Positive and Negative Symptom Response to Clozapine in Schizophrenic Patients With and Without the Deficit Syndrome
Abstract
OBJECTIVE: In a preliminary report, the authors observed that clozapine was superior to haloperidol in the treatment of positive and negative symptoms in stable outpatients with schizophrenia. In this final report, they examine the effects of clozapine on positive and negative symptoms in patients with and without the deficit syndrome to determine which patients receive the positive symptom advantage of clozapine and the extent of clozapine's therapeutic effects on negative symptoms. In addition, they examine the long-term effects of clozapine on positive, negative, and affective symptoms, social and occupational functioning, and quality of life. METHOD: Seventy-five outpatients with schizophrenia, who met retrospective and prospective criteria for residual positive or negative symptoms, were entered into a 10-week double-blind, parallel-groups comparison of clozapine and haloperidol. Patients who completed the double-blind study were then entered into a 1-year open-label clozapine study. RESULTS: For patients who completed the 10-week double-blind study, clozapine was superior to haloperidol in treating positive symptoms. This effect was not observed in the intent-to-treat analyses. There was no evidence of any superior efficacy or long-term effect of clozapine on primary or secondary negative symptoms. Long-term clozapine treatment was associated with significant improvements in social and occupational functioning but not in overall quality of life. CONCLUSIONS: For schizophrenic patients who are able to tolerate clozapine therapy, clozapine has superior efficacy for positive symptoms but not negative symptoms and is associated with long-term improvements in social and occupational functioning for patients with and without the deficit syndrome
The efficacy of clozapine for positive and negative symptoms of schizophrenia has been examined in a series of studies. Early studies failed to detect significant therapeutic differences between clozapine and conventional antipsychotics in acutely psychotic patients (1), but clinical observations suggested that clozapine may have superior efficacy for positive symptoms in treatment-resistant patients. These observations were supported in randomized, controlled double-blind studies (2, 3), which demonstrated that clozapine is superior to conventional antipsychotics for residual positive symptoms in treatment-refractory inpatients with schizophrenia. The results of open-label uncontrolled studies have provided further support for clozapine's superior efficacy for positive symptoms (4–6). In an initial report from our double-blind study (7), we presented analyses suggesting that clozapine's superior efficacy for positive symptoms extended to partially responsive outpatients with schizophrenia.
In contrast to the evidence for clozapine's efficacy for positive symptoms, there is considerable debate about its efficacy for negative symptoms (8, 9). In the Kane et al. multicenter study (2), significant improvement in negative symptoms was observed. However, in that study and in another double-blind inpatient study (3), the changes in negative symptoms occurred concurrently with significant reductions in positive, depressive, and extrapyramidal symptoms. In addition, open-label studies have demonstrated that changes in negative symptoms are often significantly associated with changes in positive symptoms and/or extrapyramidal symptoms (6, 10, 11). The results of these studies raise the possibility that the observed benefit of clozapine for negative symptoms is due to its ability to ameliorate secondary causes of these symptoms. However, the failure of these studies to differentiate primary from secondary negative symptoms precludes the resolution of this issue.
The deficit form of schizophrenia is defined by the presence of primary, enduring negative or deficit symptoms; patients without deficit symptoms are termed “nondeficit” (12). Nondeficit patients may have negative symptoms, but the symptoms are judged clinically to be secondary to other factors. The deficit/nondeficit categorization can be made reliably (13, 14), is stable over time (14 and unpublished data of Amador et al.), and is more stable than other negative symptom subtypes (14). Use of the deficit syndrome concept provides an approach for examining whether clozapine's efficacy for negative symptoms includes both primary and secondary negative symptoms. If deficit and nondeficit patients exhibit a negative symptom response, then this would provide strong evidence that clozapine is effective for both primary and secondary negative symptoms. In contrast, if nondeficit patients, but not deficit patients, exhibit a negative symptom response, then this would suggest that clozapine's efficacy for negative symptoms is limited to secondary negative symptoms.
Two studies have used this approach. In our earlier article on the present study (7), we reported that clozapine treatment produced only modest improvements in negative symptoms, which were restricted to nondeficit patients. In an open-label 12-month study of treatment-resistant inpatients with schizophrenia (15), both deficit and nondeficit groups exhibited a significant reduction in positive symptoms, but neither group exhibited a negative symptom response. Although these results would suggest that clozapine is not effective for deficit symptoms, the small number of deficit patients in our double-blind study and the open-label nature of the latter study preclude definitive conclusions.
The current study was designed to examine the comparative efficacy and long-term effect of clozapine for positive and negative symptoms in partially responsive outpatients with schizophrenia. We used a 10-week double-blind, parallel-groups design to examine the comparative efficacy of clozapine and haloperidol. The long-term effect of clozapine was then examined in an open-label 1-year descriptive study. Patients were categorized as having deficit and nondeficit forms of schizophrenia in order to examine 1) clozapine's efficacy for primary and secondary negative symptoms and 2) whether positive symptoms were equally responsive in deficit and nondeficit patients.
METHOD
Patients meeting the DSM-III-R criteria for schizophrenia or schizoaffective disorder were selected from the Maryland Psychiatric Research Center Outpatient Research Program for entry into the study. Patients were diagnosed according to a best-estimate diagnostic approach that used all available information from a structured diagnostic interview (the Structured Clinical Interview for DSM-III-R [16]), direct assessment, family informants, and past medical records. Patients with concurrent drug abuse or alcoholism, organic brain disorders, mental retardation, or a medical condition that contraindicated use of clozapine were excluded from the study. All patients provided written informed consent before participating in the study.
Patients were required to meet retrospective and prospective criteria for partial response to conventional neuroleptics (7). The retrospective criteria were 1) a history of residual positive and/or negative symptoms after at least two 6-week trials of therapeutic dosages of conventional neuroleptics from at least two different classes and 2) a minimum level of positive and/or negative symptoms at the time of evaluation for participation in the study. The minimum positive symptom level was a total score of at least 8 on the Brief Psychiatric Rating Scale (BPRS) (17) items for conceptual disorganization, hallucinations, unusual thought content, and suspiciousness (item scores on the BPRS range from 1 to 7) or a score of at least 4 on any one of the items. The minimum negative symptom level was a total score of at least 20 on the Scale for the Assessment of Negative Symptoms (SANS) (18) (item scores range from 0 to 5) or a score of at least 2 on at least one SANS global item. Sixty-seven patients met the positive symptom criterion, and 71 patients met the negative symptom criterion. The prospective evaluation of partial responsiveness consisted of a 6-week trial of 20 mg/day of open-label fluphenazine, with dose adjustments between 10 and 30 mg/day allowed. Subjects were excluded from the double-blind study if they demonstrated a 30% or greater improvement in positive or negative symptoms during this 6-week trial.
In initial reports of this study, we presented the results of the first 39 patients who completed the 10-week double-blind study (7) and entered the 1-year open-label study (19). We now present the results of the 64 patients who completed the 10-week double-blind study, and the 61 patients who completed the 1-year open-label study. This is the first report of the 75 patients in the intent-to-treat analyses.
Full details of the 10-week double-blind and 1-year open-label study designs have been previously reported (7, 19). In brief, patients who met the retrospective criteria for partial response and continued to meet admission criteria upon completion of the 6-week open-label fluphenazine trial were randomly assigned to a 10-week double-blind, parallel-groups comparison of clozapine versus haloperidol. Over the first 4 weeks of the study, doses of clozapine and haloperidol were gradually increased to 400 mg/day and 20 mg/day, respectively; fluphenazine was gradually tapered off during the first 2 weeks of the study. Clozapine and haloperidol doses could be adjusted over the next 2 weeks within fixed limits—for clozapine, 200–600 mg/day; for haloperidol, 10–30 mg/day—to maximize efficacy or to minimize side effects. Study medication dosages were then fixed for the remainder of the 10-week trial. A double-blind fixed dose of benztropine (4 mg/day) was prescribed for patients assigned to haloperidol treatment to minimize extrapyramidal symptoms and the potential for revealing treatment assignment. Patients randomly assigned to clozapine received placebo benztropine tablets.
At the end of the double-blind study, patients assigned to clozapine were offered an opportunity to continue taking clozapine and enter the year-long descriptive study. Patients assigned to haloperidol were offered a 6-week open-label clozapine trial, following which they were entered into the year-long descriptive study.
Compliance was assessed weekly by a pill count and medication review. In addition, all patients had a compliance plan that consisted of medication checks by family members and/or mental health care providers who had extensive contact with the patients. All patients who were judged to have received 75% or more of their assigned study medication were considered compliant.
Clinical Assessments
The patients were categorized into deficit and nondeficit subgroups with use of the Schedule for the Deficit Syndrome (13), a semistructured interview that provides specific criteria for assessing the presence of negative symptoms, the duration of symptoms, and whether the symptoms are primary or secondary. Additional information is obtained from clinicians with long-standing contact with the patients and from family members. All patients were categorized by B.K. and/or R.W.B. The kappa for interrater agreement on the global categorization was 0.73 (13). Positive symptoms were assessed by the sum of scores on the four BPRS positive symptom items: conceptual disorganization, hallucinations, unusual thought content, and suspiciousness. Since the Schedule for the Deficit Syndrome is not designed to measure change in negative symptoms, the SANS, which assesses both primary and secondary negative symptoms, was used to assess this change. The BPRS and SANS ratings were obtained weekly during the 10-week double-blind study and monthly during the 1-year open-label study. Social and occupational functioning and quality of life were assessed with use of the Level of Functioning Scale (20) and Quality of Life Scale (21), respectively. The Level of Functioning Scale and Quality of Life Scale ratings were obtained at baseline (before entry into the 10-week double-blind study) and at 6 and 12 months in the 1-year open-label study. The symptom and functioning ratings were conducted by master's-level and doctoral-level clinicians. Intraclass correlation coefficients for these four instruments ranged from 0.76 to 0.90.
The Simpson-Angus Rating Scale (22) and the Maryland Psychiatric Research Center Tardive Dyskinesia Scale (23) were used to assess extrapyramidal symptoms and dyskinetic movements, respectively. These scales were administered weekly during the 10-week double-blind study and monthly during the 1-year open-label study by research nurses.
Side effects and vital signs were ascertained weekly; side effects were rated as either absent or present. These ratings were conducted by a nonblind research nurse. WBC counts were obtained weekly. During the 10-week double-blind study, blood for monitoring WBC counts was collected from the patients receiving clozapine and those receiving haloperidol. The weekly WBC counts were reviewed by the nonblind research nurse.
All raters, except the nonblind research nurse, were blind to treatment assignment and deficit/nondeficit categorization.
Statistical Analyses
We used two major analytic strategies to examine the comparative efficacy of clozapine and haloperidol: intent-to-treat analyses and completer analyses. All patients who completed the 6-week open-label fluphenazine trial, were randomly assigned to treatment, and received at least one dose of study medication were included in the intent-to-treat analyses. These analyses best approximate actual clinical care situations, but they are vulnerable to early terminations and may result in an underestimation of treatment effects. Therefore, we also conducted completer analyses. These analyses may be vulnerable to differential attrition, potentially leading to a loss of representativeness of the study sample and biased estimates of treatment effect.
The two primary outcome measures were positive and negative symptom response. The BPRS positive symptom item scores and the SANS total score were used to assess these outcome measures. The SANS total score included all items except for the inappropriate affect, attentional, and global items. The inappropriate affect and attentional items were excluded because recent factor analytic study results have suggested that these items are not closely related to negative symptoms (24). The global items were excluded because they are redundant with the individual items.
10-week double-blind study. Analyses of covariance (ANCOVAs) were used to compare week 10 end-of-study ratings. The last available ratings were used for patients who dropped out of the study. Treatment assignment was the between-subjects factor, and the baseline symptom measure was used as the covariate. The baseline symptom measure was the average of the last two ratings in the 6-week open-label fluphenazine trial. These same analyses were repeated with the addition of the deficit/nondeficit categorization as a second between-groups factor. In this two-way ANCOVA, the group sizes were smaller, since all patients could not be unambiguously placed in the deficit or nondeficit group. The use of the deficit/nondeficit categorization as an additional grouping variable enables the examination of primary and secondary negative symptom response. Moreover, it enables the examination of whether deficit and nondeficit patients share the same positive symptom response. In an exploratory framework, the effect of clozapine and haloperidol was examined for BPRS total and factor scores and SANS “factor” scores.
The same ANCOVA procedure was used to examine the effect of clozapine and haloperidol for the Simpson-Angus Rating Scale total score and the Maryland Psychiatric Research Center Tardive Dyskinesia Scale dyskinesia score. Demographic and clinical characteristics, side effect frequencies, and vital signs were compared by either t tests or chi-square statistics. All probability values are two-tailed.
1-year open-label descriptive study. Positive and negative symptom response was examined with a repeated measures analysis of variance (ANOVA), with baseline, 6-month, and 12-month BPRS positive symptom scores and SANS total score as the repeated measures. The baseline BPRS and SANS measures were the average of the last two ratings in the 6-week open-label fluphenazine trial. For the patients assigned to clozapine treatment during the 10-week double-blind study, the 6-month and 12-month time points represent 10 weeks (i.e., the duration of the double-blind study) plus 6 months and 12 months, respectively. For the patients assigned to haloperidol during the 10-week double-blind study, the 6-month and 12-month time points represent 16 weeks (i.e., the duration of the double-blind study and the 6-week open-label clozapine trial following the double-blind study) plus 6 months and 12 months, respectively.
In addition, the number of patients with sustained clinical response was examined by using the following criteria: 1) a 20% or greater decrement in BPRS positive symptom score was achieved, and 2) at least 50% of subsequent BPRS positive symptom ratings met the 20% improvement criterion. The repeated measures ANOVA approach was also used to examine the long-term effect of clozapine on functional and side effect measures. Simpson-Angus Rating Scale, Quality of Life Scale, and Level of Functioning Scale total scores and the dyskinesia scores were used as the repeated measures. The baseline, 6-month, and 12-month time points for these analyses were the same as defined above.
Either repeated measures ANOVA or the Cochran Q test was used to examine the time course of side effects and vital signs. In these analyses, since the question of interest was the persistence of side effects and changes in vital signs associated with acute clozapine treatment, baseline was defined as the level of side effects or vital sign measures either after 10 weeks of clozapine treatment (for the patients randomly assigned to clozapine during the 10-week double-blind study) or after 6 weeks of open-label clozapine (for the patients randomly assigned to haloperidol in the 10-week study).
RESULTS
Eighty patients entered the 6-week open-label fluphenazine trial. Seventy-five patients completed the 6-week trial, and none met the improvement criteria. Of the five patients who failed to complete this phase, three dropped out because they could not be stabilized within the dosage range (N=1) or decompensated (N=2), one was removed because of drug abuse, and one declined further study participation. These five patients were continued in clinical care, and the 75 patients who completed the fluphenazine trial were entered into the 10-week double-blind study.
10-Week Double-Blind Study
Thirty-eight of the 75 patients who entered the 10-week double-blind trial were randomly assigned to clozapine treatment, and 37 were randomly assigned to haloperidol. Sixty-four patients completed the study. Of the 11 patients who failed to complete the study, eight had been assigned to clozapine and three had been assigned to haloperidol. Patients assigned to clozapine dropped out for the following reasons: noncompliance (N=3), relapse (N=3), low RBC count (N=1), and seizures (N=1). Patients assigned to haloperidol dropped out for the following reasons: relapse (N=2) and declining to continue study participation (N=1). There were no significant differences between the patients who completed the study and those who dropped out in age (completers: mean=36 years, SD=8; noncompleters: mean=36 years, SD=6); sex (completers: 70% male; noncompleters: 64% male); duration of illness (completers: mean=16 years, SD=7; noncompleters: mean=16 years, SD=6); baseline BPRS positive symptom score (completers: mean=3.0, SD=1.1; noncompleters: mean=3.2, SD=1.0); and baseline SANS total score (completers: mean=26.3, SD=12.4; noncompleters: mean=26.0, SD=9.9).
Demographic and clinical characteristics of the 75 patients who entered the 10-week double-blind study are presented in table 1. The two treatment groups were very similar on these variables, with no significant differences observed. There were also no significant differences between groups in the demographic and clinical characteristics of the 64 patients who completed the study (data not shown).
The deficit/nondeficit categorizations of the 75 patients who entered the study were as follows: in the clozapine group, 10 deficit patients and 27 nondeficit patients; in the haloperidol group, 11 deficit patients and 26 nondeficit patients. The deficit/nondeficit categorizations of the 64 patients who completed the study were as follows: in the clozapine group, eight deficit and 21 nondeficit patients; in the haloperidol group, 11 deficit and 23 nondeficit patients. One patient who was randomly assigned to clozapine and completed the study could not be unambiguously categorized. There were no significant interactions between treatment assignment and deficit/nondeficit categorization for any of the demographic or clinical variables for either the patients who entered the study or those who completed it.
The end-of-study medication dosages for the patients who entered the study and those who completed it are presented in table 2. At the end of the study, there were no significant differences in clozapine or haloperidol dosage between the deficit and nondeficit patients who either entered the study or completed it.
Positive symptom response. In the intent-to-treat analyses, there were no significant differences between clozapine and haloperidol for positive symptoms (table 3). The failure to find a difference between clozapine and haloperidol patients was observed in both the analyses with the deficit/nondeficit categorization as a grouping variable (F=1.70, df=1,69, p=0.20) and those without it (F=1.46, df=1,72, p=0.23).
In contrast, in the completer analyses, clozapine was significantly superior to haloperidol for positive symptoms (table 4). In the ANCOVA with the deficit/nondeficit categorization as an additional grouping variable, there was also a significant effect of treatment assignment. The interaction between treatment assignment and the deficit/nondeficit categorization was not significant (F=0.15, df=1,58, p=0.70).
Negative symptom response. In the intent-to-treat analyses, there were no significant differences between clozapine and haloperidol for negative symptoms in either the analyses with the deficit/nondeficit categorization as a grouping variable (F=0.54, df=1,69, p=0.32) or those without it (F=0.41, df=1,72, p=0.52) (table 3). There were no significant clozapine/haloperidol differences for any of the SANS factor scores. Similar results were observed in the completer analyses. There were no significant treatment effects observed in either the analyses with the deficit/nondeficit categorization as a grouping variable (F=2.38, df=1,58, p=0.13) or those without it (F=1.82, df=1,61, p=0.18) (table 4). However, there was a significant difference between clozapine and haloperidol for the SANS anhedonia factor score (F=6.96, df=1,61, p=0.01). This difference was primarily due to a worsening of this score for patients who received haloperidol (for clozapine patients, percent change=–2.0%; for haloperidol patients, percent change=8.3%). The interaction between treatment assignment and the deficit/nondeficit categorization for the SANS anhedonia factor was not significant (F=0.31, df=1,58, p=0.58).
Other measures. In the intent-to-treat analyses, there were no significant differences between clozapine and haloperidol for BPRS total score in the analyses with and without the deficit/nondeficit categorization as a grouping variable (table 3) (F=0.45, df=1,69, p=0.41, and F=1.56, df=1,72, p=0.22, respectively).
Clozapine treatment was associated with a significant reduction in Simpson-Angus Rating Scale total score. Significant differences between clozapine and haloperidol were observed in the analyses with and those without the deficit/nondeficit categorization as a grouping variable (table 3). The interaction between treatment assignment and the deficit/nondeficit categorization was not significant (F=2.16, df=1,69, p=0.15). There were no significant effects observed for the Maryland Psychiatric Research Center Tardive Dyskinesia Scale dyskinesia score in either the analyses with the deficit/nondeficit categorization as a grouping variable (F=1.16, df=1,69, p=0.29) or those without it (F=1.10, df=2,72, p=0.30) (table 3).
The same pattern of results for BPRS and Simpson-Angus Rating Scale total scores and for dyskinesia score was observed in the completer analyses (table 4).
There were no significant differences between clozapine and haloperidol in either the intent-to-treat or the completer analyses for the BPRS anxiety/depression, hostility, or activation factors.
Side effects and vital signs. The end-of-study side effect and vital sign data for the 75 patients who entered the study are listed in table 5. The numbers are based on the last available rating. There were no significant differences between the two groups in baseline level of side effects. Dizziness, salivation, and nausea were significantly more common in patients treated with clozapine (table 5). In contrast, dry mouth was significantly more common in the haloperidol-treated patients. The same pattern of results was observed in patients who completed the study.
The baseline and end-of-study (10-week) mean weights of the clozapine-treated patients were 171.5 lb (SD=39.4) and 180.6 lb (SD=41.4), respectively. For the haloperidol-treated patients the baseline mean was 174.4 lb (SD=37.4) and the end-of-study mean was 174.8 lb (SD=37.4). The difference between groups in weight gain was significant (F=29.6, df=1,71, p<<0.0001). The difference between groups among the patients who completed the study was also significant (F=33.2, df=1,60, p<<0.0001).
There were no significant differences between the two medication groups in baseline vital sign values. In both the group who entered the study and the group who completed the study, clozapine-treated patients had significantly higher pulse rates (t=4.31, df=72, p<0.0001, and t=4.22, df=62, p<0.0001, respectively). There were no significant differences between the clozapine-treated patients and the haloperidol-treated patients in either systolic or diastolic blood pressure.
One-Year Open-Label Study
Sixty-one of the 64 patients who completed the double-blind study entered the 1-year open-label descriptive study. All three patients who did not enter the year-long descriptive study had been randomly assigned to haloperidol treatment in the double-blind study; two patients declined the 6-week open-label clozapine trial and chose to remain on a haloperidol regimen, and one patient developed marked liver enzyme elevation during the 6-week open-label clozapine trial. Fifty-eight patients completed the study; the three patients who dropped out failed to complete the study because of relapse associated with noncompliance (N=2) or lack of response to treatment (N=1).
The deficit/nondeficit categorizations for the 58 patients who completed the study were as follows: deficit patients, N=19; nondeficit patients, N=38. As mentioned above, one patient could not be unambiguously categorized.
The mean end-of-study clozapine dose was 463.8 mg/day (SD=111.3). The end-of-study dose did not differ between the deficit group (mean=467.1 mg/day, SD=112.7) and the nondeficit group (mean=457.9 mg/day, SD=110.3) (t=0.30, df=55, p=0.77).
Positive and negative symptom response. Open-label clozapine treatment was associated with significant improvement in positive symptoms; the time effect was significant in the analyses with the deficit/nondeficit categorization as a grouping variable and those without it (table 6). The interaction between time and deficit/nondeficit categorization was not significant (F=0.47, df=2,110, p=0.63). In contrast, open-label clozapine treatment was not associated with a significant improvement in negative symptoms. The time effect was not significant in the analyses both with and without the deficit/nondeficit categorization as a grouping variable (F=0.45, df=2,110, p=0.64, and F=0.53, df=2,114, p=0.59, respectively). The interaction between time and deficit/nondeficit categorization was also not significant (F=0.15, df=2,110, p=0.86).
Fifty-three of the 61 patients who entered the 1-year open-label study had met the 10-week double-blind minimum positive symptom level entry criteria. Twenty-six of the 53 patients (49%) met the criteria for sustained clinical response. The responders had a significantly earlier age at onset (mean=18.3 years, SD=5.0) than the nonresponders (mean=22.3 years, SD=6.2) (F=6.52, df=1,51, p=0.01). There were no significant differences between responders and nonresponders in age, sex, race, length of illness, clozapine dosage, or proportion of deficit patients. The time course for response is depicted in figure 1.
Other measures. There was a significant time effect for BPRS total score. This effect was observed in both the analyses with the deficit/nondeficit categorization as a grouping variable and those without it. The interaction between time and deficit/nondeficit categorization was not significant (F=1.74, df=2,110, p=0.18). The decline in BPRS total score occurred during the first 6 months of treatment (table 6). There were also significant reductions in the BPRS anxiety/depression factor scores (F=5.84, df=2,114, p=0.004), hostility factor scores (F=4.51, df=2,114, p=0.01), and activation factor scores (F=6.55, df=2,114, p=0.01) (data not shown). The time effects for the anxiety and activation factor scores remained significant when the deficit/nondeficit categorization was added as a grouping variable. There was a significant interaction between time and deficit/nondeficit categorization (F=3.81, df=2,110, p<<0.03) for the hostility factor, and the time effect was no longer significant (F=2.68, df=2,110, p=0.07), which suggests that the effect of clozapine on hostility was limited to the nondeficit patients.
There was a significant time effect for the Level of Functioning Scale total score, with patients showing a linear improvement over the course of the study (table 6). The time effect was significant for both the analyses with the deficit/nondeficit categorization as a grouping variable and those without it. The interaction between time and deficit/nondeficit categorization was not significant (F=0.91, df=2,110, p=0.47). To assess whether the observed change in Level of Functioning Scale score was related to the observed improvements in positive, affective, or extrapyramidal symptoms, we examined the correlations between change in these measures and change in the Level of Functioning Scale scores. There were no significant relationships.
The time effect for Quality of Life Scale total score was not significant in the analyses with and without the deficit/nondeficit categorization as a grouping variable (F=1.19, df=2,110, p=0.31, and F=2.08, df=2,114, p=0.13, respectively). The interaction between time and deficit/nondeficit categorization was also not significant (F=1.01, df=2,110, p=0.37).
There was a significant reduction in Simpson-Angus Rating Scale total scores (table 6). This significant time effect was observed in both the analyses with the deficit/nondeficit categorization as a grouping variable and those without it. The interaction between time and deficit/nondeficit categorization was not significant (F=0.01, df=2,110, p=0.99). There was no significant time effect for dyskinesia scores in either the analyses with the deficit/nondeficit categorization as a grouping variable (F=1.93, df=2,110, p=0.15) or those without it (F=1.13, df=2,114, p=0.33), and the interaction between time and deficit/nondeficit categorization was not significant (F=2.50, df=2,110, p=0.09).
Side effects and vital signs. In general, there was a mild decrease in side effects from baseline levels over the course of the year (table 7) (baseline represents the level of side effects after 10 weeks of clozapine treatment for the patients assigned to clozapine during the 10-week double-blind study or after 6 weeks of open-label clozapine for the patients first assigned to haloperidol). There was a significant decrease in the occurrence of dry mouth. The only side effect that significantly increased over the baseline level was salivation.
There was a significant time effect for weight gain (F=7.94, df=2,100, p=0.001), with patients continuing to gain weight over the first 6 months of treatment (baseline: mean weight=182.7 lb, SD=42.5; 6 months: mean=188.7 lb, SD=43.3; 12 months: mean=187.1 lb, SD=41.0). Vital signs were relatively constant over the course of the year.
DISCUSSION
The first major finding of this study was that clozapine had superior efficacy for positive symptoms in partially responsive outpatients with schizophrenia. This advantage of clozapine over haloperidol was observed in patients who were able to tolerate clozapine treatment and complete the 10-week double-blind study. The same observation was reported in our earlier presentation of the initial subset of study subjects (7). The intent-to-treat analyses failed to demonstrate a significant advantage for clozapine. The difference between the completer and intent-to-treat analyses reflects the relatively large number of patients randomly assigned to clozapine who failed to complete the study and the effect of carrying the last value forward, which obscures the benefit that is observed when only subjects who complete the study are examined. The positive symptom advantage for clozapine was observed in patients both with and without the deficit syndrome. The observation of similar positive symptom responses in the two schizophrenia patient groups argues against the notion that patients with prominent negative symptoms are less responsive to antipsychotic medications (25).
The superior efficacy of clozapine for positive symptoms was reinforced by the results of the 1-year open-label study. This effect was largely due to improvement in these symptoms during the first 6 months of the study; there was essentially no change in positive symptoms during the second 6 months. This time course of response to treatment is also reflected in the analysis of treatment responders. Forty-nine percent of the patients met the criteria for sustained positive symptom response, and the majority of these patients (N=23 of 26) met the criteria within the first 4 months of treatment. Of the three patients who had a delayed response, one patient met response criteria within 3 months of attaining the eventual therapeutic dosage, one exhibited clear evidence of symptom reduction before 4 months, and one met response criteria within 3 months of a 20% dosage reduction. The first two cases fit with previous observations of early evidence of response, with accumulating effect over time or delayed response secondary to prolonged dosage titration (7, 15, 26). The one candidate for a truly delayed response may represent ordinary clinical variability in course rather than a clozapine effect. Even if the 1-year study had been a random-assignment controlled study, one or two “late” responders would not exceed chance expectation.
The second major finding was the lack of efficacy of clozapine for negative symptoms. There was no significant difference between clozapine and haloperidol for SANS total score in the analyses comparing the total groups or the deficit/nondeficit subgroups. The lack of a significant interaction between treatment assignment and deficit/nondeficit categorization suggests that there was not a selective benefit of clozapine for either primary or secondary negative symptoms. The lack of a significant benefit of clozapine for secondary negative symptoms occurred despite the fact that patients were treated with adequate doses and clozapine exhibited superior benefit for extrapyramidal symptoms. This lack of efficacy for negative symptoms was also observed in the examination of the individual SANS factors. The only comparison that revealed a significant difference was the completer analysis for the SANS anhedonia factor. However, the difference between the two treatment groups was largely due to worsening of these symptoms in the haloperidol-treated patients. The lack of efficacy of clozapine for negative symptoms was also observed in the 1-year open-label study. Although it was an open-label study, the inclusion of 19 deficit patients provided a relatively reasonable assessment of the efficacy of clozapine for deficit symptoms and corroborates the observations from the double-blind study. The results are also in agreement with the results from an open-label study of inpatients with the deficit syndrome (15). The lack of differential efficacy of clozapine for negative symptoms, either primary or secondary, is consistent with oral reports from other recent double-blind studies (27 and unpublished data of J.M. Kane, 1996).
The conflicting negative symptom results between this and other more recent studies and earlier double-blind studies may reflect differences in baseline severity of positive and extrapyramidal symptoms. In the earlier inpatient studies, there were more robust reductions in both positive and extrapyramidal symptoms (2, 3). The patients in the current study had a significant but modest reduction in positive symptoms and, perhaps more important, a low level of baseline extrapyramidal symptoms. Taken together, the double-blind studies, in combination with open-label studies (6, 10, 11), argue that clozapine's effect for negative symptoms occurs largely in the context of patients with high levels of extrapyramidal symptoms and/or marked changes in positive symptoms.
In the examination of other measures, there were two differences between our initial and final presentations of results. First, in contrast to our earlier report of the double-blind study, we did not observe superior efficacy of clozapine for the BPRS hostility factor score. There was a tendency for this measure to decrease during the 10-week double-blind study, but the reduction did not reach significance. In the 1-year open-label study, clozapine treatment was observed to be effective for hostility symptoms, with the benefit for these symptoms largely observed in nondeficit inpatients. Second, despite the low baseline level of extrapyramidal symptoms, clozapine had superior efficacy and a long-term beneficial effect for these symptoms.
We continued to observe a significant improvement in social and occupational functioning as assessed by the Level of Functioning Scale. In contrast to positive and extrapyramidal symptoms, where changes during the 10-week double-blind study support the interpretation that observed changes in the 1-year open-label study are associated with clozapine treatment, the lack of similar controlled data for the Level of Functioning Scale requires a more conservative approach for attributing the observed improvement to clozapine treatment. The need for a conservative interpretation of these data is underscored by the recent 1-year open-label study by Essock and colleagues (28), who found that although clozapine improved functional status and subjective quality of life, there was no significant difference between clozapine and usual treatment.
If improvement in Level of Functioning Scale scores was associated with clozapine treatment, then what mechanisms might underlie the observed change in social and occupational functioning? There were no significant relationships among improvement in positive, affective, or extrapyramidal symptom measures and improvement in Level of Functioning Scale scores. This finding is consistent with our observation in the preliminary study group that Level of Functioning Scale total score was associated with memory performance (29). The differential association of symptom and cognitive measures with measures of functioning underscores the importance of developing effective treatments for cognitive impairments if enhanced social and occupational functioning is to be a goal of treatment (30).
Clozapine treatment was not associated with significant improvement in tardive dyskinesia, although this may have been related to the relatively low baseline levels of tardive dyskinesia in the study group. In a 1-year controlled study of patients with tardive dyskinesia (31), clozapine was shown to have superior efficacy for both dyskinetic movements and withdrawal dyskinesia. In our 10-week study, clozapine treatment was associated with increased dizziness, nausea, salivation, tachycardia, and weight gain and decreased extrapyramidal symptoms. In contrast, haloperidol treatment was associated with significant increases in dry mouth. Although there was a tendency for side effects to decrease over the course of our open-label study, there was a significant decrease only in the occurrence of dry mouth. There was actually a significant increase in the occurrence of salivation. These results suggest that there is a limited development of tolerance to the major side effects of clozapine treatment.
In summary, clozapine was more effective than haloperidol for positive symptoms in a group of stable, partially responsive outpatients with schizophrenia. This effect was observed in patients both with and without the deficit syndrome, and the majority of the patients exhibited a positive symptom response in the first 4 months of treatment. There was no evidence of any benefit of clozapine for either primary or secondary negative symptoms in this group, which strongly suggests that the field still lacks an effective treatment for primary negative symptoms.
 |
 |
 |
 |
 |
 |
 |
Received Aug. 7, 1997; revision received Dec. 12, 1997; accepted Dec. 23, 1997. From the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland at Baltimore, and the Section on Clinical Studies, NIMH, Bethesda, Md. Address reprint requests to Dr. Buchanan, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228. Supported in part by NIMH grants MH-45074 and MH-40279. Study medications were provided by the Sandoz Pharmaceuticals Corporation.
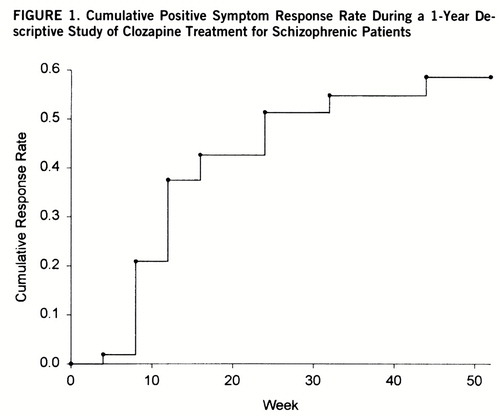
FIGURE 1. Cumulative Positive Symptom Response Rate During a 1-Year Descriptive Study of Clozapine Treatment for Schizophrenic Patients
1 Buchanan RW: Clozapine: efficacy and safety. Schizophr Bull 1995; 21:579–591Crossref, Medline, Google Scholar
2 Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
3 Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH: Clinical and biologic response to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Arch Gen Psychiatry 1992; 49:345–353Crossref, Medline, Google Scholar
4 Kuoppasalmi K, Rimon R, Naukkarinen H, Lang S, Sandqvist A, Leinonen E: The use of clozapine in treatment-refractory schizophrenia. Schizophr Res 1993; 10:29–32Crossref, Medline, Google Scholar
5 Lindenmayer J-P, Grochowski S, Mabugat L: Clozapine effects on positive and negative symptoms: a six-month trial in treatment-refractory schizophrenics. J Clin Psychopharmacol 1994; 14:201–204Crossref, Medline, Google Scholar
6 Lieberman JA, Safferman AZ, Pollack S, Syzmanski S, Johns C, Howard A, Kronig M, Bookstein P, Kane JM: Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry 1994; 151:1744–1752Link, Google Scholar
7 Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT Jr: Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 1994; 151:20–26Link, Google Scholar
8 Meltzer HY: Clozapine: is another view valid? (editorial). Am J Psychiatry 1995; 152:821–825Link, Google Scholar
9 Carpenter WT Jr, Conley RR, Buchanan RW, Breier A, Tamminga CA: Patient response and resource management: another view of clozapine treatment of schizophrenia. Am J Psychiatry 1995; 152:827–832Link, Google Scholar
10 Tandon R, Goldman R, DeQuardo JR, Goldman M, Perez M, Jibson M: Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatr Res 1993; 27:341–347Crossref, Google Scholar
11 Miller DD, Perry PJ, Cadoret RJ, Andreasen NC: Clozapine's effect on negative symptoms in treatment-refractory schizophrenics. Compr Psychiatry 1994; 35:8–15Crossref, Medline, Google Scholar
12 Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Link, Google Scholar
13 Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
14 Fenton WS, McGlashan TH: Testing systems for assessment of negative symptoms in schizophrenia. Arch Gen Psychiatry 1992; 49:179–184Crossref, Medline, Google Scholar
15 Conley R, Gounaris C, Tamminga C: Clozapine response varies in deficit versus non-deficit schizophrenic subjects. Biol Psychiatry 1994; 35:746–747Crossref, Google Scholar
16 Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
17 Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
18 Andreasen NC: Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry 1982; 39:784–788Crossref, Medline, Google Scholar
19 Breier A, Buchanan RW, Irish D, Carpenter WT Jr: Clozapine treatment of outpatients with schizophrenia: outcome and long-term response patterns. Hosp Community Psychiatry 1993; 44:1145–1149Abstract, Google Scholar
20 Hawk AB, Carpenter WT, Strauss JS: Diagnostic criteria and five-year outcome in schizophrenia: a report from the International Pilot Study of Schizophrenia. Arch Gen Psychiatry 1975; 32:343–347Crossref, Medline, Google Scholar
21 Heinrichs DW, Hanlon TE, Carpenter WT Jr: The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 1984; 10:388–398Crossref, Medline, Google Scholar
22 Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
23 Cassady SL, Thaker GK, Summerfelt A, Tamminga CA: The Maryland Psychiatric Research Center scale and the characterization of involuntary movements. Psychiatry Res 1997; 70:21–37Crossref, Medline, Google Scholar
24 Buchanan RW, Carpenter WT: Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis 1994; 182:193–204Crossref, Medline, Google Scholar
25 Crow TJ: The two-syndrome concept: origins and current status. Schizophr Bull 1985; 11:471–486Crossref, Medline, Google Scholar
26 Umbricht DG, Lieberman JA, Kane JM: The clinical efficacy of clozapine in the treatment of schizophrenia. Reviews in Contemporary Pharmacotherapy 1995; 16:165–186Google Scholar
27 Rosenheck R, Charney D, Cramer J, Xu W, Thomas J, VA CSH 17 Study Group: A randomized, double-blind trial of the efficacy and cost-effectiveness of clozapine (abstract). Schizophr Res 1997; 24:188Google Scholar
28 Essock SM, Hargreaves WA, Covell NH, Goethe J: Antipsychotics in research and clinical settings: clozapine's effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull 1996; 32:683–697Medline, Google Scholar
29 Buchanan RW, Holstein C, Breier A: The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol Psychiatry 1994; 36:717–725Crossref, Medline, Google Scholar
30 Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
31 Tamminga CA, Thaker GK, Moran M, Kakigi T, Gao X-M: Clozapine in tardive dyskinesia: observations from human and animal model studies. J Clin Psychiatry 1994; 55(9, suppl B):102–106Google Scholar



