Randomized Trial of Interventions for Smoking Cessation Among Medicaid Beneficiaries With Mental Illness
Abstract
Objective:
Medicaid beneficiaries with severe mental illnesses are a financially disadvantaged group with high rates of smoking and poor cessation outcomes. This study examined whether abstinence-contingent monetary incentives improved outcomes when added to cessation treatments at community mental health centers: prescriber visit for pharmacotherapy only (PV only), prescriber visit and facilitated quitline (PV+Q), and prescriber visit and telephone cognitive-behavioral therapy (PV+CBT).
Methods:
During 2012–2015, a total of 1,468 adult, daily smoking Medicaid beneficiaries with mental illnesses received Web-based motivational tobacco education. Eligible participants who wanted cessation treatment (N=661) were randomly assigned to treatment with or without abstinence-contingent incentives for four weeks after a quit attempt and assessed for biologically verified abstinence at three, six, nine, and 12 months. To examine intervention effect on abstinence over time, logistic generalized linear models estimated with generalized estimating equations were used, with missing observations imputed as smoking.
Results:
Participants included smokers with schizophrenia disorders (N=148), bipolar disorder (N=150), major depressive disorder (N=158), and anxiety and other disorders (N=205). There was no significant effect of intervention (PV only, PV+Q, and PV+CBT). However, participants who received monetary incentives were more likely to be abstinent from smoking over time (adjusted odds ratio [AOR]=1.77, p=.009). Post hoc comparisons indicated greater abstinence at 12 months in PV+Q with incentives than in PV+Q without incentives (14% versus 4% abstinent, AOR=3.94, p=.014). Treatment participation and cessation outcomes did not differ significantly between diagnostic groups.
Conclusions:
Abstinence-contingent incentives improved cessation outcomes among financially disadvantaged smokers with mental illness receiving tobacco treatment at community mental health centers.
Among people with low incomes, those with mental illness have some of the highest rates of smoking and lowest rates of quitting (1). Cessation treatment can help these smokers quit. Structured cognitive-behavioral therapy (CBT) for smoking cessation has been tailored, manualized, and tested with various pharmacotherapies in many studies among smokers with severe mental illnesses (2–5). Because in-person counseling is not easily accessible, providing counseling over the phone can improve access (6,7). Quitlines provide free, multisession telephone cessation counseling and nicotine replacement therapy (NRT) through a centralized service (8), but initial reports indicate that outcomes are worse for smokers with mental illness (7). One study demonstrated that 7% of smokers with schizophrenia achieved biologically confirmed abstinence at six-month follow-up (9). Thus additional controlled research is needed to evaluate more potent strategies among financially disadvantaged smokers with mental illnesses.
Providing monetary incentives contingent on abstinence is one potential approach to improve outcomes (10). Based on reinforcement theory, principles of contingency management (11), and behavioral economics (12), incentives serve to reinforce behavior, increasing the likelihood of the recurrence of that behavior in the future. The efficacy of incentives for smoking cessation has been shown for a range of economically disadvantaged smokers (13–18). The Affordable Care Act created the Medicaid Incentives for the Prevention of Chronic Diseases (MIPCD) program to test the effectiveness of incentives for the adoption of healthy behaviors specifically among Medicaid beneficiaries. New Hampshire received a MIPCD award to address obesity and smoking.
With this award, funded by the Centers for Medicare and Medicaid Services, we tested the ability of monetary incentives to improve outcomes among Medicaid beneficiaries receiving outpatient community mental health smoking cessation treatment services. Participants were randomly assigned to one of three clinical cessation conditions in their community mental health centers (CMHCs): prescriber visit (PV only), prescriber visit and facilitated quitline (PV+Q), or prescriber visit and telephone CBT (PV+CBT). Participants in each cessation intervention group were also randomly assigned to receive a postquit four-week program of monetary incentives contingent on biologically verified smoking abstinence or no incentives. We hypothesized that the 12-session PV+CBT condition would result in the highest rate of smoking cessation, compared with the other two intervention conditions, and that assignment to monetary incentives would result in greater smoking abstinence than no incentives across all cessation treatment conditions.
Methods
Study Sites
The ten New Hampshire CMHCs participated in the study. Approximately 75 psychiatrists and advanced practice nurses deliver psychiatric care with psychotropic medication. They were trained (19) to provide “5 As” brief counseling (ask, advise, assess, assist, and arrange) (20) and cessation pharmacotherapy tailored for people with severe mental illness (4,5).
Participants
Participants were community-dwelling adult Medicaid beneficiaries with a mental illness diagnosis who were receiving services at a CMHC. Eligibility for Medicaid required a yearly income less than $1,317 per month; thus all participants had low income. All English-speaking daily smokers were eligible to receive the initial Web-based education for smokers. Upon completion, eligibility for the cessation intervention study was limited to those who smoked 10 or more cigarettes per day and who had a breath carbon monoxide sample ≥8 ppm and a willingness to initiate cessation treatment within 30 days. Exclusion criteria were being pregnant or nursing or having dementia, a terminal illness, or an active alcohol or drug dependence diagnosis.
Procedures
Medicaid beneficiaries were recruited from 2012 through 2015 via flyers, clinician referral, and direct mail. After providing informed consent, they received Web-based motivational tobacco education. Interested and eligible participants could then enroll in the treatment study after providing a second written informed consent.
We used equipoise randomization (21,22) that allowed participants to opt out of one of the cessation treatment conditions or allowed randomization to any of the three options. This strategy is recommended for comparative effectiveness trials that include more than two treatments (23). Randomization strata were defined by conditions to which the participant was willing to be randomly assigned. Within the stratum, a participant was then randomly assigned with equal probability to the selected treatment condition options. Computer-generated tables for each strata within each site were used for random assignment. In addition, participants were randomly assigned to receive incentives for biologically verified abstinence or no incentives. After randomization, participants were encouraged to initiate their assigned interventions, but interventions could be accessed for the one-year study period.
Participants were assessed at baseline and at three, six, nine, and 12 months for abstinence, symptoms and adverse events by trained program coordinators. Intervention participation was assessed via treatment and administrator record verification as described below. Participants received a $15 payment for each research assessment. The Committees for the Protection of Human Subjects at Dartmouth College and the New Hampshire Department of Health and Human Services reviewed and approved all study materials and monitored the study.
Interventions
Program coordinators.
Full-time, trained bachelor’s-level staff facilitated linkage to study interventions and delivered all incentives following manualized procedures, intervention manuals, and participant handouts.
Motivational tobacco education.
The “Let’s Talk About Smoking” program is a Web-based motivational enhancement tool tailored for smokers with severe mental illnesses (24,25). The program includes a guided self-assessment of the pros and cons of smoking and interactive education provided in video, text, and audio, with a linear format that encourages completion. Participants who completed this program received $50 and could enroll in the cessation intervention study if they met the aforementioned eligibility criteria.
Usual care prescriber visit for smoking cessation.
All conditions included a visit with participants’ existing CMHC psychiatrist or nurse practitioner to discuss cessation medications and NRT and to obtain a prescription if they decided to use pharmacotherapy (PV). CMHC prescribers were trained with a yearly 45-minute session of group academic detailing (19) regarding safety, efficacy, and techniques for providing brief tobacco cessation counseling (20) with evidence-based pharmacotherapy (4,5) tailored to smokers with mental illnesses. NRT (single product) and cessation medications (varenicline and bupropion) were covered by Medicaid. Upon completion of this visit, prescribers provided written verification.
Cessation condition 1: usual care PV only (PV only).
Participants met with their usual community mental health prescriber as described above and received a participation reward of $30.
Cessation condition 2: PV plus facilitated quitline counseling (PV+Q).
Participants met with their prescriber as described above, for which $15 was provided, and received a supported referral to the New Hampshire Tobacco Helpline, which provides an average of three manualized telephone counseling sessions to help smokers quit and to support abstinence. Program coordinators explained how to use the helpline and facilitated an initial call. New Hampshire Tobacco Helpline counseling services were delivered per the usual protocol. Participants’ cellphone records or helpline staff verified participation, enabling rewards for up to three calls ($20 each).
Cessation condition 3: PV plus CBT (PV+CBT).
Participants met with their prescriber as described above for which a $15 participation reward was provided. Program coordinators explained how to use telephone counseling and forwarded a referral to the telephone CBT therapist, who initiated the first call. The CBT used in this study was a manualized adaptation of the 12-session Freedom From Smoking program for people with severe mental illnesses (2,26,27) provided by experienced tobacco treatment specialists. Participants received a $5 participation reward for each completed session, confirmed by counselors’ records. Training and certification for telephone CBT providers included a series of didactic meetings and role-playing of sessions. Weekly group supervision provided ongoing coaching on using the manual and handouts consistently.
Incentives for smoking abstinence.
Within each intervention, half of participants were randomly assigned to receive monetary incentives contingent upon abstinence during one four-week cessation attempt. Program coordinators explained how to use the abstinence incentive intervention. Participants agreed to come in to the clinic for abstinence confirmation after they initiated a quit attempt. Participants in the abstinence rewards conditions received $50 in cash for verified abstinence on Mondays, Wednesdays, and Fridays in the first two weeks of the quit attempt. The incentives were contingent on breath carbon monoxide ≤6 ppm on the first day and ≤4 ppm on subsequent days and urine cotinine sample <100 ng/mL in the second week for those not using NRT. Participants could return in the third and fourth weeks for additional incentives (that is, $75 for verified abstinence with the same criteria). Participants could earn up to $450 during the four weeks after quitting.
Measures
Trained research staff assessed participants at baseline with structured interviews to obtain demographic and questionnaire information. Physician-completed DSM-IV-TR current diagnoses of mental illnesses were obtained from CHMC record review.
Abstinence.
The primary research outcome was self-reported seven-day abstinence biologically confirmed with expired breath carbon monoxide ≤4 ppm and urine cotinine <100 ng/mL (28,29) (or solely breath carbon monoxide if a participant was using NRT). We used the Smokerlyzer breath monitor to measure carbon monoxide level and the Accutest NicAlert test strip to measure urine cotinine (a metabolite of nicotine). For the longitudinal analyses, missed assessments of smoking status were imputed as smoking.
Other smoking-related measures.
Research staff also obtained smoking history at baseline and self-reported smoking and other tobacco product use over the past three months with the timeline follow-back method (30). Dependence was assessed with the Fagerström Test for Nicotine Dependence (31,32).
Mental and general medical health measures.
Psychiatric symptom distress was assessed with the modified Colorado Symptom Index (33), a 14-item questionnaire (0–4 scale). General medical comorbidity was assessed with the self-reported Katz Comorbidity Scale (34). We also measured height, weight, and blood pressure.
Participant Flow
A total of 1,468 participants used the Web-based motivational education program at their CMHC; 661 were eligible and wanted to enroll in the treatment study. Among those enrolled, 90% were assessed at least once; however, all were included in the longitudinal analyses, with missing abstinence assessments imputed as smoking. Just over a third (35%, N=231) of participants discontinued the study. [A figure in an online supplement to this article shows participant flow and reasons for discontinuation.]
Statistical Analyses
Descriptive analyses.
Participants in the three treatment conditions were compared with respect to baseline measures. Continuous variables were compared with analysis of variance and categorical variables with chi-square tests.
Stratum effect.
With an equipoise-stratified design, random assignment to available treatments within a stratum should produce balance between programs within a stratum, but overall, there may be an imbalance between arms in sample size or composition of participants from each stratum. In this case, if there is a large stratum effect on the outcome, there is a possibility of confounding the treatment and stratum effect. Therefore, we tested for a stratum effect on the abstinence outcome. Although we did not find a significant effect, we adjusted for stratum in all analyses.
Outcome analyses.
To compare programs with respect to abstinence over time, we fit logistic generalized linear models estimated with generalized estimating equations, which account for the nonindependence of repeated observations within individuals. We performed these analyses by imputing missing observations as smoking. For the comparison of programs, we included a program main effect, along with a time effect and an interaction between program and time. Significance of the program × time interaction would indicate a significant difference between programs in the change in likelihood of biologically confirmed abstinence over time. Because we did not expect a linear change in abstinence over time, we included time point as a categorical predictor to allow for nonlinear effects over time. This allowed for group comparisons at each follow-up time point. To test whether monetary incentives improved the likelihood of abstinence across all program arms and whether they differentially improved likelihood of abstinence in the program arms, we tested, respectively, both a main effect of incentives and a program × incentives effect. All analyses adjusted for gender, psychiatric diagnostic group, severity of nicotine dependence, and equipoise stratum.
Safety analyses.
We used chi-square tests and Fisher’s exact tests to evaluate serious adverse events between groups. We modeled psychiatric symptom severity over time via mixed-effects models. Analyses were conducted with SPSS, version 22.0.2.
Results
Study Participants
Participant demographic and clinical characteristics are presented in Table 1.
| Total sample (N=661) | PV only (N=146) | PV+Q (N=303) | PV+CBT (N=212) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % |
| Age (M±SD)b | 45.0±10.8 | 43.0±10.8 | 45.0±10.7 | 46±11.0 | ||||
| Female | 426 | 64 | 82 | 56 | 200 | 66 | 144 | 67 |
| White | 610 | 93 | 131 | 91 | 821 | 93 | 198 | 93 |
| High school graduate | 549 | 83 | 116 | 80 | 247 | 81 | 186 | 87 |
| Not employed | 545 | 82 | 113 | 77 | 258 | 85 | 174 | 81 |
| Diagnosis | ||||||||
| Schizophrenia spectrum disorder | 148 | 22 | 31 | 21 | 74 | 24 | 43 | 20 |
| Bipolar disorder | 150 | 23 | 29 | 20 | 68 | 22 | 56 | 26 |
| Major depression | 158 | 24 | 36 | 25 | 69 | 23 | 53 | 25 |
| Anxiety and other disorders | 205 | 31 | 50 | 34 | 94 | 31 | 62 | 29 |
| Modified Colorado Symptom Index score (M±SD)c | 49.1±10.9 | 49.5±11.9 | 49.0±10.8 | 48.0±10.2 | ||||
| Lifetime psychiatric hospitalizations (M±SD) | 8.3±26.9 | 6.0±15.4 | 9.0±34.7 | 9.0±19.0 | ||||
| Blood pressure category | ||||||||
| Prehypertension | 303 | 46 | 61 | 42 | 147 | 49 | 95 | 45 |
| Hypertension | 170 | 26 | 45 | 31 | 73 | 24 | 52 | 25 |
| Obese | 371 | 56 | 90 | 62 | 160 | 53 | 121 | 57 |
| Tobacco use | ||||||||
| Breath carbon monoxide (M±SD ppm)d | 25.3±16.9 | 25.0±17.3 | 27.0±16.8 | 24.0±16.8 | ||||
| Fagerström Test for Nicotine Dependence score (M±SD)e | 5.3±2.3 | 5.0±2.4 | 5.0±2.1 | 6.0±2.4 | ||||
| Cigarettes smoked per day (M±SD)b | 17.3±10.5 | 16.0±10.9 | 18.0±10.2 | 17.0±10.5 | ||||
| Quit attempt in past year | 347 | 52 | 69 | 47 | 155 | 51 | 123 | 58 |
| Confidence in quitting score (M±SD)f | 3.7±1.2 | 4.0±1.2 | 4.0±1.2 | 4.0±1.2 | ||||
TABLE 1. Baseline characteristics of 661 smokers with severe mental illnesses who were New Hampshire Medicaid beneficiaries (2012–2015), by smoking cessation interventiona
Treatment Outcomes
The effect of intervention condition on biologically confirmed abstinence did not differ significantly among the interventions across the time points (intervention × time interaction). Those assigned to receive abstinence-contingent monetary incentives were significantly more likely to be abstinent over time (adjusted odds ratio [AOR]=1.77, 95% confidence interval [CI]=1.15–2.72, p=.009) (Figure 1). However, this effect did not differ significantly by cessation intervention condition. Post hoc comparisons of intervention condition by incentive group at each time point indicated greater abstinence at 12 months among participants receiving PV+Q with incentives compared with PV+Q without incentives (14% versus 4%; AOR=3.94, CI=1.32–11.75, p=.014) (Figure 2). Gender, severity of nicotine dependence, and diagnostic group were not significantly related to abstinence.
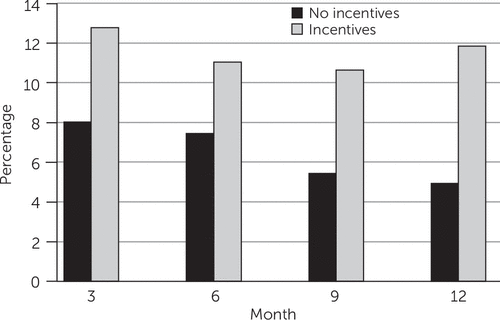
FIGURE 1. Proportion of smokers with mental illness who were abstinent at each assessment time point, by whether they received abstinence-contingent monetary incentivesa
aMissing observations were imputed as smoking. Participants who received incentives were more likely to be verified as abstinent over time (adjusted odds ratio=1.77, 95% confidence interval=1.15–2.72, p=.009).
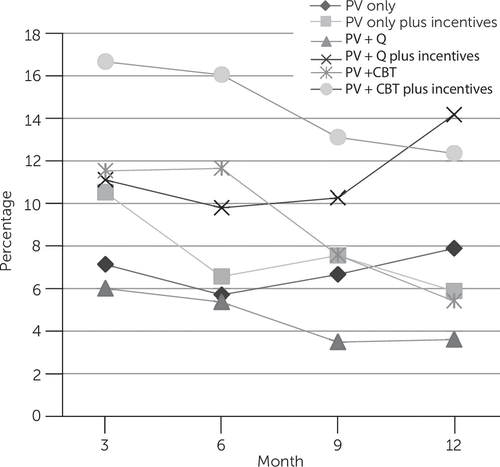
FIGURE 2. Proportion of smokers with mental illness in each smoking cessation intervention who were abstinent at each assessment time pointa
aMissing observations were imputed as smoking. PV only, prescriber visit for pharmacotherapy only; PV+Q, prescriber visit and facilitated quitline; PV+CBT, PV and cognitive-behavioral therapy. Main effect of incentives did not differ significantly by intervention. Post hoc comparisons indicated greater abstinence at 12 months in PV+Q with incentives than in PV+Q without incentives (14% versus 4% abstinent, adjusted odds ratio [OR]=3.94, 95% confidence interval=1.32–11.75, p=.014).
Treatment Safety
Mean psychiatric symptom severity did not change over time and was not significantly different between treatment groups over time. Twenty-five participants (4%) experienced a serious adverse event: 16 were hospitalized for psychiatric exacerbations, seven were hospitalized for medical reasons (pneumonia, lung cancer, and heart attack), and five study participants died. One person who was hospitalized for psychiatric exacerbation was abstinent, the rest were smoking when the serious adverse event occurred. None of these events were deemed related to study interventions or activities. The proportion of people with serious adverse events did not differ by cessation treatment condition or incentive status.
Treatment Program Participation
Treatment program participation was high, as described below. Among those who participated (N=562), over three-quarters (N=474, 84%) initiated their assigned treatment within three months of enrollment, with no significant difference between treatment groups and diagnostic groups.
All participants were advised to meet with their medical provider to discuss cessation pharmacotherapy (PV): 87% of participants (N=575) completed this visit. Those assigned to PV+CBT with incentives had the highest participation, and those assigned to PV only had the lowest (94%, N=102, versus 79%, N=55; χ2=11.6, df=5, p=.04). Participation in PV did not differ by age, gender, assignment to abstinence incentives, and diagnostic group.
Regarding pharmacological treatments, 60% of participants who had follow-up assessments (N=354 of 590) indicated that they used NRT during the study period, 18% (N=105) used bupropion, and 16% (N=92) used varenicline. Use of these treatments did not differ significantly between assigned conditions or between diagnostic groups.
Regarding behavioral cessation treatments, 82% (N=247) of those assigned to PV+Q made at least one call (mean±SD=2.00±1.24 calls), and 90% (N=191) of those assigned to PV+CBT participated in at least one CBT session (mean=9.00±4.37 sessions). Initiation of quitline calls, initiation of telephone CBT, and number of calls and sessions did not differ significantly based on assignment to abstinence incentives or by diagnostic group.
Regarding abstinence incentives, 49% (N=37) of participants in PV only, 46% (N=70) of those in PV+Q, and 61% (N=66) of those in PV+CBT received at least one abstinence incentive (for example, $50 for verified abstinence) during the four-week period after a quit attempt (χ2=6.26, df=2, p=.04). Smokers in the PV+CBT with incentives group were more likely than those in the other groups to initiate a quit attempt in the first six months (40%, N=30, in PV only with incentives; 39%, N=60, in PV+Q with incentives; and 57%, N=61, in PV+CBT with incentives (χ2=8.76, df=2, p=.013).
Thus, a greater proportion of participants in the PV only with incentives group (N=7 of 37, 19%) and the PV+Q with incentives group (N=10 of 70, 14%) had delayed quit attempts compared with the PV+CBT with incentives group (N=5 of 66, 8%).
Discussion
Monetary abstinence incentives delivered with cessation treatment increased biologically verified smoking abstinence over one year among Medicaid beneficiaries with mental illness. Mental illness symptom stability was maintained, and treatment participation was high, indicating safety and acceptability among smokers and a feasible implementation model. Our results are consistent with previous research demonstrating the efficacy of abstinence-contingent incentives for low-income smokers (14,15,17,18). Contrary to our hypothesis, this study did not find a difference between intervention conditions (PV, PV+Q, and PV+CBT). Conservative rates of biologically confirmed six-month abstinence (with missing observations assumed to be smoking) ranged from about 5% to 12%. The study was not powered to detect the magnitude of differences seen here.
In comparison, recent studies of telephone CBT with NRT initiated during psychiatric hospitalization reported 7.7%−15.8% six-month abstinence (verified by breath carbon monoxide only) among smokers with a variety of diagnoses similar to those in this study (35,36). Other previous studies of combined pharmacotherapy with six to 14 sessions of behavioral interventions for people with severe mental illnesses have reported verified abstinence for 7% to 43% (37–42). Studies of varenicline and high-intensity behavioral interventions focused on medication adherence tended to report higher rates of abstinence. Tailored, computerized interventions delivered over six months have also been successful (43). The rates of abstinence in this study may be lower because our definition of confirmed abstinence was more rigorous than in many other studies and because the interventions we studied did not include vigorous tracking and reinforcement of pharmacotherapy use.
Although lower-intensity behavioral interventions and shorter duration of pharmacotherapy are typically associated with lower rates of cessation, the scalable interventions used in this study have potential for very large impact if delivered broadly. Approximately 4.9 million smokers with severe mental illnesses may interface with U.S. CMHCs. If all were provided treatment with incentives over the next ten years and 12% quit, as did the smokers in this study, these 588,000 quitters would dramatically reduce disease burden in this group.
Post hoc analyses found a specific effect for monetary abstinence incentives at 12 months in the PV+Q group, whereas no significantly different intervention effects for added incentives were noted at the earlier assessment points. A greater proportion of participants in the PV only and PV+Q groups had delayed quit attempts (19% and 14% compared with 8% in the PV+CBT with incentives group), which may explain why the PV+Q group did better at 12 months. Given the flexibility, broad availability, and brevity of quitline services, these findings indicate that a combination of pharmacotherapy, quitline counseling, and incentives warrants further study to replicate these results.
The study had some limitations. The abstinence incentives were delivered for one month; a longer duration of incentives may be more effective. Small incentives for participation may have increased participation, but the study was not designed to test their efficacy. Additionally, a substantial minority of participants missed research assessments. This population can have difficulty attending research appointments because of psychiatric instability, disorganization, or lack of transportation. In the analyses, missed smoking assessments were counted as smoking; thus these results are conservative and probably underestimate true rates of abstinence. Finally, the study was not powered to detect small differences between groups that may be important. Nevertheless, the study’s large sample, minimal exclusion criteria, and use of equipoise randomization enhance the generalizability of our findings.
Conclusions
This research indicates the promise of combined cotinine- and breath carbon monoxide–based, abstinence-contingent incentives to assist low-income Medicaid beneficiaries with mental illness in quitting smoking. Further research is needed to establish the optimal amount, timing, and duration of incentive-based smoking cessation treatment, as well as the optimal type of behavioral treatment to use in combination with incentives for this population.
1 : Smoking and mental illness in the US population. Tobacco Control 23(e2):e147–e153, 2014Crossref, Medline, Google Scholar
2 : A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. Journal of Clinical Psychopharmacology 25:218–225, 2005Crossref, Medline, Google Scholar
3 : A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry 52:53–61, 2002Crossref, Medline, Google Scholar
4 : Smoking cessation and reduction in people with chronic mental illness. BMJ 351:h4065, 2015Crossref, Medline, Google Scholar
5 : Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: a systematic review and network meta-analysis. Addiction 111:599–612, 2016Crossref, Medline, Google Scholar
6 : Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 8:CD002850, 2013Google Scholar
7 : Smokers with self-reported mental health conditions: a case for screening in the context of tobacco cessation services. PLoS One 11:e0159127, 2016Crossref, Medline, Google Scholar
8 : Smoking cessation quitlines: an underrecognized intervention success story. American Psychologist 65:252–261, 2010Crossref, Medline, Google Scholar
9 : Smoking reduction for persons with mental illnesses: 6-month results from community-based interventions. Community Mental Health Journal 47:694–702, 2011Crossref, Medline, Google Scholar
10 : Contingency Management in Substance Abuse Treatment New York. NY, Guilford, 2008Google Scholar
11 : Contingency management: incentives for sobriety. Alcohol Research and Health 23:122–127, 1999Google Scholar
12 : Economic concepts for the analysis of behavior. Journal of the Experimental Analysis of Behavior 34:219–238, 1980Crossref, Medline, Google Scholar
13 : The use of financial incentives in promoting smoking cessation. Preventive Medicine 55(suppl):S24–S32, 2012Crossref, Medline, Google Scholar
14 : Financial incentives to promote extended smoking abstinence in opioid-maintained patients: a randomized trial. Addiction 111:903–912, 2016Crossref, Medline, Google Scholar
15 : A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiology, Biomarkers and Prevention 15:12–18, 2006Crossref, Medline, Google Scholar
16 : Effects of Large Financial Incentives for Long-Term Smoking Cessation: A Randomized Trial. Journal of the American College of Cardiology 68:777–785, 2016Crossref, Medline, Google Scholar
17 : Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. American Journal of Public Health 105:1198–1205, 2015Crossref, Medline, Google Scholar
18 : A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine 360:699–709, 2009Crossref, Medline, Google Scholar
19 : Expanding cessation pharmacotherapy via videoconference educational outreach to prescribers. Nicotine and Tobacco Research 17:960–967, 2015Crossref, Medline, Google Scholar
20 : Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine 163:622–634, 2015Crossref, Medline, Google Scholar
21 : Strengthening clinical effectiveness trials: equipoise-stratified randomization. Biological Psychiatry 50:792–801, 2001Crossref, Medline, Google Scholar
22 : Innovative clinical trial designs: toward a 21st-century health care system. Statistics in Biosciences 3:145–168, 2011Crossref, Medline, Google Scholar
23 : What have we learned about trial design from NIMH-funded pragmatic trials? Neuropsychopharmacology 35:2491–2501, 2010Crossref, Medline, Google Scholar
24 : An electronic decision support system to motivate people with severe mental illnesses to quit smoking. Psychiatric Services 62:360–366, 2011Link, Google Scholar
25 : Carbon monoxide feedback in a motivational decision support system for nicotine dependence among smokers with severe mental illnesses. Journal of Substance Abuse Treatment 45:319–324, 2013Crossref, Medline, Google Scholar
26 Join Freedom From Smoking. Chicago, American Lung Association, http://www.lung.org/stop-smoking/how-to-quit/freedom-from-smoking. Accessed July 1, 2017Google Scholar
27 : Smoking cessation treatment for patients with schizophrenia. American Journal of Psychiatry 155:974–976, 1998Link, Google Scholar
28 : Expired air carbon monoxide: a simple breath test of tobacco smoke intake. British Medical Journal 281:484–485, 1980Crossref, Medline, Google Scholar
29 : Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research 4:149–159, 2002Crossref, Medline, Google Scholar
30 : Reliability and validity of smoking timeline follow-back interview. Psychology of Addictive Behaviors 12:101–112, 1998Crossref, Google Scholar
31 : Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors 3:235–241, 1978Crossref, Medline, Google Scholar
32 : Reliability of the Fagerström Test for Nicotine Dependence, Minnesota Nicotine Withdrawal Scale, and Tiffany Questionnaire for Smoking Urges in smokers with and without schizophrenia. Drug and Alcohol Dependence 86:278–282, 2007Crossref, Medline, Google Scholar
33 : Client outcomes II: longitudinal client data from the Colorado treatment outcome study. Milbank Quarterly 72:123–148, 1994Crossref, Medline, Google Scholar
34 : Can comorbidity be measured by questionnaire rather than medical record review? Medical Care 34:73–84, 1996Crossref, Medline, Google Scholar
35 : Efficacy of a universal smoking cessation intervention initiated in inpatient psychiatry and continued post-discharge: a randomised controlled trial. Australian and New Zealand Journal of Psychiatry 51:366–381, 2017Crossref, Medline, Google Scholar
36 : Impact of a postdischarge smoking cessation intervention for smokers admitted to an inpatient psychiatric facility: a randomized controlled trial. Nicotine and Tobacco Research 16:1417–1428, 2014Crossref, Medline, Google Scholar
37 : Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 311:145–154, 2014Crossref, Medline, Google Scholar
38 : Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA 304:2485–2493, 2010Crossref, Medline, Google Scholar
39 : A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 73:654–660, 2012Crossref, Medline, Google Scholar
40 : A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. American Journal of Psychiatry 163:1934–1942, 2006Link, Google Scholar
41 : Mood management and nicotine patch for smoking cessation in adults with bipolar disorder. Nicotine and Tobacco Research 15:1805–1806, 2013Crossref, Medline, Google Scholar
42 : Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. American Journal of Public Health 96:1808–1814, 2006Crossref, Medline, Google Scholar
43 : Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: a randomized controlled trial. American Journal of Public Health 104:1557–1565, 2014Crossref, Medline, Google Scholar



