years (SD=1.9). All subjects were ill at intake; 32 (64%) of them were neuroleptic-naive. The other 18 subjects (36%) had had minimal neuroleptic treatment before their first psychiatric hospitalization (median duration=3 months, interquartile range=4 months). The median amount of neuroleptic exposure before their first hospitalization was 0.8 antipsychotic dose years (interquartile range=1.6 antipsychotic dose years) (28). (Analogous to the “pack years” concept for documentation of cigarette use, one antipsychotic dose year equals a 100-mg chlorpromazine-equivalent dose of neuroleptic per day for 1 year.)
Procedure
Assessment
During the index hospitalization, the subjects underwent evaluation with the Comprehensive Assessment of Symptoms and History (CASH) (29) and the Psychiatric Symptoms You Currently Have—Baseline Version (PSYCH-BASE) (30). PSYCH-BASE is a structured interview instrument with items designed to evaluate at intake the quality of life, previous treatments, and course of illness. All possible sources of information, including the subject, family members, hospital records, and observations during the hospitalization, were used in completing the CASH and PSYCH-BASE.
Following discharge from the first hospitalization, the subjects were evaluated at 6-month intervals by the rater who had made the initial assessment. During these follow-up evaluations, the raters used longitudinal follow-up versions of the CASH (CASH-UP) and PSYCH-BASE (PSYCH-UP) to document the subjects’ levels of symptoms and quality of life.
Symptom measurement
The Scale for the Assessment of Negative Symptoms (SANS) (31) and the Scale for the Assessment of Positive Symptoms (SAPS) (32) were used to rate the subject’s level of symptoms. The SANS and SAPS ratings form part of the CASH and PSYCH-UP. Definitions for the three symptom dimensions are summarized in the footnote of Figure 1. Higher scores represent higher levels of symptoms. The maximum scores for the negative, psychotic, and disorganized dimensions are 20, 10, and 15, respectively. “Symptoms at intake” refers to the worst level of symptoms that each subject experienced at the time of first hospitalization. At 2-year follow-up, we recorded the symptoms experienced during the week before follow-up.
Quality of life measurement
We analyzed eight measures of the subjects’ quality of life during the month preceding the 2-year follow-up: occupational impairment, main income source, impairment in performance of household duties, enjoyment of recreational activities, relationships with family members, relationships with friends, and two measures of overall psychosocial functioning—the rater’s assessment of overall level of social adjustment and the Global Assessment Scale (GAS) (33). These eight measures of quality of life are described in detail on the PSYCH-BASE and PSYCH-UP. The degree of “relationship impairment with family members” was the average of the levels of interpersonal relationships between the subject and each different family member. On the basis of the two overall psychosocial functioning measures, we considered that a subject had a poor outcome at the 2-year follow-up if he or she met both the following criteria: 1) marked or severe impairment in overall level of social adjustment and 2) a GAS score of 40 or lower.
Premorbid adjustment scale and prehospitalization quality of life measures
We retrospectively assessed the subjects’ premorbid functioning at the time of first hospitalization in two ways: by use of the modified premorbid adjustment scale (34) and through estimation of the corresponding eight measures of quality of life during the best 6-month period of functioning in the 5 years before intake.
Treatment during the 2-year follow-up period
We started all subjects on a regimen of neuroleptic treatment during the first hospitalization. In this ongoing prospective study, treatment is not controlled. During the 2-year period, the median total duration of neuroleptic treatment was 1.86 years (interquartile range=0.58 years). During these 2 years, 11 subjects were treated with an atypical neuroleptic (two with clozapine, six with risperidone, one with clozapine and risperidone, and two with olanzapine), with a mean duration of atypical neuroleptic treatment of 1.10 years (SD=0.51). At 2-year follow-up, 45 subjects were receiving neuroleptic treatment, nine of whom were still taking an atypical neuroleptic. Thus, most of the subjects had received neuroleptic treatment for most of the 2-year follow-up period.
RESULTS
Symptoms at Intake and at 2-Year Follow-Up
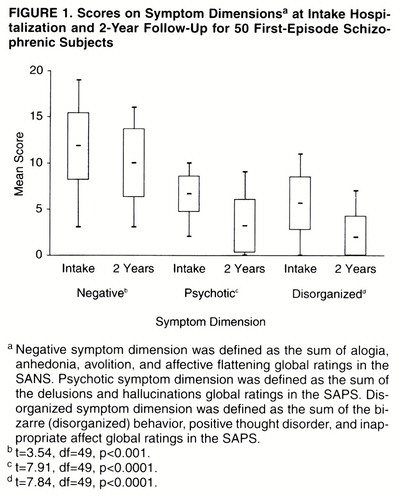
The levels of symptoms at intake and at 2-year follow-up are shown in Figure 1. Negative symptoms were prominently present at the first hospitalization in this group of acutely ill patients. At 2-year follow-up, the symptoms in all three dimensions improved significantly from intake. Both the psychotic and disorganized dimensions improved considerably more than the negative dimension.
Quality of Life at 2-Year Follow-Up
At follow-up, 60% of the patients were unemployed because of the illness. Another 12% experienced moderate to severe impairment in their capacity to work; 64% were financially supported by social service agencies; and 44% experienced at least moderate impairment in their ability to perform household duties. The level of interpersonal relationship with family members was between good and fair (mean=2.79, SD=0.75, on a 5-point scale; lower scores indicate better relations); on the other hand, 58% of the group had poor or very poor relations with friends, and 48% had “very little enjoyment” from participation in recreational activities or hobbies. Another 4% had no involvement in recreational activities. At 2-year follow-up, 60% of the group had marked or severe impairment, whereas only 12% had mild or no impairment in overall social adjustment. The mean GAS score was 44.7 (SD=11.3). Thus, in this cohort of first-episode subjects, the majority were substantially impaired. Quality of life at 2-year follow-up was generally poor; 50% of the subjects met the criteria for poor outcome as defined earlier in this article.
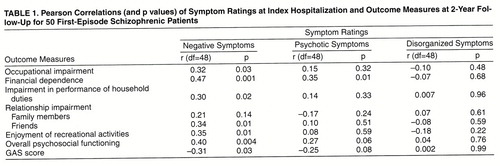
Relationship Between Symptom Dimensions at Intake and Quality of Life at 2-Year Follow-Up
We first analyzed the relationships between the three symptom dimensions and quality of life measures at 2-year follow-up by using Pearson correlation coefficients (table 1). The level of negative symptoms was moderately and significantly correlated with later occupational impairment, financial dependence on others, impairment in performance of household duties, impaired relationships with friends, impaired ability to enjoy recreational activities, and global assessments of functioning (r values=0.31 to 0.47, df=48, p values <0.03 to 0.0006). Severe negative symptoms at intake were associated with poor quality of life at 2-year follow-up.
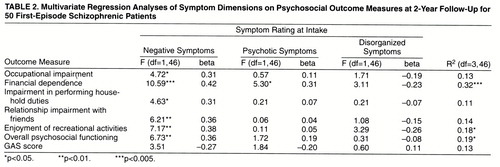
Multivariate Analyses
We further analyzed the relation between intake symptom dimensions and quality of life measures at follow-up by using multivariate regression, with each quality of life measure as the dependent variable and the three symptoms dimensions as independent variables. We removed the outcome measure “relationship impairment with family members” from these analyses since there were no significant correlations with intake symptoms. The variance, F values, and standardized regression beta coefficients are summarized in table 2.
In general, the magnitudes of correlation between psychotic symptoms or disorganized symptoms and 2-year quality of life measures were lower and not statistically significant. The only exception was between psychotic symptoms and subsequent financial dependence on others (r=0.36, df=48, p<0.01).
The scales that measure quality of life and the negative symptom dimension may overlap. Specifically, the anhedonia-asociality and the avolition-apathy global ratings on the SANS also assess occupational functioning, interpersonal relationships, and recreational interests. Instead of using all four SANS global ratings, we did further correlation analyses with only the alogia and affective flattening global ratings as measures of the negative symptom dimension. Although the magnitudes of correlation for only these two global scores (median r=0.26) were lower than those for all four SANS global scores (median r=0.33), higher negative symptoms were still associated with poor quality of life. Thus, in the subsequent analyses, we used all four SANS global ratings as the measure of the negative symptom dimension.
Although the variance in quality of life explained by intake symptoms was moderate at best (11% to 32%), the regression analyses again revealed the same distinct pattern. The beta weights for the negative symptom dimension were consistently higher than the weights given to the other two dimensions in the regression models. The negative symptom dimension was also most consistently the only significant independent variable in these analyses, with two exceptions.
Effects of Prehospitalization Quality of Life Measures and Premorbid Adjustment
Negative symptoms have been associated with poor premorbid functioning. The ability of the negative symptom dimension to predict poor quality of life may have been related to unfavorable premorbid functioning. Therefore, we examined this potential confounding factor by using partial correlation. We examined each 2-year outcome quality of life measure individually, partialing the effects of the corresponding prehospitalization quality of life measure and premorbid adjustment score.
The correlations between premorbid functioning and intake negative symptoms were low (r values=0.01 to 0.18, df=48). After we had partialed out the effects of premorbid functioning, the correlations between the negative symptom dimension at intake and later quality of life remained essentially unchanged from those in table 1. Thus, independent of premorbid functioning, severe negative symptoms at intake were associated with poorer quality of life at 2-year follow-up.
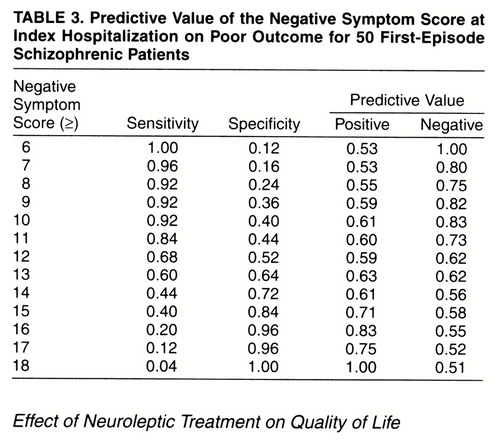
Predictive Value of the Negative Symptom Dimension on Poor Outcome
Table 3 shows the sensitivity, specificity, and positive and negative predictive values for a range of threshold negative symptom dimension scores in predicting poor outcome. A negative symptom score of 13 or greater provided the best combination of sensitivity, specificity, and positive and negative predictive values on predicting poor outcome. Given an intake negative symptom score of 13 or greater, the probability of a poor outcome at 2 years was 63%. If a subject had a negative symptom score of less than 13, the probability of a good outcome was 62%.
Effect of Neuroleptic Treatment on Quality of Life
There were no statistically significant correlations between total duration of neuroleptic medication treatment (r values=0.009 to 0.13, df=48, p values >0.36) or duration of atypical neuroleptic treatment (r values=0.03 to 0.16, df=48, p values >0.28) and quality of life measures. When we compared the 11 subjects who had atypical neuroleptic treatment with the other 39 subjects who only had typical neuroleptics, we found no statistically significant differences between the two groups in intake symptom measures, quality of life measures, or total duration of neuroleptic treatment (t values=1.42 to 0.14, df=15 to 24, p values>0.17). Subjects with poor outcome were not any more likely than the rest of the group to have been treated with a typical neuroleptic during the follow-up period χ2=1.05, df=1, p<0.31).
DISCUSSION
The relationship between symptoms and outcome in schizophrenia has been extensively studied, but the results remain inconclusive (35, 36). In the literature, there is general consensus that symptoms ascertained in the chronic stable state predict concurrent functioning (37-42), i.e., higher residual levels of positive and negative symptoms correlate with poorer functioning. However, findings regarding the predictive value of symptoms ascertained in severely or acutely ill patients have been more mixed. While some investigators have reported that such symptoms do not predict follow-up social disability (1, 38, 43, 44), others have found quite the opposite results (7, 36, 39, 45). Negative symptoms have even been associated with good short-term outcome (2, 26). These conflicting findings may be due, in part, to the fact that the subjects in these studies, although all in an “acute exacerbation,” were in different phases of the disease. Furthermore, neuroleptic treatment, which is not uniform across these studies, may also influence the predictive values of symptoms.
Lindenmayer et al. and Kay et al. (2, 46, 47) noted that the prognostic significance of the positive and negative syndromes may carry different meanings at different stages of the illness. Symptoms ascertained in acutely ill recent-onset patients may have better predictive validity than those ascertained during an acute exacerbation in patients who have been ill longer (36). Although the course of schizophrenia is variable, psychotic symptoms tend to become less florid, whereas negative symptoms often predominate the chronic phase (48). Therefore, this natural course of symptoms over time, together with the effect of maintenance neuroleptic treatment, may affect the predictive value of symptoms.
In this study, we were interested to know if the symptoms of schizophrenia, measured during the acutely disturbed state at the first psychiatric hospitalization, could predict subsequent quality of life. We examined a cohort of first-episode patients, most of whom had been neuroleptic-naive. Such a group permitted us to evaluate outcome in schizophrenia without the confounds of chronicity or prior treatment.
We found that the magnitudes of correlation with quality of life measures were generally greater in the negative symptom dimension compared with the other two dimensions. Negative symptoms moderately predicted poorer quality of life early in the course of schizophrenia, even after we controlled for the effects of the other two dimensions and premorbid functioning. The negative symptoms also had relatively greater predictive value than the other two dimensions, with one exception where there was comparable strength of association between psychotic symptoms and financial dependence. Although less than 20% of the variance in quality of life at 2 years was explained by the initial symptoms, this finding is not surprising since outcome in schizophrenia is related to many factors.
A threshold of 13 on the intake negative symptom score best discriminated the poor outcome patients in our group. However, we derived this cutoff score from a relatively small cohort of 50 subjects at a single center, and we used trained raters. This finding needs replication before generalization to the clinical setting can be made.
Nevertheless, the ability to identify around the time of initial diagnosis which patients will likely have poor outcome has advantages. Knowing that negative symptoms are a portent of poor quality of life may influence the clinician to opt for atypical neuroleptic treatment and stress the need for more intensive psychosocial interventions for patients with prominent initial negative symptoms.
Atypical neuroleptics have been shown to be more effective in reducing negative symptoms (49-52) and, therefore, may potentially improve outcome of patients with prominent initial negative symptoms. In this study, we did not find any differences in outcome between subjects who had received atypical neuroleptic treatment and those who had not. This may be related, in part, to the small number of subjects who had received atypical neuroleptics. Furthermore, we did not control neuroleptic treatment in this study. Subjects who had been treated with atypical neuroleptics, particularly the three who received clozapine, might have a more severe form of the illness than subjects who had been maintained on regimens of only typical neuroleptics.
Symptoms during the acute illness early in the presentation of schizophrenia have some prognostic value. The negative symptom dimension has relatively greater predictive value than the other two symptom dimensions. Severe negative symptoms at the time of first hospitalization may be a portent of poor outcome. In general, the psychotic and the disorganized symptom dimensions do not appear to predict subsequent quality of life.
Presented in part at the 150th annual meeting of the American Psychiatric Association, San Diego, May 17–22, 1997.Received Oct. 2, 1997; ; revision received Feb. 19, 1998; accepted April 2, 1998. From the Department of Psychiatry, Mental Health Clinical Research Center, and the Department of Preventive Medicine and Environmental Health, University of Iowa.. Address reprint requests to Dr. Ho, University of Iowa Hospitals and Clinics, Department of Psychiatry, MHCRC 2911 JPP, 200 Hawkins Dr., Iowa City, IA 52242. Supported in part by NIMH grants MH-31593 and MHCRC-43271; the Nellie Ball Trust Research Fund, Iowa State and Bank Trust Company, Trustee; and NIMH Research Scientist Award MH-00625.
 |
 |
 |
1. Moller HJ, von Zerssen D, Werner-Eilert K, Wuschner-Stockheim M: Outcome in schizophrenic and similar paranoid psychoses. Schizophr Bull 1982; 8:99–108Crossref, Medline, Google Scholar
2. Kay SR, Lindenmayer JP: Outcome predictors in acute schizophrenia: prospective significance of background and clinical dimensions. J Nerv Ment Dis 1987; 175:152–160Crossref, Medline, Google Scholar
3. Prudo R, Blum HM: Five-year outcome and prognosis in schizophrenia: a report from the London Field Research Centre of the International Pilot Study of Schizophrenia. Br J Psychiatry 1987; 150:345–354Crossref, Medline, Google Scholar
4. Strauss JS, Carpenter WT Jr: Prediction of outcome in schizophrenia, III: five-year outcome and its predictors. Arch Gen Psychiatry 1977; 34:159–163Crossref, Medline, Google Scholar
5. McGlashan TH: Predictors of shorter-, medium-, and longer-term outcome in schizophrenia. Am J Psychiatry 1986; 143:50–55Link, Google Scholar
6. Bailer J, Brauer W, Rey ER: Premorbid adjustment as predictor of outcome in schizophrenia: results of a prospective study. Acta Psychiatr Scand 1996; 93:368–377Crossref, Medline, Google Scholar
7. Thara R, Eaton WW: Outcome of schizophrenia: the Madras longitudinal study. Aust NZ J Psychiatry 1996; 30:516–522Crossref, Medline, Google Scholar
8. Angermeyer MC, Kuhn L, Goldstein JM: Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull 1990; 16:293–307Crossref, Medline, Google Scholar
9. Westermeyer JF, Harrow M: Course and outcome in schizophrenia, in Handbook of Schizophrenia: Nosology, Epidemiology and Genetics. Edited by Tsuang MT, Simpson JC. Amsterdam, Elsevier, 1988, pp 205–244Google Scholar
10. Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE: The natural course of schizophrenia: a review of first-admission studies. Schizophr Bull 1992; 18:185–207Crossref, Medline, Google Scholar
11. Loebel AD, Lieberman JA, Alvir JMJ, Mayerhoff DI, Geisler SH, Szymanski SR: Duration of psychosis and outcome in first episode schizophrenia. Am J Psychiatry 1992; 149:1183–1188Link, Google Scholar
12. Jackson H: Selected Writings. London, Hodder & Stoughton, 1931Google Scholar
13. Berrios GE: Positive and negative symptoms and Jackson: a conceptual history. Arch Gen Psychiatry 1985; 42:95–97Crossref, Medline, Google Scholar
14. Berrios GE: Positive and negative signals: a conceptual history, in Negative vs Positive Schizophrenia. Edited by Marneros A, Andreasen NC, Tsuang MT. New York, Springer, 1991, pp 8–27Google Scholar
15. Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK: Symptomatic and neuropsychological components of defect states. Schizophr Bull 1985; 11:409–419Crossref, Medline, Google Scholar
16. Liddle PF: The symptoms of chronic schizophrenia: a re-examination of the positive-negative dichotomy. Br J Psychiatry 1987; 151:145–151Crossref, Medline, Google Scholar
17. Kulhara P, Kota SK, Joseph S: Positive and negative subtypes of schizophrenia: a study from India. Acta Psychiatr Scand 1986; 74:353–359Crossref, Medline, Google Scholar
18. Gur RE, Mozley PD, Resnick SM, Levick S, Erwin R, Saykin AJ, Gur RC: Relations among clinical scales in schizophrenia. Am J Psychiatry 1991; 148:472–478Link, Google Scholar
19. Minas IH, Stuart GW, Klimidis S, Jackson HJ, Singh BS, Copolov DL: Positive and negative symptoms in the psychoses: multidimensional scaling of SAPS and SANS items. Schizophr Res 1992; 8:143–156Crossref, Medline, Google Scholar
20. Brown KW, White T: Syndromes of chronic schizophrenia and some clinical correlates. Br J Psychiatry 1992; 161:317–322Crossref, Medline, Google Scholar
21. Peralta V, de Leon J, Cuesta MJ: Are there more than two syndromes in schizophrenia? a critique of the positive-negative dichotomy. Br J Psychiatry 1992; 161:335–343Crossref, Medline, Google Scholar
22. Arndt S, Alliger RJ, Andreasen NC: The distinction of positive and negative symptoms: the failure of a two-dimensional model. Br J Psychiatry 1991; 158:317–322Crossref, Medline, Google Scholar
23. Miller DD, Arndt S, Andreasen NC: Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry 1993; 34:221–226Crossref, Medline, Google Scholar
24. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
25. Brekke JS, DeBonis JA, Graham JW: A latent structure analysis of the positive and negative symptoms in schizophrenia. Compr Psychiatry 1994; 35:252–259Crossref, Medline, Google Scholar
26. Gureje O, Aderibigbe YA, Obikoya O: Three syndromes in schizophrenia: validity in young patients with recent onset of illness. Psychol Med 1995; 25:715–725Crossref, Medline, Google Scholar
27. Flaum MA, Andreasen NC, Arndt S: The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr Bull 1992; 18:481–490Crossref, Medline, Google Scholar
28. Miller DD, Flaum MA, Nopoulos P, Arndt S, Andreasen NC: The concept of dose years: a reliable method for calculating lifetime psychotropic drug exposure (abstract). Schizophr Res 1995; 15:159Crossref, Google Scholar
29. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
30. Andreasen NC: PSYCH-BASE. Iowa City, University of Iowa, 1989Google Scholar
31. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
32. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
33. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766–771Crossref, Medline, Google Scholar
34. Gupta S, Rajaprabhakaran R, Arndt S, Flaum M, Andreasen NC: Premorbid adjustment as a predictor of phenomenological and neurobiological indices in schizophrenia. Schizophr Res 1995; 16:189–197Crossref, Medline, Google Scholar
35. Andreasen NC, Roy M-A, Flaum MA: Positive and negative symptoms, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Cambridge, England, Blackwell Science, 1995, pp 28–45Google Scholar
36. Wieselgren IM, Lindstrom E, Lindstrom LH: Symptoms at index admission as predictor for 1–5 year outcome in schizophrenia. Acta Psychiatr Scand 1996; 94:311–319Crossref, Medline, Google Scholar
37. Pogue-Geile MF, Harrow M: Negative and positive symptoms in schizophrenia and depression: a followup. Schizophr Bull 1984; 10:371–87Crossref, Medline, Google Scholar
38. Breier A, Schreiber JL, Dyer J, Pickar D: National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48:239–246Crossref, Medline, Google Scholar
39. Addington J, Addington D: Premorbid functioning, cognitive functioning, symptoms and outcome in schizophrenia. J Psychiatry Neurosci 1993; 18:18–23Medline, Google Scholar
40. Johnstone EC, Frith CD, Gold A, Stevens M: The outcome of severe acute schizophrenic illnesses after one year. Br J Psychiatry 1979; 134:28–33Crossref, Medline, Google Scholar
41. Kolakowska T, Williams AO, Jambor K, Ardern M: Schizophrenia with good and poor outcome, III: neurological “soft” signs, cognitive impairment and their clinical significance. Br J Psychiatry 1985; 146:348–357Crossref, Medline, Google Scholar
42. Keefe RSE, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Kendler KS, Horvath TB, Nora R, Davis KL: Characteristics of very poor outcome schizophrenia. Am J Psychiatry 1987; 144:889–895Link, Google Scholar
43. Schubart C, Krumm B, Biehl H, Schwarz R: Measurement of social disability in a schizophrenic patient group: definition, assessment and outcome over 2 years in a cohort of schizophrenic patients of recent onset. Soc Psychiatry 1986; 21:1–9Crossref, Medline, Google Scholar
44. Hwu HG, Tan H, Chen CC, Yeh LL: Negative symptoms at discharge and outcome in schizophrenia. Br J Psychiatry 1995; 166:61–67Crossref, Medline, Google Scholar
45. Munk-Jorgensen P, Mortensen PB: Schizophrenia: a 13-year follow-up: diagnostic and psychopathological aspects. Acta Psychiatr Scand 1989; 79:391–399Crossref, Medline, Google Scholar
46. Lindenmayer JP, Kay SR, Friedman C: Negative and positive schizophrenic syndromes after the acute phase: a prospective follow-up. Compr Psychiatry 1986; 27:276–286Crossref, Medline, Google Scholar
47. Kay SR, Fiszbein A, Lindenmayer JP, Opler LA: Positive and negative syndromes in schizophrenia as a function of chronicity. Acta Psychiatr Scand 1986; 74:507–518Crossref, Medline, Google Scholar
48. McGlashan TH, Fenton WS: The positive-negative distinction in schizophrenia: review of natural history validators. Arch Gen Psychiatry 1992; 49:63–72Crossref, Medline, Google Scholar
49. Miller DD, Perry PJ, Cadoret RJ, Andreasen NC: Clozapine’s effect on negative symptoms in treatment-refractory schizophrenics. Compr Psychiatry 1994; 35:8–15Crossref, Medline, Google Scholar
50. Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825–835Link, Google Scholar
51. Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Arnott W: A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 1993; 13:25–40Crossref, Medline, Google Scholar
52. Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S: Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 1996; 14:111–123Crossref, Medline, Google Scholar



