Intellectual Disability and Psychotic Disorders in Children: Association With Maternal Severe Mental Illness and Exposure to Obstetric Complications in a Whole-Population Cohort
Abstract
Objective:
Children of mothers with severe mental illness are at significantly increased risk of developing intellectual disability. Obstetric complications are also implicated in the risk for intellectual disability. Moreover, children of mothers with severe mental illness are more likely to be exposed to obstetric complications. The purpose of this study was to examine the independent and joint contributions of familial severe mental illness and obstetric complications to the risk of intellectual disability.
Method:
Record linkage across Western Australian whole-population psychiatric, inpatient, birth, and midwives’ registers identified 15,351 children born between 1980 and 2001 to mothers with severe mental illness and 449,229 children born to mothers with no mental illness. Multivariable models were adjusted for paternal psychiatric status, parental intellectual disability, and other family and sociodemographic covariates.
Results:
The risk of intellectual disability was increased among children of mothers with severe mental illness compared with children of unaffected mothers. The impact varied across maternal diagnostic groups. For children of mothers with schizophrenia, the unadjusted odds ratio was 3.8 (95% CI=3.0, 4.9) and remained significant after simultaneous adjustment for exposure to obstetric complications and other covariates (odds ratio=1.7, 95% CI=1.3, 2.3). The odds ratio for exposure to obstetric complications also remained significant after adjustment (odds ratio=1.7, 95% CI=1.6, 1.8). For intellectual disability of a genetic basis, the adjusted odds ratio for maternal schizophrenia was elevated but not statistically significant. Among children with intellectual disability, 4.2% later developed a psychotic disorder, compared with 1.1% of children without intellectual disability.
Conclusions:
Maternal severe mental illness and exposure to obstetric complications contribute separately to the risk of intellectual disability, suggesting potentially different causal pathways.
There is a growing literature on the overlap between schizophrenia and intellectual disability as well as other neuropsychiatric disorders, such as autism and epilepsy, with evidence of shared genetic contributions and common phenotypic features (1–4). Observations of a link between schizophrenia and intellectual disability are not novel. Kraepelin estimated that intellectual disability was the etiology for 3.5% of cases of early-onset dementia praecox, which he called Pfropfschizophrenie (or engrafted schizophrenia) (5). In 1938, Penrose reported documented evidence of familial overlap (6). Concurrently, work by Rosanoff and colleagues underscored the role of early cerebral trauma in the etiology of schizophrenia, intellectual disability, and epilepsy (7, 8). By the 1950s, Barbara Fish was describing neurological and neurodevelopmental abnormalities in young children of mothers with schizophrenia, which she referred to as “pandysmaturation” (9). By using whole-population record-linked data from Western Australia, we previously estimated that 4.9% of people with schizophrenia have intellectual disability (10). A number of studies have also shown that the risk of intellectual disability is increased among children of parents with schizophrenia (11–13).
The mechanisms underlying this elevation remain unclear. Findings may reflect a shared genetic basis for both schizophrenia and intellectual disability. Alternatively, they may point toward an increased liability for the offspring of parents with schizophrenia to be exposed to environmental risks for intellectual disability. In this context, obstetric complications could play a critical role: not only are obstetric complications implicated as risk factors for idiopathic intellectual disability (14–17), but women with schizophrenia are at increased risk of obstetric trauma during delivery compared with unaffected women (18–20).
In previous work, we demonstrated that children of mothers with schizophrenia, bipolar disorder, or unipolar major depression were up to three times more likely to have intellectual disability compared with children of mothers with no known psychiatric history and that both maternal severe mental illness and obstetric complications acted separately on risk of intellectual disability among these offspring (13). Similarly, prenatal environmental risk factors and parental psychopathology reportedly act independently on risk for autism (21). However, our previous study was based on a small sample of 6,303 children. To our knowledge, there are no whole-population epidemiological studies investigating whether the association between maternal schizophrenia and intellectual disability in offspring is influenced by exposure to obstetric complications. In the present study, we used a larger, whole-population cohort of 464,580 live-born offspring to examine such associations.
Our aims in this study were to investigate the following:
Whether children born to mothers with a severe mental illness (familial heightened risk) are more likely to have an intellectual disability compared with children born to mothers with no known psychiatric history (generic risk);
Whether any increased risk of intellectual disability among offspring with heightened risk varies according to the mothers’ specific psychiatric diagnosis (schizophrenia, bipolar disorder, unipolar major depression, or other psychotic illness); and
Whether exposure to obstetric complications modifies the risk of intellectual disability predicted by maternal severe mental illness.
We analyzed separately two outcomes: any intellectual disability and intellectual disability of a genetic basis. Additionally, we investigated onset of psychotic disorders in offspring up to ages 9.5–31.5, depending on year of birth, to determine the degree of overlap between intellectual disability and psychotic disorder in the offspring.
Method
Study Population
In this register-based study, we investigated a whole-population cohort of 464,580 children born in Western Australia between January 1, 1980, and December 31, 2001. Children with familial heightened risk (of later psychosis, in adulthood) were those born to mothers diagnosed with severe mental illness. Children with generic risk were those born to mothers with no recorded history of a psychiatric disorder. Children were identified through state-mandated midwives’ records. Linkage was carried out by the Data Linkage Branch of the Western Australian Department of Health. Full details on the linked registers used to establish and characterize the study cohort have been published elsewhere (22).
Outcome Variable: Intellectual Disability
The intellectual disability status of a child was determined with the Intellectual Disability Exploring Answers database (23), which includes all Western Australians with intellectual disability registered with the Disability Services Commission since 1953 and children with intellectual disability identified through the Western Australian Department of Education from 1983 onward. Children were identified as having intellectual disability if they met the criteria set by the American Association on Intellectual and Developmental Disabilities, including a full-scale IQ score ≥2 standard deviations below the population mean in combination with limitations in adaptive behaviors and skills. A small number of children with a borderline IQ were included if their limitations in adaptive behaviors and skills were sufficiently severe (23). For children identified solely via the Department of Education, only the IQ criterion was applied. Additional children were identified through hospital admission records from all hospitals in Western Australia for the period from January 1, 1980, to June 30, 2010, and from mental health ambulatory and outpatient records and community mental health records for the same period (ICD-9 codes 317, 318.8, 318.2, 318.3, or 319; ICD-10 codes F70–F73 or F79 [24, 25]). Finally, several children were identified through the Birth Defects Registry of Western Australia (26), which records all malformations, including syndromes causing intellectual disability, diagnosed in children up to age 6. Malformations were classified by using the British Paediatric Association Classification of Diseases (codes included were 75880–75885 and 75888). For children registered with the Disability Services Commission, Heber diagnoses pertaining to the basis of the intellectual disability were available (27). We classified intellectual disabilities of genetic bases (chromosomal, autosomal, and X-linked) according to the categories developed by Yeargin-Allsopp et al. (28), which indicate the etiological basis of biomedical origin of the intellectual disability, and which we previously applied to the Heber classification system (10). For further details, see the online supplement.
Psychotic Disorders in Children
Psychiatric records for children were available up to June 30, 2011. Children were classified as having a psychotic disorder if they had any inpatient, outpatient, or community mental health record with a diagnosis of ICD-9 codes 295–298 or if they had an ICD-10 diagnosis mapping to these codes.
Exposure Variables
Maternal severe mental illness.
The primary exposure variable of maternal severe mental illness identified children with familial heightened risk. An algorithm based on the most recent diagnosis was applied to inpatient and outpatient and community mental health psychiatric records to determine maternal severe mental illness, namely, maternal schizophrenia, bipolar disorder, unipolar major depression, paranoid states, and other nonorganic psychoses (ICD-9 codes 295–298; ICD-8 and ICD-10 codes were mapped to ICD-9 codes.). We also examined schizophrenia separately (ICD-9 code 295) to assess the specificity of our findings for schizophrenia.
Obstetric complications.
Data were extracted from midwives’ records, which included infants’ gestational age and weight; complications during pregnancy, labor and delivery, or the early neonatal period; and maternal demographic characteristics. Obstetric complications were scored with the McNeil-Sjöström Scale for Obstetric Complications (29), adapted for automated scoring in Australian electronic databases. This clinically derived 6-point severity scale reflects the potential of each obstetric complication for a negative impact on the exposed offspring’s CNS during specific stages of development (during pregnancy, labor and delivery, or the neonatal period). The same obstetric complication can contribute to the scale during more than one period, with different weightings reflecting the severity of effect for that period. Children were defined as having been exposed to obstetric complications of critical severity if they had at least one exposure of “potentially clearly harmful or relevant” severity with a score ≥4. Binary scores were computed for each birth for each developmental period (pregnancy, labor and delivery, or neonatal) affected by harmful exposure as well as for the three periods combined.
Other covariates.
Additional variables thought to potentially influence the association between maternal severe mental illness, exposure to obstetric complications, and intellectual disability in children were included in adjusted models. Sociodemographic and socioeconomic factors were extracted from midwives’ records and linked birth registration records. An indicator of whether the father was registered on the birth record was included, because nonregistration (0.7% of the study sample) was more frequent among children with heightened risk. Socioeconomic status and geographical remoteness of the mother’s residence at the child’s birth were determined with measures from the Australian Bureau of Statistics (the Index of Relative Socio-Economic Disadvantage [30] and the Australian Standard Geographic Classification–Remoteness Area [31]). Indigenous status of the child was scored positive if the child or either parent was identified as an Aboriginal or a Torres Strait Islander. Competing etiological influences included the intellectual disability status of the parents and the father’s psychiatric morbidity. For children born to mothers with severe mental illness, a binary variable coded whether conception occurred before or after the diagnosis.
Data Analyses
We used multiple logistic regressions to examine associations between a child’s exposure to obstetric complications, maternal severe mental illness (as a proxy for familial heightened risk), and intellectual disability outcome. Estimates of odds ratios with 95% confidence intervals were computed.
Unadjusted analyses modeled associations between heightened familial risk classification and the child’s intellectual disability status and associations between the mothers’ specific diagnoses and the child’s intellectual disability status. These associations were then adjusted in model 1 for demographic and socioeconomic variables and exposure to obstetric complications and in model 2 with additional adjustment for potentially competing etiological factors. Parallel analyses were run for intellectual disability with a genetic basis.
Statistical interaction between maternal severe mental illness and exposure to obstetric complications was examined by including additional interaction terms in model 2. We used a likelihood ratio test for multiplicative interaction and relative excess risk due to interaction (32, 33) for additive interaction. Interaction terms allowed odds ratios for exposure to obstetric complications to be estimated separately for both children with familial heightened risk and children with generic risk for each period of exposure (pregnancy, labor and delivery, and neonatal). Robust standard errors were computed in all analyses to protect against erroneous deflation of standard errors due to clustering of maternal sibships. Missing data levels were low and not considered to introduce bias. Analyses were conducted with Stata, version 13 (34).
The study was approved by the Western Australian Department of Health Human Research Ethics Committee and the University of Western Australia Human Research Ethics Committee.
For further details on the study methods, see the online supplement.
Results
Sample Characteristics
Data linkage identified 15,351 children with heightened risk for developing a psychotic disorder who were born to 7,492 mothers with severe mental illness, including 1,653 children born to 887 mothers with schizophrenia. The 449,229 children born to 238,880 mothers with no psychiatric history comprised the generic risk group. The children’s demographic characteristics are summarized in Table 1. Compared with children in the generic risk group, children in the heightened-risk group were more likely to be classified as Indigenous, have younger parents, have parents born in Australia, have fathers who were unknown, have single mothers, have a mother residing in an area of greater disadvantage or outside a major city at the time of birth, have parents with intellectual disability, and have fathers with a history of psychiatric illness. Approximately one-fifth of the children in the heightened-risk group (20.6%) were conceived after their mother’s diagnosis of severe mental illness. The characteristics of the children with heightened risk whose mothers had a diagnosis other than schizophrenia are summarized in Table S1 in the online supplement.
| Children With Intellectual Disability | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cohort (N=464,580) | Maternal Severe Mental Illness (N=15,351) | Maternal Schizophrenia (N=1,653) | Any (N=6,217) | Genetic Basis (N=860) | ||||||
| Characteristic | N | % | N | % | N | % | N | % | N | % |
| Child with intellectual disability | 6,217 | 1.3 | 510 | 3.3 | 77 | 4.7 | ||||
| Child with intellectual disability and information on the cause | 3,179 | 262 | 43 | |||||||
| Child with intellectual disability of a genetic basis | 860 | 0.2 | 54 | 0.4 | 7 | 0.4 | ||||
| Exposure to obstetric complications of critical severity | ||||||||||
| During pregnancy | 125,542 | 27.0 | 5,239 | 34.1 | 541 | 32.7 | 2,380 | 38.3 | 406 | 47.2 |
| During labor and delivery | 219,934 | 47.3 | 7,551 | 49.2 | 777 | 47.0 | 3,311 | 53.3 | 439 | 51.0 |
| During the neonatal period | 200,665 | 43.2 | 7,720 | 50.3 | 865 | 52.3 | 3,768 | 60.6 | 636 | 74.0 |
| During pregnancy, labor and delivery, or the neonatal period | 327,452 | 70.5 | 11,552 | 75.3 | 1,232 | 74.5 | 5,013 | 80.6 | 749 | 87.1 |
| Sex | ||||||||||
| Male | 238,381 | 51.3 | 7,962 | 51.9 | 875 | 52.9 | 3,937 | 63.3 | 486 | 56.5 |
| Female | 226,199 | 48.7 | 7,389 | 48.1 | 778 | 47.1 | 2,280 | 36.7 | 374 | 43.5 |
| Maternal age (years) | ||||||||||
| ≤19 | 25,979 | 5.6 | 1,557 | 10.1 | 198 | 12.0 | 574 | 9.2 | 36 | 4.2 |
| 20–34 | 389,483 | 83.8 | 12,341 | 80.4 | 1,249 | 75.6 | 4,957 | 79.7 | 663 | 77.1 |
| ≥35 | 49,102 | 10.6 | 1,453 | 9.5 | 206 | 12.5 | 686 | 11.0 | 161 | 18.7 |
| Unknown | 16 | 0.6 | ||||||||
| Maternal place of birth | ||||||||||
| Outside Australia | 136,016 | 29.3 | 3,632 | 23.7 | 382 | 23.1 | 1,551 | 24.9 | 284 | 33.0 |
| Australia | 327,560 | 70.5 | 11,679 | 76.1 | 1,267 | 76.6 | 4,650 | 74.8 | 576 | 67.0 |
| Unknown | 1,004 | 0.2 | 40 | 0.3 | 4 | 0.2 | 16 | 0.3 | ||
| Father’s age (years) | ||||||||||
| ≤19 | 6,553 | 1.4 | 363 | 2.4 | 45 | 2.7 | 155 | 2.5 | 10 | 1.2 |
| 20–54 | 435,934 | 93.8 | 13,174 | 85.8 | 1,211 | 73.3 | 5,437 | 87.5 | 813 | 94.5 |
| ≥55 | 1,034 | 0.2 | 43 | 0.3 | 3 | 0.2 | 27 | 0.4 | 5 | 0.6 |
| Unknown | 17,795 | 3.8 | 1,577 | 10.3 | 356 | 21.5 | 532 | 8.6 | 29 | 3.4 |
| Father unknown | 3,264 | 0.7 | 194 | 1.3 | 38 | 2.3 | 66 | 1.1 | 3 | 0.3 |
| Father’s place of birth (if the father is known) | ||||||||||
| Outside Australia | 140,099 | 30.2 | 3,741 | 24.4 | 423 | 25.6 | 1,615 | 26.0 | 285 | 33.1 |
| Australia | 303,189 | 65.3 | 9,837 | 64.1 | 835 | 50.5 | 4,004 | 64.4 | 543 | 63.1 |
| Unknown | 18,028 | 3.9 | 1,579 | 10.3 | 357 | 21.6 | 532 | 8.6 | 29 | 3.4 |
| Birth cohort | ||||||||||
| 1980–1982 | 56,062 | 12.1 | 1,938 | 12.6 | 266 | 16.1 | 395 | 6.4 | 109 | 12.7 |
| 1983–1986 | 80,197 | 17.3 | 2,744 | 17.9 | 327 | 19.8 | 1,099 | 17.7 | 177 | 20.6 |
| 1987–1992 | 130,685 | 28.1 | 4,478 | 29.2 | 511 | 30.9 | 2,469 | 39.7 | 261 | 30.3 |
| 1993–1996 | 87,708 | 18.9 | 2,917 | 19.0 | 277 | 16.8 | 1,346 | 21.7 | 152 | 17.7 |
| 1997–2001 | 109,928 | 23.7 | 3,274 | 21.3 | 272 | 16.5 | 908 | 14.6 | 161 | 18.7 |
| Birth order | ||||||||||
| 1 | 180,960 | 39.0 | 5,449 | 35.5 | 628 | 38.0 | 2,241 | 36.0 | 297 | 34.5 |
| 2 | 156,878 | 33.8 | 4,795 | 31.2 | 496 | 30.0 | 1,853 | 29.8 | 256 | 29.8 |
| 3 | 80,423 | 17.3 | 2,842 | 18.5 | 281 | 17.0 | 1,151 | 18.5 | 166 | 19.3 |
| ≥4 | 46,319 | 10.0 | 2,265 | 14.8 | 248 | 15.0 | 972 | 15.6 | 141 | 16.4 |
| Mother’s marital status at time of child’s birth | ||||||||||
| Married or de facto marriage | 422,622 | 91.0 | 12,321 | 80.3 | 1,110 | 67.2 | 5,190 | 83.5 | 787 | 91.5 |
| Single or other | 41,697 | 9.0 | 3,006 | 19.6 | 539 | 32.6 | 1,023 | 16.5 | 73 | 8.5 |
| Unknown | 261 | 0.1 | 24 | 0.2 | 4 | 0.2 | 4 | 0.1 | ||
| Socioeconomic index of mother’s residence at time of child’s birth | ||||||||||
| Lowest quintile (most disadvantaged) | 90,433 | 19.5 | 4,322 | 28.2 | 581 | 35.1 | 1,854 | 29.8 | 161 | 18.7 |
| Second quintile | 102,855 | 22.1 | 3,801 | 24.8 | 399 | 24.1 | 1,479 | 23.8 | 202 | 23.5 |
| Third quintile | 90,257 | 19.4 | 2,869 | 18.7 | 274 | 16.6 | 1,139 | 18.3 | 167 | 19.4 |
| Fourth quintile | 83,589 | 18.0 | 2,315 | 15.1 | 208 | 12.6 | 884 | 14.2 | 145 | 16.9 |
| Highest quintile (least disadvantaged) | 94,502 | 20.3 | 1,968 | 12.8 | 184 | 11.1 | 823 | 13.2 | 179 | 20.8 |
| Missing data | 2,944 | 0.6 | 76 | 0.5 | 7 | 0.4 | 38 | 0.6 | 6 | 0.7 |
| Remoteness of mother’s residence at time of child’s birth | ||||||||||
| Major city | 308,520 | 66.4 | 8,991 | 58.6 | 1,086 | 65.7 | 4,087 | 65.7 | 588 | 68.4 |
| Inner regional | 45,047 | 9.7 | 1,625 | 10.6 | 122 | 7.4 | 590 | 9.5 | 85 | 9.9 |
| Outer regional | 56,136 | 12.1 | 2,564 | 16.7 | 222 | 13.4 | 772 | 12.4 | 104 | 12.1 |
| Remote | 35,842 | 7.7 | 1,466 | 9.5 | 140 | 8.5 | 495 | 8.0 | 61 | 7.1 |
| Very remote | 18,853 | 4.1 | 704 | 4.6 | 82 | 5.0 | 272 | 4.4 | 22 | 2.6 |
| Missing data | 182 | 0.0 | 1 | 0.0 | 1 | 0.0 | ||||
| Indigenous status: of Aboriginal or Torres Strait Islander descent | 30,551 | 6.6 | 2,056 | 13.4 | 354 | 21.4 | 871 | 14.0 | 42 | 4.9 |
| Mother with an intellectual disability | 712 | 0.2 | 273 | 1.8 | 97 | 5.9 | 153 | 2.5 | 10 | 1.2 |
| Father with an intellectual disability | 532 | 0.1 | 46 | 0.3 | 7 | 0.4 | 99 | 1.6 | 7 | 0.8 |
| Father with any mental health contact other than for a psychotic disorder | 45,538 | 9.8 | 2,801 | 18.2 | 305 | 18.5 | 1,066 | 17.1 | 111 | 12.9 |
| Father with any diagnosis of a psychotic disorder | 7,779 | 1.7 | 769 | 5.0 | 103 | 6.2 | 187 | 3.0 | 19 | 2.2 |
| Child’s conception after the mother’s diagnosis of severe mental illness | 3,161 | 20.6 | 676 | 40.9 | 2,380 | 38.3 | 13 | 1.5 | ||
TABLE 1. Demographic and Clinical Characteristics of Children in the Study Cohort by Maternal Severe Mental Illness Category
Child Intellectual Disability by Maternal Severe Mental Illness
The total number of children with an intellectual disability was 6,217 (1.3%). The incidence proportion of intellectual disability was higher for children in the heightened-risk group (3.3%) compared with children in the generic risk group (1.3%), representing an odds ratio of 2.7 (95% CI=2.4, 3.0) (Table 2). After adjustment for demographic and socioeconomic effects and exposure to obstetric complications (model 1), the adjusted odds ratio was reduced but remained significantly elevated. After further adjustment for competing etiological influences in model 2, the odds ratio remained significant (odds ratio=1.7, 95% CI=1.5, 1.9). (For further details, see Table S2 in the online supplement.) There was no evidence of differing rates of intellectual disability attributable to a child’s conception occurring before or after the mother’s diagnosis: the odds ratio for children conceived after their mother’s diagnosis compared with those conceived before diagnosis, estimated as an additional term in model 2, was 0.86 (95% CI=0.66, 1.11). A sensitivity analysis of model 2, excluding the 3,161 children conceived after their mother’s diagnosis, produced no discernible differences in parameter estimates other than modest elevations in the odds ratios attributable to parental intellectual disability.
| Intellectual Disability and Maternal Severe Mental Illness | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Any intellectual disability | ||||||
| Child with heightened risk | ||||||
| Any maternal severe mental illness | 2.7 | 2.4, 3.0 | 2.0 | 1.8, 2.3 | 1.7 | 1.5, 1.9 |
| Schizophrenia | 3.8 | 3.0, 4.9 | 2.4 | 1.9, 3.2 | 1.7 | 1.3, 2.3 |
| Bipolar disorder | 2.0 | 1.6, 2.5 | 1.6 | 1.3, 2.1 | 1.3b | 1.0, 1.7 |
| Unipolar major depression | 2.6 | 2.3, 3.0 | 2.0 | 1.8, 2.4 | 1.9 | 1.6, 2.1 |
| Other psychoses | 3.4 | 2.5, 4.6 | 2.3 | 1.7, 3.1 | 1.8 | 1.3, 2.4 |
| Child with generic risk | Reference | Reference | Reference | |||
| Intellectual disability of genetic basis | ||||||
| Child with heightened risk | ||||||
| Any maternal severe mental illness | 2.0 | 1.5, 2.7 | 1.8 | 1.3, 2.4 | 1.6 | 1.2, 2.2 |
| Schizophrenia | 2.4 | 1.2, 5.1 | 2.1b | 1.0, 4.4 | 1.6 | 0.7, 3.6 |
| Bipolar disorder | 1.5 | 0.8, 2.9 | 1.3 | 0.7, 2.6 | 1.2 | 0.6, 2.3 |
| Unipolar major depression | 2.3 | 1.5, 3.1 | 2.1 | 1.4, 3.0 | 2.0 | 1.3, 2.9 |
| Other psychoses | 1.1 | 0.4, 3.4 | 0.9 | 0.3, 2.9 | 0.8 | 0.3, 2.5 |
| Child with generic risk | Reference | Reference | Reference | |||
TABLE 2. Child Intellectual Disability by Maternal Diagnosisa
Child Intellectual Disability by Maternal-Specific Diagnosis
Among children with heightened risk, children of mothers with schizophrenia had the highest unadjusted odds of intellectual disability (odds ratio=3.8, 95% CI=3.0, 4.9) (Table 2). Children of mothers with bipolar disorder, unipolar major depression, or other psychotic disorders also had significantly increased odds ratios. After adjustment for demographic and socioeconomic effects and obstetric complications during pregnancy, labor and delivery, or the neonatal period, odds ratios were reduced in model 1 but remained significant. When further adjusted for potentially competing etiological factors (parental intellectual disability and paternal psychiatric health) in model 2, odds ratios were further reduced but remained significant (schizophrenia: odds ratio=1.7, 95% CI=1.3, 2.3). There was no evidence of differing odds due to timing of the child’s conception relative to the mother’s onset of mental illness of any specific diagnosis.
Combined Effect of Exposure to Maternal Severe Mental Illness and Obstetric Complications
There was evidence of increased risk of intellectual disability associated with obstetric complications during each period of exposure (pregnancy, labor and delivery, and neonatal) as well as with any exposure during the three periods combined. These variables remained significant in model 2 (Table 3).
| Intellectual Disability and Exposure to Obstetric Complications | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Any intellectual disability | ||||||
| Obstetric complications | ||||||
| Pregnancy | 1.7 | 1.6, 1.8 | 1.4 | 1.3, 1.5 | 1.4 | 1.3, 1.5 |
| Labor and delivery | 1.3 | 1.2, 1.3 | 1.1 | 1.1, 1.2 | 1.1b | 1.0, 1.2 |
| Neonatal | 2.0 | 1.9, 2.2 | 1.7 | 1.6, 1.8 | 1.7 | 1.6, 1.8 |
| Any | 1.8 | 1.6, 1.9 | 1.7 | 1.6, 1.8 | 1.7 | 1.6, 1.8 |
| No exposure of critical severity | Reference | Reference | Reference | |||
| Intellectual disability of genetic basis | ||||||
| Obstetric complications | ||||||
| Pregnancy | 2.4 | 2.1, 2.8 | 1.9 | 1.6, 2.1 | 1.9 | 1.6, 2.1 |
| Labor and delivery | 1.2b | 1.0, 1.3 | 0.9 | 0.8, 1.0 | 0.9 | 0.8, 1.0 |
| Neonatal | 3.8 | 3.2, 4.4 | 3.4 | 2.9, 4.0 | 3.4 | 2.9, 3.9 |
| Any | 2.8 | 2.3, 3.5 | 2.9 | 2.3, 3.5 | 2.9 | 2.3, 3.5 |
| No exposure of critical severity | Reference | Reference | Reference | |||
TABLE 3. Child Intellectual Disability by Exposure to Obstetric Complicationsa
The introduction of interaction terms in model 2 allowed for the computation of odds ratios of no exposure to either maternal severe mental illness or obstetric complications (reference=1.0); exposure to maternal severe mental illness only; exposure to obstetric complications only; exposure to both maternal severe mental illness and obstetric complications; exposure to maternal schizophrenia only; and exposure to maternal schizophrenia and obstetric complications. These are displayed in Figure 1. For the combined effect of maternal severe mental illness and exposure to obstetric complications, the confidence intervals did not span the estimates of exposure to obstetric complications for children with generic risk. For children of mothers with schizophrenia who were exposed to obstetric complications during pregnancy or the neonatal period, the lower bounds of the confidence intervals were only slightly lower than the estimates of exposure to obstetric complications for children with generic risk. For further details, see the online supplement.
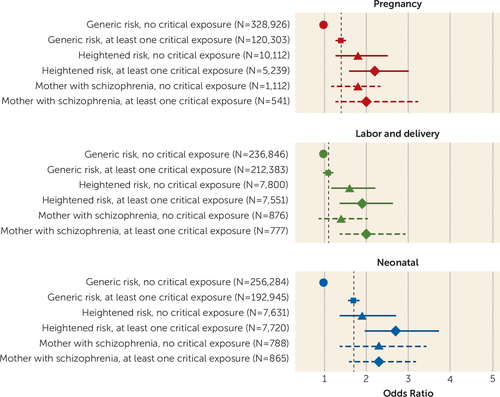
FIGURE 1. Intellectual Disability in Children by Maternal Severe Mental Illness and Exposure to Obstetric Complications of Critical Severitya
a The odds ratios for the periods listed were adjusted simultaneously for exposure to obstetric complications of critical severity in nonlisted periods (for example, the odds ratio for pregnancy was adjusted for exposure in labor and delivery and in the neonatal period; the odds ratio for labor and delivery was adjusted for exposure in pregnancy and in the neonatal period; the odds ratio for the neonatal period was adjusted for exposure in pregnancy and during labor and delivery) and mother’s psychiatric diagnosis. Demographic and socioeconomic covariates were sex, year of birth, parents’ ages, parents’ places of birth, birth order, mother’s marital status, area-level socioeconomic and remoteness measures of the mother’s residence, and Indigenous status. Model 2 adjusted further for potentially competing etiological factors, such as the intellectual disability status of both the mother and father and the father’s psychiatric morbidity status. Odds ratios were calculated from linear combination of relevant regression terms and their standard errors.
Intellectual Disability of Genetic Basis
We were able to determine the Heber diagnosis for 3,179 children with intellectual disability. Of these, 860 had a genetic basis for their disability. The odds ratio of intellectual disability of a genetic basis was 2.0 (95% CI=1.5, 2.7) for children with familial heightened risk compared with children with generic risk (Table 2). After adjustment in model 1, the odds ratio was reduced but remained significantly elevated. After further adjustment in model 2, the odds ratio continued to remain significant (odds ratio=1.6, 95% CI=1.2, 2.2). Across maternal diagnostic groups (schizophrenia, bipolar disorder, unipolar major depression, or other psychotic illness), only children of mothers with schizophrenia or unipolar major depression showed significantly elevated risk. After adjustment in model 2, only children of mothers with unipolar major depression remained at significantly elevated risk (odds ratio=2.0, 95% CI=1.3, 2.9). When we examined the combined effect of exposure to maternal severe mental illness and obstetric complications on intellectual disability with a genetic basis, we observed results that were similar to or stronger than those for any intellectual disability.
Psychotic Disorders in Children
A total of 4,972 children developed a psychotic disorder, ranging from 1.1% among children with generic risk without intellectual disability to 24.4% among children with intellectual disability whose mothers had schizophrenia (Table 4 and Figure 2).
| Outcome | Mother With Schizophrenia | Children With Generic Risk | ||||||
|---|---|---|---|---|---|---|---|---|
| Children With Intellectual Disability | Children Without Intellectual Disability | Children With Intellectual Disability | Children Without Intellectual Disability | |||||
| N | % | N | % | N | % | N | % | |
| Child with psychotic disorder | 10 | 24.4 | 86 | 6.5 | 159 | 4.0 | 4,717 | 1.1 |
| Child with no mental illness | 31 | 75.6 | 1,236 | 93.5 | 3,843 | 96.0 | 415,286 | 98.9 |
| Total | 41 | 100.0 | 1,322 | 100.0 | 4,002 | 100.0 | 420,003 | 100.0 |
TABLE 4. Children’s Psychotic Disorder Outcome by the Mother’s Schizophrenia Diagnosis and Child’s Intellectual Disability Status
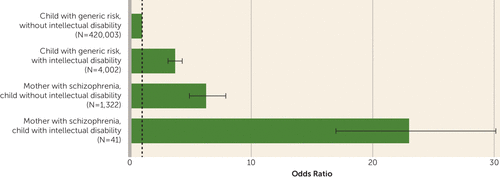
FIGURE 2. Children With Psychotic Disorders by Maternal Schizophrenia Diagnosis and the Child’s Intellectual Disability Statusa
a The reference group was the child with generic risk without intellectual disability group (odds ratio=1.0). The odds ratio for the child with generic risk with intellectual disability group is 3.7 (95% CI=3.1, 4.3). The odds ratio for the mother with schizophrenia, child without intellectual disability group is 6.2 (95% CI=4.9, 7.9). The odds ratio for the mother with schizophrenia, child with intellectual disability group is 23.0 (95% CI=17.3, 30.5). Error bars indicate 95% confidence intervals.
Discussion
For children of mothers with severe mental illness (familial heightened risk), we observed significantly elevated odds ratios of intellectual disability compared with children with generic risk (born to mothers with no history of psychiatric disorders). This result held true for any intellectual disability and intellectual disability of a genetic basis. For any intellectual disability, the odds ratios for all subgroups of children with heightened risk (those born to mothers with schizophrenia, bipolar disorder, unipolar major depression, and other psychotic disorders) were significantly increased compared with the odds ratios for children with generic risk. For intellectual disability with a genetic basis, the odds ratio remained significant only for those children whose mothers had unipolar major depression.
For any intellectual disability, we observed significantly elevated odds ratios attributable to exposure to obstetric complications of critical severity at each of three observation periods (pregnancy, labor and delivery, and neonatal). For each period, the effect sizes for exposure to obstetric complications were reduced but remained significant when adjusted for maternal severe mental illness. This remained true for any intellectual disability and intellectual disability of a genetic basis (with the exception of labor and delivery in the latter). The reduction in effect sizes indicates some overlap of the two influences. This is not surprising, because these offspring may have been exposed to other insults in common. Given that each type of influence displays a significant effect on the risk of intellectual disability even when controlling for the effects of the other influence, we inferred that the impact of obstetric complications should be considered separately from the effect of maternal severe mental illness: not all of the contribution to risk of intellectual disability attributed to obstetric complications can be ascribed to the contribution of maternal severe mental illness.
The only evidence of statistical interaction between the effects was when children with heightened risk were exposed to obstetric complications in the neonatal period. Here, the estimated size of the joint effect was less than expected if the individual effects had been accrued.
Our results suggest that maternal schizophrenia and obstetric complications lead to individual but not necessarily mutually exclusive causal pathways toward intellectual disability, including intellectual disability with a genetic basis. We observed that for any intellectual disability, the risk attributable to the combined exposure to obstetric complications and maternal severe mental illness was significantly greater than the risk attributable to obstetric complications alone, with the confidence interval of the elevated odds ratio of the former not overlapping with the latter estimate. For children of mothers with schizophrenia specifically, this accumulation was significant for exposure to obstetric complications during labor and delivery and nearly reached statistical significance for exposure during pregnancy and during the neonatal period.
Finally, we observed in our cohort of children that intellectual disability itself was a risk factor for later psychotic disorder. Moreover, among children with intellectual disability, those who were also exposed to maternal schizophrenia had four times greater odds of developing a psychotic disorder compared with children with generic risk. These data provide whole-population phenotypic evidence of an overlap between intellectual disability and schizophrenia, supporting published genetic findings (1–3).
Our study design has several strengths. Our large sample size ensured adequate power for simultaneous investigation of the effects of maternal severe mental illness and exposure to obstetric complications during distinct periods of development (pregnancy, labor and delivery, and neonatal). Access to whole-population registers enabled inclusion of prospectively recorded data, avoiding recall bias. Use of multiple registers allowed access to a wide range of covariates, providing robust estimates.
One limitation of the study is the possibility of differential rates of ascertainment of intellectual disability, depending on maternal severe mental illness. Children of mothers with severe mental illness may be more likely to be identified as having intellectual disability by health care and educational services as a result of having greater contact with such services. Ascertainment rates for mothers and fathers may be similarly affected. Ascertainment of fathers’ intellectual disability was likely marginally lower for children with heightened risk than for children with generic risk, because not all fathers were registered on birth certificates and missing data were greater in the heightened-risk group. Additionally, although we adjusted for an extensive number of covariates, we lacked data on maternal smoking and nonsevere maternal alcohol or illicit substance abuse during pregnancy. We also could not adjust for maternal psychotropic medication use during pregnancy, although our sensitivity analysis with similar patterns of results for births conceived before and after diagnosis of maternal illness suggests no effect of medication use. Many children with intellectual disability did not have a Heber diagnosis, which reduced the statistical power to assess influences on intellectual disability of a genetic basis as comprehensively as influences on any intellectual disability. Finally, a substantial proportion of offspring had not reached the age of greatest risk for developing a psychotic disorder when we assessed this outcome at ages 9.5–31.5 years. In general, these limitations attenuate differences between children with familial heightened risk and those with generic risk.
There have been few published studies in this area. Our findings replicate those of Morgan and colleagues (13) in a sample of 6,303 children; however, we examined a cohort of almost half a million children. We have extended previous findings to obtain separate estimates for simultaneous effects of exposure to obstetric complications during pregnancy, labor and delivery, and the neonatal period. Consistent with previous findings, unadjusted estimates of odds ratios attributable to a mother’s schizophrenia were 3.8 compared with 3.2 in the study by Morgan and colleagues. Odds ratios for exposure to obstetric complications during pregnancy and labor and delivery were also consistent with those of Morgan and colleagues (1.7 and 1.3, respectively, compared with 1.5 and 1.2). This is close to the figure of 1.4 reported by Langridge and colleagues for the risk of autism with intellectual disability following labor and delivery complications (15). The observed odds ratios attributable to maternal severe mental illness and exposure to obstetric complications when estimated in our multivariate model are also consistent with those of Morgan and colleagues (13). The odds ratios presented here for schizophrenia, bipolar disorder, and unipolar major depression (1.7, 1.3, and 1.9, respectively) are lower than those estimated by Morgan and colleagues (2.2, 2.6, and 2.7, respectively), as is the adjusted odds ratio for complications during labor and delivery (1.1 compared with 1.4) (13). These discrepancies are consistent with the ability in our study to adjust for a wider range of covariates. In addition, Morgan and colleagues did not adjust for exposure to obstetric complications during pregnancy or the neonatal period in their adjusted model, and they did not include other psychotic disorders (ICD-9 codes 297.x and 298.x) in the umbrella category of maternal severe mental illness. Our large sample allowed simultaneous estimation of these latter effects.
We observed that the risk of intellectual disability is affected by both maternal severe mental illness and exposure to obstetric complications, with the presence of one not excluding the effect of the other. The only evidence of significant overlap between these influences was when exposure occurred during the neonatal period. Common obstetric complications during this period include required resuscitation and being small for gestational age. Some of these may reflect unobserved adverse events earlier in pregnancy, with their consequences becoming evident at birth.
The introduction of covariates resulted in reductions in the odds associated with maternal severe mental illness but had little impact on the odds associated with obstetric complications. The stable estimates for obstetric complications across models highlight the importance of good antenatal care for the long-term well-being of all mothers and their children. The lowering of estimates for severe mental illness with inclusion of covariates underscores the additional impact of the sociodemographic and cognitive disadvantages that these women often face.
The association between intellectual disability and maternal schizophrenia extended to maternal affective and other psychoses, suggestive of genetic overlap. However, much remains to be learned about common genetic and neurodevelopmental mechanisms influencing disease risk across these neuropsychiatric phenotypes. Although the rate of intellectual disability of a genetic basis was also increased among children of mothers with a severe mental illness as a whole, we were not able to determine the question of specificity to maternal diagnosis, possibly because of the subsample’s small size and lower statistical power. However, the increased rate of psychotic disorders in children with intellectual disability provides further evidence that phenotypically different neuropsychiatric disorders cluster within pedigrees.
This study fills an important gap in the epidemiological research on risk factors for adverse outcomes when mothers with severe mental illness give birth, and it has important implications for understanding shared genetic risk and covariation of severe mental illness and intellectual disability. We observed that maternal severe mental illness and obstetric complications in pregnancy, labor and delivery, and the neonatal periods each contribute to the risk of intellectual disability, with only some overlap in their contributions, even when adjusted for important covariates, suggesting potentially distinct causal pathways.
1 : The penetrance of copy number variations for schizophrenia and developmental delay. Biol Psychiatry 2014; 75:378–385Crossref, Medline, Google Scholar
2 : Analysis of intellectual disability copy number variants for association with schizophrenia. JAMA Psychiatry 2016; 73:963–969Crossref, Medline, Google Scholar
3 : The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev 2015; 55:173–183Crossref, Medline, Google Scholar
4 : Evidence for shared susceptibility to epilepsy and psychosis: a population-based family study. Biol Psychiatry 2012; 71:836–839Crossref, Medline, Google Scholar
5 : Dementia Praecox and Paraphrenia. New York, R E Krieger Publishers, 1919Google Scholar
6 Penrose LS: A clinical and genetic study of 1280 cases of mental defect (Medical Research Council: Special Report Number 229). London, H M Stationary Office, 1938Google Scholar
7 : The etiology of so-called schizophrenic psychoses: with special reference to their occurrence in twins. Am J Psychiatry 1934; 91:247–286Link, Google Scholar
8 : Relation of premature birth and under-weight condition at birth to mental deficiency. Am J Psychiatry 1934; 13:829–852Link, Google Scholar
9 : The detection of schizophrenia in infancy; a preliminary report. J Nerv Ment Dis 1957; 125:1–24Crossref, Medline, Google Scholar
10 : Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry 2008; 193:364–372Crossref, Medline, Google Scholar
11 : Mental retardation in a North Swedish isolate. Clin Genet 1986; 30:374–380Crossref, Medline, Google Scholar
12 : Elevated rates of schizophrenia in a familial sample with mental illness and intellectual disability. J Intellect Disabil Res 2004; 48:531–539Crossref, Medline, Google Scholar
13 : Intellectual disability and other neuropsychiatric outcomes in high-risk children of mothers with schizophrenia, bipolar disorder and unipolar major depression. Br J Psychiatry 2012; 200:282–289Crossref, Medline, Google Scholar
14 : The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 2002; 8:117–134Crossref, Medline, Google Scholar
15 : Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One 2013; 8:e50963Crossref, Medline, Google Scholar
16 : Prenatal and perinatal factors associated with intellectual disability. Am J Intellect Dev Disabil 2013; 118:156–176Crossref, Medline, Google Scholar
17 : Prenatal, perinatal and neonatal risk factors for intellectual disability: a systemic review and meta-analysis. PLoS One 2016; 11:e0153655Medline, Google Scholar
18 : Obstetric complications in children born to parents with schizophrenia: a meta-analysis of case-control studies. Psychol Med 1996; 26:279–287Crossref, Medline, Google Scholar
19 : Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry 2005; 162:79–91Link, Google Scholar
20 : Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG 2014; 121:566–574Crossref, Medline, Google Scholar
21 : Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005; 161:916–925, discussion 926–928Crossref, Medline, Google Scholar
22 : Cohort profile: pathways of risk from conception to disease: the Western Australian schizophrenia high-risk e-Cohort. Int J Epidemiol 2011; 40:1477–1485Crossref, Medline, Google Scholar
23 : Inaugural Report of the IDEA Database: Intellectual Disability in Western Australia. Perth, Western Australia, Institute for Child Health Research, 2004Google Scholar
24 : International Classification of Diseases, 9th Revision, Clinical Modification. Geneva, World Health Organization, 1979Google Scholar
25 : The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, World Health Organization, 1992Google Scholar
26 Bower C, Rudy E, Callaghan A, et al: Report of the Birth Defects Registry of Western Australia 1980–2008. Perth, Australia, King Edward Memorial Hospital, Health Department of Western Australia, 2009Google Scholar
27 : A manual on terminology and classification in mental retardation. Am J Ment Defic 1959; 64(Suppl 64):1–111Medline, Google Scholar
28 : Reported biomedical causes and associated medical conditions for mental retardation among 10-year-old children, metropolitan Atlanta, 1985 to 1987. Dev Med Child Neurol 1997; 39:142–149Crossref, Medline, Google Scholar
29 : McNeil-Sjöström Scale for Obstetric Complications. Malmö, Sweden, Department of Psychiatry, Lund University, 1995Google Scholar
30 Australian Bureau of Statistics: Socio-Economic Indexes for Areas (SEIFA)–technical paper 2006 (Catalogue no 2039.0.55.001). Canberra, Australia, Australian Bureau of Statistics, 2008Google Scholar
31 Australian Bureau of Statistics. Statistical geography, vol 1: Australian Standard Geographical Classification (ASGC) (Catalogue no 1216.0). Canberra, Australia, Australian Bureau of Statistics, 2006Google Scholar
32 : Confidence interval estimation of interaction. Epidemiology 1992; 3:452–456Crossref, Medline, Google Scholar
33 : Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol 2007; 36:1111–1118Crossref, Medline, Google Scholar
34 : Stata: Release 13, Statistical Software. College Station, Tex, Stata Corp, 2013Google Scholar



