Increased Depression Screening and Treatment Recommendations After Implementation of a Perinatal Collaborative Care Program
Abstract
Objective:
The study evaluated whether implementation of perinatal collaborative care is associated with improvements in screening and treatment recommendations for perinatal depression by obstetric clinicians.
Methods:
This cohort study, conducted from January 2015 to January 2019, included all women who received prenatal care in five obstetric clinics and delivered at a single quaternary care hospital in Chicago. In January 2017, a perinatal collaborative care program (COMPASS) was implemented. Completion of depression screening and recommendations for treatment following a positive depression screen were compared before and after COMPASS implementation. Adjusted analyses included inverse probability weighting by using propensity scores to impose control over imbalance between exposure groups with respect to prespecified covariates.
Results:
A total of 7,028 women were included in these analyses: 3,227 (46%) before and 3,801 (54%) after COMPASS implementation. Women who received obstetric care after implementation were significantly more likely than those who received care before implementation to receive antenatal screening for depression (81% versus 33%; adjusted odds ratio [aOR]=8.5, 95% confidence interval [CI]=7.6–9.5). After implementation, women with a positive antenatal screen for depression were more likely to receive a treatment recommendation (61% versus 44%; aOR=2.1, 95% CI=1.2–3.7). After implementation of perinatal collaborative care, combined psychotherapy and pharmacotherapy were more frequently recommended, compared with before implementation.
Conclusions:
Implementation of a perinatal collaborative care program was associated with improvements in perinatal depression screening and recommendations for treatment by obstetric clinicians.
HIGHLIGHTS
Whether implementation of collaborative care is effective in the unique context of perinatal care has not been established.
After a perinatal collaborative care program was implemented, women were significantly more likely to receive screening for depression, compared with before implementation, and when a positive screen was identified, they were also more likely to receive a treatment recommendation by their obstetric clinician.
Perinatal collaborative care may be a health services approach to improving obstetric clinician engagement in the perinatal mental health care cascade.
Perinatal depression is one of the most common complications of pregnancy. Annually in the United States, a new episode of perinatal depression affects approximately 300,000 women during pregnancy and an additional 260,000 women in the first 3 months postpartum (1, 2). Untreated or undertreated perinatal depression can have devastating maternal consequences. For example, one in five women with perinatal depression endorse suicidal ideation (2), and suicide remains a leading cause of maternal mortality (3–5). Not only does depression incur serious maternal risks, but it also has been associated with adverse perinatal outcomes, including fetal growth restriction (6–9) and preterm birth (7, 9, 10). These complications are responsible for a majority of neonatal morbidity and mortality and are an enormous economic burden to the health care system. Untreated perinatal depression is independently associated with long-term adverse neurodevelopmental consequences for the offspring, with effects particularly pronounced in socioeconomically disadvantaged populations (11).
Although universal screening for depression during pregnancy and postpartum is recommended (12), fewer than half of women with perinatal depression are identified by their obstetric clinician (13). Numerous obstacles to routine screening exist, and many obstetricians report that time constraints to evaluate women with positive screen results are a leading barrier (14). Thus, substantial support is required for depression screening programs to be successful (15).
Screening in the absence of adequate care planning and treatment has little impact on depression outcomes (16). Obstetric clinicians often do not feel comfortable managing a positive depression screen because of inadequate training in pharmacotherapy and limited access to scarce psychiatric referral sources (17). Consequently, only a minority of women with perinatal depression receive mental health care (18), and meta-analytic data suggest that only 3%−5% of women with perinatal depression are treated to remission (13). These data underscore the absence of a critical system of health services for women with perinatal depression.
Collaborative care (CC), a health systems approach to care for individuals with depression, integrates mental health care into ongoing primary care. Mental health benefits are achieved through adherence to its core principles: patient-centered team care, population-based care, measurement-based treatment to target remission, and evidence-based interventions (19). A care manager serves as the cornerstone of CC and facilitates initial treatment planning, brief behavioral care, longitudinal symptom monitoring, and implementation of specialist-informed stepped-care recommendations. Data from primary care settings suggest that CC is both successful and cost-effective (20–25). Whether these results can be generalized to the perinatal period is uncertain (26,27). Pregnant and postpartum women have unique challenges, such as the physical demands of pregnancy, delivery, breastfeeding, and caring for a new infant and the marked change in interpersonal relationships and employment status. Despite hurdles, the perinatal period is a time of opportunity for mental health interventions because of frequent antenatal contact with clinicians and women’s motivation for positive health changes during pregnancy (28–30).
Two small randomized trials evaluating CC programs in obstetric clinics showed improved depression outcomes among women who received the CC intervention (31, 32). However, the improved outcomes seen with CC in primary care trial settings have not been replicated consistently when CC has been implemented in the actual care setting (33, 34). Evidence to support the effectiveness of perinatal CC when incorporated into an obstetrical setting rather than a highly controlled randomized trial has not been published but is needed prior to broad dissemination (35). In addition, most women with perinatal depression are neither identified nor counseled to seek treatment by their obstetric clinician (13). The impact of CC on these obstetric clinician behaviors has not been examined. Our objective was to evaluate whether implementation of perinatal CC was associated with improvements in screening for and provision of depression treatment recommendations by obstetric clinicians.
Methods
COMPASS Program
COMPASS (Collaborative Care Model for Perinatal Depression Support Services) was implemented in January 2017 within five obstetric care offices affiliated with an urban academic medical center. These practices serve approximately 3,500 women annually. Three practices are staffed by obstetrician-gynecologist specialists, one by maternal-fetal medicine subspecialists, and one by certified nurse midwives.
All women who are patients of these practices are eligible for COMPASS services during pregnancy or up to 1 year postpartum. Women with a history of depression or current depressive symptoms as assessed by a clinical evaluation or a positive routine antenatal or postpartum screen are referred either by their obstetric clinician or via self-referral. All pregnant women are given written information about the program at their first prenatal visit. Once a woman is referred, a care manager completes a psychiatric diagnostic evaluation, along with screening for other psychiatric morbidities (i.e., anxiety, posttraumatic stress, and bipolar disorders), and discusses an initial care plan with the patient and her obstetric clinician.
Options for treatment with antidepressants and psychotherapy are discussed with all women. A shared decision–making model, including the obstetric clinician, care manager, and the patient herself, is used for all treatment recommendations. Psychiatric consultation, if clinically indicated, is performed within the obstetric clinic by instructor-level perinatal psychiatry trainees (i.e., women’s mental health psychiatric fellows) supervised by perinatal psychiatry attending physicians. Psychotherapy, if clinically indicated, is performed by the care manager or another licensed clinical social worker located within the obstetric clinic. Women who require primary psychiatric management (e.g., women with bipolar disorder) are enrolled in the COMPASS program, but the COMPASS psychiatrist provides longitudinal psychiatric care within the obstetric offices. For women who require a higher level of care, such as intensive outpatient care or partial hospitalization, referrals are made and the care manager continues to follow them in COMPASS within the patient registry. Women who enter the COMPASS program are followed within the patient registry through 12 months postpartum with Web-based electronic depressive symptom screens to monitor for response, remission, and relapse of their depression.
The core COMPASS care team includes the program director, a clinical liaison, two perinatal psychiatry fellows, an L.C.S.W. therapist, and two L.C.S.W. care managers. This team meets weekly to review new referrals and discuss all patients whose depressive symptoms are not improving with treatment.
The team meets monthly to ensure that the program is maintaining fidelity to the principles of CC (36). Specifically, the care pathways of CC that are embedded into the COMPASS workflow are described herein. All referred patients have perinatal depression symptoms tracked in a patient registry. Self-reported measures of depression (i.e., the nine-item Patient Health Questionnaire [PHQ-9] [37]) are sent every 2 weeks during the time that the patient endorses depressive symptoms and then monthly once the symptoms are in remission (i.e., a PHQ-9 score <5). The trajectory of symptom response is used to inform the care plan, via measurement-based practice. The goal for all referred individuals is remission of symptoms, and changes to the care plan for individuals who do not achieve remission are discussed in the weekly patient care meeting, led by the care manager and supervised by the consulting perinatal psychiatrist. These care recommendations are then communicated by the care manager to both the patient and her obstetric clinician for implementation.
Sample
All women who received prenatal care in the five clinics within which COMPASS was embedded were included in these analyses. Women were identified through a single, comprehensive, and integrated repository of all electronic health records (EHRs) at Northwestern Medicine. Women were excluded from this analysis if they delivered prior to 20 weeks gestation (given the limited opportunities for antenatal screening and treatment) or if they delivered at an outside hospital (because postpartum care was often then provided by other clinicians not affiliated with COMPASS).
Women were divided into two cohorts, based on their delivery date, to reflect when the COMPASS program was implemented (Figure 1). The 1-year period prior to COMPASS implementation included women who delivered from September 1, 2015, to August 31, 2016. Obstetric clinician instruction regarding the principles of CC and the COMPASS program began in September 2016. This training was included in a department grand rounds, and individualized training sessions were provided in at least two of the practices’ clinical meetings. These sessions included the core principles of CC, the mental health resources available, and the role of obstetric clinicians in supporting and comanaging pharmacotherapy for perinatal depression. Notably, there was no coordination with the obstetric practices to facilitate depression screening workflows. In addition, there were no systems changes to the EHR, such as best alerts, to support depression screening completion. Rather, each practice was advised on the importance of depression screening, but practices were left to implement processes within their own clinics to facilitate change.
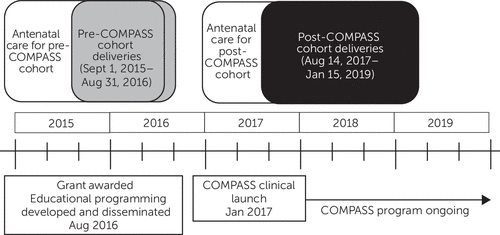
FIGURE 1. Study cohorts before and after COMPASS collaborative care program implementation
The full COMPASS program (i.e., the ability to receive referrals and provide clinical care) was initiated on January 30, 2017. For these analyses, we excluded women whose pregnancy or postpartum visit occurred during the rollout period from September 2016 through January 2017 (38). Accordingly, the post-COMPASS cohort included women who delivered from August 14, 2017, to January 15, 2019.
Data Acquisition
For all women eligible for inclusion in the analysis, detailed prenatal, intrapartum, and postpartum data were abstracted from their EHR by use of a combination of computer-generated systematic queries and manual abstraction for data points not systematically accessible for data analytics (e.g., free-text descriptions of psychiatric care plans). Data were abstracted under the supervision of the COMPASS program director, and the team housed the data in Northwestern University’s Research Electronic Data Capture (39). To ensure data integrity, a chart auditor with robust obstetric and perinatal depression EHR experience reviewed a random sample of records to provide iterative feedback to minimize inaccuracies or missing data. The Northwestern University Institutional Review Board approved the study, with a waiver of informed consent. Principles outlined in the Declaration of Helsinki were followed.
The primary objective was to evaluate whether implementation of COMPASS was associated with changes in obstetric clinician behaviors (e.g., screening for depression and recommendation for mental health treatment). We hypothesized that when a supportive infrastructure for clinical care was in place, obstetric clinicians would be more likely to engage in perinatal mental health care delivery. Accordingly, the primary outcomes were the performance of depression screening by the obstetric clinician and provision of recommendations for treatment. Across obstetric practices, depression screening occurred with the PHQ-9 (37) and was recorded in the EHR in a flowsheet. Free-text searches of prenatal and postpartum outpatient obstetric visits were performed to identify screening data that were not entered into the flowsheet. Any depression screen performed by the obstetric clinician during the prenatal or postpartum periods was documented.
For all women with a PHQ-9 score ≥10, detailed information on recommendations for treatment was abstracted from the patient’s EHR (e.g., free-text search surrounding the visit or visits when screening was performed, medication summaries, or the problem list). For women with more than one positive screen, we recorded the plan with the highest intensity of treatment (i.e., pharmacotherapy+psychotherapy>pharmacotherapy>psychotherapy>other) as their ascribed care plan. Although sustained linkage to mental health care would be a clinically important outcome, because of the fragmented nature of the health care system and privacy restrictions within the EHR, data on mental health care visits were not consistently available. Thus, the presence of a recommendation for mental health treatment documented by the obstetric clinician, an important step in the mental health care cascade, was used for analysis.
Statistical Analysis
We used descriptive statistics to characterize sociodemographic and clinical characteristics of COMPASS-eligible women, stratified by exposure cohort (i.e., before or after implementation of COMPASS). Bivariable comparisons involved Student’s t test or the Mann-Whitney U test for continuous variables or chi-square analysis for categorical variables.
Simple logistic regression was used to examine the unadjusted association between COMPASS implementation cohort (independent variable) and depression screening completion (dependent variable). Adjusted analyses included inverse probability weighting using propensity scores to impose control over imbalance between cohorts with respect to prespecified covariates. We chose the covariates for inclusion in adjusted analyses a priori based on clinical judgment. Covariates included maternal age, estimated gestational age at first prenatal visit (for antenatal analyses), insurance, parity, maternal race, maternal ethnicity, use of tobacco, history of substance use, and any maternal chronic medical problem. For postpartum analyses, we also considered as covariates gestational diabetes, hypertensive disorders of pregnancy, route of delivery, and gestational age at delivery (40). Preterm birth and birthweight were excluded because of their overlap with gestational age at delivery. Mental health diagnosis prior to pregnancy was also excluded, given anticipated information bias that selectively enhances acquisition of these clinical data by the COMPASS care team.
As is consistent with methodology in propensity score (PS) weighting, the post-COMPASS cohort received a weight of 1/PS (where PS=probability of exposure to COMPASS given the above list of covariates), and the pre-COMPASS cohort received a weight of 1/(1−PS). We obtained confidence limits around inverse probability–weighted estimates with bootstrapping, whereby we resampled from the existing data set 5,000 times. In each iteration, we performed inverse probability weighting to estimate the effect of COMPASS exposure on screening rate, and after the 5,000 iterations, we used the 2.5th and 97.5th percentiles of these estimates to approximate the 95% confidence limits around the weighted estimate.
All analyses were conducted with R, version 3.5.3 and assumed a two-sided, 5% significance level. We did not adjust for multiple hypothesis testing.
Results
During the study period, 7,028 women met eligibility criteria. A total of 3,227 (46%) women received prenatal care before and 3,801 (54%) women received prenatal care after implementation of COMPASS. Table 1 displays participant characteristics stratified by COMPASS implementation cohort. A number of statistically significant, but likely clinically insignificant, differences were noted between the two cohorts with respect to demographic and clinical characteristics. Specifically, compared with women who received care before COMPASS, women who received care after implementation were more likely to have public insurance (p=0.001), to be nulliparous (p=0.04), and to self-identify as being from a racial-ethnic minority group (p=0.001). Women who received care after implementation of COMPASS were also more likely to have a documented mental health diagnosis prior to pregnancy (p<0.001) and to have a documented chronic medical problem (p<0.001).
| Preimplementation | Postimplementation | ||||
|---|---|---|---|---|---|
| (N=3,227) | (N=3,801) | ||||
| Characteristic | N | % | N | % | p |
| Maternal age at first prenatal visit (M±SD) | 32.8±4.5 | 32.9±4.7 | .48 | ||
| Gestational age at first prenatal visit (weeks)a | 9.14 | 9.43 | <.001 | ||
| Insurance type | .001 | ||||
| Private | 2,840 | 88.0 | 3,263 | 85.8 | |
| Public | 379 | 11.7 | 509 | 13.4 | |
| Other | 8 | .2 | 29 | .8 | |
| Nulliparous | 1,588 | 49.2 | 1,965 | 51.7 | .04 |
| Race | <.001 | ||||
| White or Caucasian | 1,897 | 58.8 | 2,146 | 56.5 | |
| Black or African American | 399 | 12.4 | 550 | 14.5 | |
| Asian, Native Hawaiian, or Other Pacific Islander | 303 | 9.4 | 413 | 10.9 | |
| Unknown or refused to answer | 151 | 4.7 | 385 | 10.1 | |
| Other | 477 | 14.8 | 307 | 8.1 | |
| Hispanic ethnicity | 407 | 13.0 | 472 | 13.3 | .72 |
| Mental health diagnosis prior to pregnancy | 576 | 17.8 | 982 | 25.9 | <.001 |
| Tobacco use | .002 | ||||
| Never | 2,716 | 84.2 | 3,280 | 86.6 | |
| Past | 484 | 15.0 | 464 | 12.2 | |
| Current | 27 | .8 | 44 | 1.2 | |
| Other substance use | 116 | 3.6 | 92 | 2.4 | .005 |
| Chronic medical condition | 1,047 | 32.4 | 1,592 | 41.9 | <.001 |
| Gestational diabetes | 153 | 4.7 | 245 | 6.4 | .002 |
| Hypertensive disorder of pregnancy | 237 | 7.3 | 340 | 8.9 | .02 |
| Gestational age at delivery (weeks)b | 39.43 | 39.29 | <.001 | ||
| Preterm birth | 286 | 8.9 | 417 | 11.0 | .004 |
| Birthweight (M±SD kg) | 3.30±.62 | 3.26±.61 | .003 | ||
| Route of delivery | .99 | ||||
| Operative vaginal delivery | 220 | 6.8 | 262 | 6.9 | |
| Spontaneous vaginal delivery | 2,203 | 68.3 | 2,596 | 68.3 | |
| Cesarean delivery | 804 | 24.9 | 943 | 24.8 | |
TABLE 1. Characteristics of women receiving prenatal care, by collaborative care implementation cohort
Screening for antenatal depression improved significantly after implementation of COMPASS (80.9% versus 32.7%; p<0.001, odds ratio [OR]=8.82) (Table 2). The increased odds of screening were similar after propensity score–weighted analyses. When screening was performed, no clinically significant differences were noted in the frequency of a positive screen before and after COMPASS implementation. However, after COMPASS implementation women with a positive screen were significantly more likely to receive a recommendation for mental health treatment (61.4% versus 43.6%, p=0.021, OR=2.1). Findings remained similar after propensity score weighting (Table 2).
| Unadjusted | Propensity score– | |||||||
|---|---|---|---|---|---|---|---|---|
| Preimplementation | Postimplementation | analysis | weighted analysis | |||||
| Variable | N | % | N | % | OR | 95% CI | aOR | 95% CI |
| Antenatal depression | ||||||||
| Screen performed | 1,054 | 32.7 | 3,075 | 80.9 | 8.82 | 7.89–9.87 | 8.46 | 7.61–9.47 |
| Screen positivea | 101 | 9.6 | 272 | 8.9 | .98 | .77–1.26 | 1.36 | 1.05–1.79 |
| Care plan recommendedb | 44 | 43.6 | 167 | 61.4 | 2.10 | 1.31–3.38 | 2.13 | 1.23–3.71 |
| Postpartum depression | ||||||||
| Screen performed | 2,779 | 92.8 | 3,237 | 94.9 | 1.46 | 1.18–1.80 | 1.52 | 1.22–1.91 |
| Screen positivea | 100 | 3.6 | 148 | 4.6 | 1.34 | 1.03–1.74 | 1.31 | 1.00–1.74 |
| Care plan recommendedb | 63 | 63.0 | 104 | 70.3 | 1.29 | .74–2.25 | 1.47 | .78–2.78 |
TABLE 2. Depression screening and care plan recommendations made by obstetric clinicians for women receiving obstetric care, comparisons by collaborative care implementation cohort
Of the 7,028 women included in the study, 6,405 (91.1%) attended their postpartum visit. Among women who attended their postpartum visit, screening for postpartum depression significantly increased after implementation of COMPASS (94.9% versus 92.8%, p<0.001; OR=1.46) (Table 2). The frequency of a positive screen was slightly higher among women screened after implementation of COMPASS. For women with a postpartum positive screen point estimates of obstetric clinician recommendation for a care plan increased after COMPASS implementation (70.3% versus 63.0%, p=0.029, OR=1.29); however, this difference was not statistically significant in propensity score–weighted analyses. As in the antenatal outcomes, the odds of association were similar for all unadjusted and propensity score–weighted analyses.
When a care plan was developed in response to a positive depression screen, the type of care plan significantly differed by implementation cohort (Table 3). For both antenatal and postpartum care, women with moderate depression (i.e., PHQ-9 score ≥10) were more likely after implementation to receive a recommendation for both psychotherapy and pharmacotherapy.
| Recommended | Preimplementation | Postimplementation | |||
|---|---|---|---|---|---|
| care plan | N | % | N | % | p |
| Antenatal depression care plan | 44 | 100 | 167 | 100 | .008 |
| Psychotherapy | 11 | 25.0 | 60 | 35.9 | |
| Pharmacotherapy | 10 | 22.7 | 17 | 10.2 | |
| Both psychotherapy and pharmacotherapy | 5 | 11.4 | 46 | 27.5 | |
| Social work consultation or other care | 18 | 40.9 | 44 | 26.3 | |
| Postpartum depression care plan | 63 | 100 | 104 | 100 | .01 |
| Psychotherapy | 26 | 41.3 | 28 | 26.9 | |
| Pharmacotherapy | 15 | 23.8 | 13 | 12.5 | |
| Both psychotherapy and pharmacotherapy | 17 | 27.0 | 42 | 40.4 | |
| Social work consultation or other care | 5 | 7.9 | 21 | 20.2 | |
TABLE 3. Depression care plans recommended by obstetric clinicians, by collaborative care implementation cohort
Discussion
Obstetric clinicians are the first-line providers in perinatal depression care. Unfortunately, only 50% of women with antenatal depression and only 33% of women with postpartum depression are identified in the care model used in most practices (13). Data from this implementation project demonstrate that perinatal CC is a health systems approach that enhances identification of perinatal depression. After implementation of CC, 81% and 95% of women were screened for antenatal and postpartum depression, respectively. Although screening alone cannot improve outcomes, these data suggest that after implementation of CC, most women who were identified as having perinatal depressive symptoms also received a recommendation for mental health treatment from their obstetric clinician. After implementation of CC, the recommended mental health care plans were more likely to include a combination of psychotherapy and pharmacotherapy. This may reflect both improved patient access to psychotherapy within the COMPASS program, as well as increased obstetric clinician comfort with discussing and prescribing pharmacotherapy.
In the United States, nearly half of adults with depression are managed by primary care clinicians (41, 42). Robust data support the notion that CC improves depression outcomes, compared with usual care in the primary care setting (20). However, these data have established CC as a standard of care only after depression has been identified. Studies of CC, to date, have not examined whether this system of care improves antecedent components (e.g., screening and initiation of treatment) of the depression care cascade. From a public health perspective, two of the largest gaps in the depression care cascade lie in the identification of symptoms and recommendation for treatment (43). Depression is identified by nonpsychiatrists in fewer than half of cases (44), and when it is diagnosed by a nonpsychiatrist, only 37% of individuals receive mental health care within a year of symptom onset (45). Data from this study suggest that implementation of CC overcomes the initial barriers to mental health care entry. Because no additive process supports were provided and no mandates for changes in care disseminated over the study period, we theorize that obstetric clinicians were more willing and able to do both, given the additional psychiatric resources and support inherent in the CC model.
This study was strengthened by the large and diverse population of women within which implementation occurred. The COMPASS program included practices based in midwifery, perinatology, and obstetrics in which socioeconomically diverse women received peripartum care. These characteristics increased the likelihood of external generalizability. The observational design improved our ability to assess the effectiveness of CC in the real-world setting. Moreover, the validity of the results was strengthened by propensity score–weighting analytic techniques that adjusted for potential confounders, which could have accounted for differences in screening or treatment recommendations. Nevertheless, we recognize that even with the use of these analytic tools for causal inference, causality cannot be established. Fueled by these findings, future research should used randomized methodologies, such as a cluster-randomized trial design, to evaluate the true impact of perinatal CC. In addition, this study took place in a state that legislatively mandated postpartum depression screening in 2008 (46), which likely contributed to higher baseline rates of postpartum screening and treatment, compared with women seen in other areas of the United States (13). This legislation may have increased obstetric clinicians’ awareness of postpartum depression and thereby muted potential impacts of CC on postpartum depression screening and treatment recommendations. Finally, the data available were limited to recommendations for treatment by the obstetric clinician. Although this represents an important, and often neglected, step in the care cascade, it does not reflect whether treatment was initiated or continued.
Our data are related to process outcomes. Future research should examine whether these depression care changes translate into improvements in clinical and psychiatric outcomes. In addition, randomized trials of CC in the primary care setting have demonstrated improvements in several somatic health outcomes, including reduced hemoglobin A1c levels among individuals with diabetes, improved cholesterol profiles, lower systolic blood pressure, and improved quality of life from arthritis (47, 48). The small sample sizes in the existing literature of perinatal CC do not allow for meaningful assessment of somatic perinatal outcomes, such as preterm birth or neonates who are small for gestational age. Future research should evaluate whether perinatal CC is a means to improve somatic obstetric or perinatal outcomes. Finally, COMPASS was implemented in five clinics within a single university setting. Future research should evaluate the implementation strategies used and adapt them to other obstetric contexts.
Conclusions
Public health steps toward remission of perinatal depression require specific targeting of multiple gaps in the depression care cascade. CC supports a culture of mental health awareness and support that enables obstetric clinicians to initiate the critical first steps on the path to remission. Future research should investigate implementation strategies to optimize dissemination.
1 : Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess 2005; 2005(119):1–8Google Scholar
2 : Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 2013; 70:490–498Crossref, Medline, Google Scholar
3 : Maternal deaths from suicide and overdose in Colorado, 2004–2012. Obstet Gynecol 2016; 128:1233–1240Crossref, Medline, Google Scholar
4 : Maternal health in pregnancy: messages from the 2014 UK Confidential Enquiry Into Maternal Death. Br J Gen Pract 2015; 65:444–445Crossref, Medline, Google Scholar
5 : Maternal self-harm deaths: an unrecognized and preventable outcome. Am J Obstet Gynecol 2019; 221:295–303Crossref, Medline, Google Scholar
6 : Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry 2012; 69:706–714Crossref, Medline, Google Scholar
7 : A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010; 67:1012–1024Crossref, Medline, Google Scholar
8 : Does fetal exposure to SSRIs or maternal depression impact infant growth? Am J Psychiatry 2013; 170:485–493Link, Google Scholar
9 : Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 2006; 63:898–906Crossref, Medline, Google Scholar
10 : Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol 2002; 156:797–802Crossref, Medline, Google Scholar
11 : Effects of perinatal mental disorders on the fetus and child. Lancet 2014; 384:1800–1819Crossref, Medline, Google Scholar
12 The American College of Obstetricians and Gynecologists Committee opinion no 630: screening for perinatal depression. Obstet Gynecol 2015; 125:1268–1271Crossref, Medline, Google Scholar
13 : The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry 2016; 77:1189–1200Crossref, Medline, Google Scholar
14 : Australian midwives’ awareness and management of antenatal and postpartum depression. Women Birth 2012; 25:23–28Crossref, Medline, Google Scholar
15 : Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Med Care 2004; 42:1211–1221Crossref, Medline, Google Scholar
16 Screening and case-finding instruments for depression: a meta-analysis. CMAJ 2008; 178:997–1003Crossref, Medline, Google Scholar
17 : Depression screening attitudes and practices among obstetrician-gynecologists. Obstet Gynecol 2003; 101:892–898Crossref, Medline, Google Scholar
18 : Success of mental health referral among pregnant and postpartum women with psychiatric distress. Gen Hosp Psychiatry 2009; 31:155–162Crossref, Medline, Google Scholar
19 : Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the academy of psychosomatic medicine research and evidence-based practice committee. Psychosomatics 2014; 55:109–122Crossref, Medline, Google Scholar
20 : Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012; 10:CD006525Medline, Google Scholar
21 : Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med 2012; 42:525–538Crossref, Medline, Google Scholar
22 : Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry 2006; 28:101–107Crossref, Medline, Google Scholar
23 : Cost and quality impact of Intermountain’s mental health integration program. J Healthc Manag 2010; 55:97–113Crossref, Medline, Google Scholar
24 : Partnership research: a practical trial design for evaluation of a natural experiment to improve depression care. Med Care 2010; 48:576–582Crossref, Medline, Google Scholar
25 : Initiation of Primary Care–Mental Health Integration programs in the VA health system: associations with psychiatric diagnoses in primary care. Med Care 2010; 48:843–851Crossref, Medline, Google Scholar
26 : Patterns of depression and treatment in pregnant and postpartum women. Can J Psychiatry 2012; 57:161–167Crossref, Medline, Google Scholar
27 : Seeking help for perinatal psychological distress: a meta-synthesis of women’s experiences. Br J Gen Pract 2017; 67:e692–e699Crossref, Medline, Google Scholar
28 : Motivation for smoking cessation among pregnant women. Psychol Addict Behav 2001; 15:126–132Crossref, Medline, Google Scholar
29 : Giving offspring a healthy start: parents’ experiences of health promotion and lifestyle change during pregnancy and early parenthood. BMC Public Health 2011; 11:936Crossref, Medline, Google Scholar
30 : Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol 2010; 202:135.e1–135.e8Crossref, Google Scholar
31 : Collaborative care for perinatal depression in socioeconomically disadvantaged women: a randomized trial. Depress Anxiety 2015; 32:821–834Crossref, Medline, Google Scholar
32 : Improving care for depression in obstetrics and gynecology: a randomized controlled trial. Obstet Gynecol 2014; 123:1237–1246Crossref, Medline, Google Scholar
33 : The DIAMOND initiative: implementing collaborative care for depression in 75 primary care clinics. Implement Sci 2013; 8:135Crossref, Medline, Google Scholar
34 : A stepped-wedge evaluation of an initiative to spread the collaborative care model for depression in primary care. Ann Fam Med 2015; 13:412–420Crossref, Medline, Google Scholar
35 : Integrated perinatal mental health care: a national model of perinatal primary care in vulnerable populations. Prim Health Care Res Dev 2018; 20:1–8Google Scholar
36
37 : Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282:1737–1744Crossref, Medline, Google Scholar
38 : Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health 2018; 39:5–25Crossref, Medline, Google Scholar
39 : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381Crossref, Medline, Google Scholar
40 : An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424Crossref, Medline, Google Scholar
41 : The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105Crossref, Medline, Google Scholar
42 : Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA 1998; 279:526–531Crossref, Medline, Google Scholar
43 : The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep 2012; 14:328–335Crossref, Medline, Google Scholar
44 : Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009; 374:609–619Crossref, Medline, Google Scholar
45 : Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:603–613Crossref, Medline, Google Scholar
46 : Perinatal depression: a review of US legislation and law. Arch Women Ment Health 2013; 16:259–270Crossref, Medline, Google Scholar
47 : Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010; 363:2611–2620Crossref, Medline, Google Scholar
48 : Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA 2003; 290:2428–2429Crossref, Medline, Google Scholar



