Improving the Rates of Quitting Smoking for Veterans With Posttraumatic Stress Disorder
Abstract
OBJECTIVE: Smoking is highly prevalent and refractory among people with posttraumatic stress disorder (PTSD). This study aimed to improve the rate of quitting smoking for veterans with PTSD by integrating treatment for nicotine dependence into mental health care. METHOD: Smokers undergoing treatment for PTSD (N=66) were randomly assigned to 1) tobacco use treatment delivered by mental health providers and integrated with psychiatric care (integrated care) versus 2) cessation treatment delivered separately from PTSD care by smoking-cessation specialists (usual standard of care). Seven-day point prevalence abstinence was the primary outcome, measured at 2, 4, 6, and 9 months after random assignment. Data were analyzed by using a generalized estimating equations approach following the intent-to-treat principle. RESULTS: Subjects assigned to integrated care were five times more likely than subjects undergoing the usual standard of care to abstain from smoking across follow-up assessment intervals (odds ratio=5.23). Subjects in the integrated care condition were significantly more likely than subjects in usual standard of care to receive transdermal nicotine and nicotine gum. They also received a greater number of smoking-cessation counseling sessions. Stopping smoking was not associated with worsening symptoms of PTSD or depression. CONCLUSIONS: Smoking-cessation interventions can be safely incorporated into routine mental health care for PTSD and are more effective than treatment delivered separately by a specialized smoking-cessation clinic. Integrating cessation treatment into psychiatric care may have the potential for improving smoking quit rates in other populations of chronically mentally ill smokers.
Posttraumatic stress disorder (PTSD) is one of the most prevalent mental disorders (1, 2), particularly among Veterans Administration (VA) health care enrollees (3, 4). In the general population, PTSD is associated with high rates of smoking (45% [5] versus the national average of 23% [6]) and a fourfold increased risk for nicotine dependence (7). The rates of smoking in veterans with PTSD (53%–66% [8–10]) are approximately double those of VA enrollees in general (30% [11]). Moreover, a greater proportion of smokers with combat-related PTSD smoke heavily (>25 cigarettes per day) compared with veterans who smoke but do not have PTSD (48% versus 28% [8]). PTSD is associated with a smoking quit rate of only 23% (5), about half that of lifetime smokers without a mental disorder (12), and falls third from the bottom in a ranking of quit rates for 13 mental disorders (5). Smokers with PTSD also experience nicotine withdrawal symptoms in response to encounters with trauma-related stimuli (13) and report smoking in order to relieve anxiety and tension (8). Taken together, this research suggests a dynamic relationship between PTSD and tobacco use that argues for a coordinated approach to the treatment of both disorders.
Several pharmacological and behavioral treatments for nicotine dependence have shown efficacy in controlled clinical trials (12, 14). However, these treatments are only as useful as the ability of health care organizations to deliver them effectively to individuals who need them most. Primary care providers only infrequently apply even brief, cost-effective tobacco-cessation interventions to smokers (15–18), despite the fact that 60%–70% of smokers want to quit (6, 11). Nicotine-dependence treatment in patients with mental disorders may be particularly neglected since psychiatric patients receive cessation counseling at only 38% of primary care visits and 12% of visits with a psychiatrist (16). Referral of patients to specialty smoking-cessation clinics is a commonly used alternative to primary care-based delivery of tobacco use treatment. However, the effectiveness of these clinics is compromised by poor patient compliance, with rates of attendance as low as 13%–14% (19, 20) and limited capacity to provide repeated intervention for a chronic, relapsing disorder such as nicotine dependence. These limitations create a situation in which only 17% of smokers in the nation’s largest health care system (the VA) report receiving desired cessation treatment in the previous year (11).
The simultaneous treatment of two or more interwoven disorders by a single provider team has shown promising clinical effectiveness for patients with severe mental illness and comorbid substance use disorders (21, 22). This study accordingly tested an approach for improving the effectiveness of tobacco-cessation service delivery by integrating treatment for smoking into the routine mental health care of patients with PTSD. Specifically, this randomized, controlled clinical trial compared the effectiveness of two different methods for delivering guideline-based smoking-cessation treatment to patients receiving VA mental health care: 1) brief smoking-cessation interventions integrated with ongoing mental health care and delivered by mental health providers (integrated care) versus 2) smoking-cessation interventions delivered separately from mental health care by smoking-cessation specialists (usual standard of care). It was hypothesized that integrated care would result in higher rates of smoking cessation than would usual standard of care. A secondary objective of this study was to determine if smoking cessation was associated with worsening PTSD or depression symptoms. Anticipating this possibility is important, given the high co-occurrence of depression with PTSD (23) and prior research showing that smoking abstinence can exacerbate depression in individuals with a history of this disorder (24–27).
Method
Subjects
Subjects (N=66) were recruited from the VA Puget Sound Health Care System PTSD clinic, which provides specialized outpatient treatment for chronic PTSD. Table 1 reports demographic and smoking characteristics for the study group as well as psychometric data showing moderate to severe levels of symptoms on the PTSD Checklist (28) and the Beck Depression Inventory (29). Subjects in the integrated care and usual standard of care conditions did not differ significantly on baseline characteristics (all p values >0.05).
Subjects were included if they smoked ≥10 cigarettes per day, expressed a willingness to receive smoking-cessation treatment, and met DSM-IV criteria for PTSD. Exclusion criteria were 1) the use of smokeless tobacco, pipes, or cigars, 2) the presence of unstable axis I disorders, and 3) current substance dependence disorder other than tobacco use. After providing a complete description of the study, written informed consent was obtained. The subjects were then randomly assigned to integrated care (N=33) versus the usual standard of care (N=33) for smoking cessation. The rate of refusal to participate in this study was 3% for help-seeking smokers.
Treatment Conditions
All study subjects received psychotropic medications for PTSD throughout their participation in the study, as prescribed by two PTSD clinic psychiatrists and a nurse practitioner. Each subject also received psychotherapy from an assigned case manager who coordinated their mental health care. Case managers included four psychologists, one social worker, an addictions therapist, and a technician.
Integrated Care: Experimental Condition
The subjects randomly assigned to integrated care received smoking-cessation interventions administered by their assigned PTSD clinic prescriber and case manager. Integrated care was modeled after the brief clinical interventions for primary care practitioners published in the U.S. Public Health Service’s clinical practice guideline titled Treating Tobacco Use and Dependence(14). PTSD clinic staff received approximately 3 hours of training, plus as-needed consultation in smoking-cessation treatment from the clinic director (M.M.). They then delivered interventions using a treatment manual (available upon request from the first author) that operationalized interventions for each session.
The subjects received smoking-cessation protocol medications (bupropion, transdermal nicotine, nicotine polacrilex gum, and nicotine spray) from the psychiatrist or nurse practitioner managing their pharmacological treatment of PTSD. Prescribers in the integrated care and the usual standard of care conditions agreed to adopt the practice of routinely prescribing bupropion, transdermal nicotine, and nicotine gum or spray in order to standardize the medication protocol across conditions. However, the prescribers were allowed to use their discretion to select only some of these medications, depending on patient preferences and medical contraindications.
The core behavioral counseling components of integrated care, administered by case managers, consisted of 1) the assessment of tobacco use status, abstinence history, and individualized reasons for quitting; 2) education about the health risks of smoking and the benefits of quitting; 3) advice to quit smoking; 4) application of motivational interventions for ambivalent smokers; 5) setting a date to quit; 6) behavioral counseling to help subjects prepare to quit smoking, coping with smoking urges and barriers to abstinence, and developing problem-solving skills; 7) self-help reading materials; 8) intrasession support and assistance in identifying extrasession social support; and 9) self-directed behavioral methods for reducing anxiety, consisting of a relaxation training tape and written materials on stress management. The protocol required case managers to administer five individual behavioral counseling sessions on a once-weekly basis, plus one follow-up contact (the sessions averaged 20 minutes each). These interventions were rolled into regularly scheduled visits addressing PTSD and comorbid mental disorders for subjects whose treatment plan ordinarily included individual sessions with a case manager. For subjects receiving only group therapy for PTSD, case managers scheduled separate individual smoking-cessation sessions. After delivering the six core behavioral counseling sessions, clinicians used their discretion to periodically assess smoking status and reinstate cessation treatment for subjects who relapsed.
Usual Standard of Care: Comparison Condition
The subjects randomly assigned to the usual standard of care were referred to the VA Puget Sound Health Care System’s Smoking Cessation Clinic. Nursing personnel who staffed this clinic were trained at Mayo Clinic’s Nicotine Dependence Treatment Center and had extensive experience in cessation treatment. This clinic delivered U.S. Public Health Service guideline-adherent cessation treatment (14). Clinic providers used the same algorithm as integrated care providers for prescribing smoking-cessation medications. The subjects attended one group orientation class, followed by individual sessions in which they received medications and behavioral counseling. Unrestricted access to usual standard of care treatment sessions was provided to all subjects. That is, the number of treatment contacts received was determined by clinical recommendations of usual standard of care providers and the preferences and initiative of subjects. The subjects assigned to the usual standard of care condition received absolutely no tobacco-cessation interventions from their PTSD clinic providers.
Measures
Delivery of study treatments
The Consumer Health Information and Performances Sets, an administrative database accounting for services delivered by the VA, was used to track smoking-cessation medications actually received by the subjects. This database provided information about the proportion of subjects who filled prescriptions for study medications as well as doses for these medications. The number of smoking cessation behavioral counseling sessions delivered to the subjects was extracted from the VA’s computerized patient record system, where clinicians are required to document all patient visits.
Adherence of integrated care case managers to the treatment manual was assessed by independent review of the computerized patient record system. These records contained provider documentation of interventions delivered during each visit on specially prepared charting templates that listed a total of 31 specific protocol interventions prescribed by the manual. These interventions constituted the core behavioral counseling components of integrated care (e.g., “set and recorded a quit date,” “identified strategies for coping with withdrawal symptoms”). After each session, case managers checked off interventions delivered on the charting template and provided a narrative summary of smoking status and treatment progress. The study coordinator (D.Y.) independently reviewed chart notes for all providers and treatment sessions in order to compute clinicians’ self-recorded adherence to protocol interventions as a percentage of the total recommended. This review showed that 80% of behavioral counseling interventions prescribed by the treatment manual were reportedly administered.
Smoking outcomes
Seven-day point prevalence abstinence, verified by expired carbon monoxide (≤10 ppm), was the primary outcome measure used to compare integrated care and the usual standard of care at 2, 4, 6, and 9 months after random assignment. The period after randomization provided a consistent marker across subjects of the initiation of the treatment process. The convention of using an end-of-treatment phase or an initial quit date as a starting point for abstinence measurements was not followed because this study aimed to compare tobacco cessation treatments as they are actually practiced in a clinical setting. Specifically, guideline-adherent treatment for a chronic relapsing condition, such as tobacco use disorder, is a continuous process requiring multiple quit attempts and reapplication of interventions aimed at “recycling” patients who relapse (14, 30, 31). Consistent with expert recommendations (12, 14, 31), study treatments were not defined by a discrete episode of care marked by a fixed endpoint, number of sessions, or single quit date.
Repeated 7-day point prevalence abstinence was used as a proxy for prolonged abstinence, consistent with prior research (32–36). Repeated 7-day point prevalence abstinence was based on three consecutive follow-up assessment intervals (4, 6, and 9 months after random assignment). The 2-month assessment was not included in this computation, allowing subjects a stabilization period to recover from lapses after initial intervention (37). Although repeated 7-day point prevalence abstinence is a less conservative measure than prolonged abstinence, the two measures nevertheless correlate highly (r=0.85–0.94 [38, 39]).
Mental health quality assurance outcomes
The PTSD Checklist (28) and the Beck Depression Inventory (29) were completed by subjects at baseline and 6- and 9-month follow-ups. The subjects rated symptoms that occurred during the prior week. The PTSD Checklist and the Beck Depression Inventory were used to assess changes in mental health symptoms over time in order to determine if smoking cessation was associated with worsening symptoms.
Patient satisfaction outcomes
The subjects in each study condition rated their satisfaction with the amount and quality of smoking-cessation treatment received by using a 4-point Likert scale (1=“very dissatisfied” and 4=“very satisfied”). The ratings were gathered at the terminal (9-month) study assessment.
Data Analysis
Abstinence data were analyzed in an intent-to-treat analysis by using marginal logistic regression with the method of generalized estimating equations. This statistical approach to analyzing smoking-cessation outcome data conforms to recommendations by an expert panel from the Society for Research on Nicotine and Tobacco (39). The generalized estimating equation method has several advantages in that it accounts for the correlation of repeated measurement, uses all available data points, and is sensitive to the pattern of change over time (40). Covariates in the analysis were baseline measures of severity of nicotine dependence (the Fagerstrom Test for Nicotine Dependence [41]) and depression (the Beck Depression Inventory). Depression was included as a covariate because of evidence showing that it may adversely affect smoking-cessation outcomes (42–44).
The effect of smoking cessation on mental health symptoms at 6- and 9-month follow-up assessments was analyzed by computing change from baseline scores for the PTSD Checklist and the Beck Depression Inventory at these assessment intervals. Separate Mann-Whitney U tests were then used to compare these change scores for continuing smokers versus subjects who were abstinent at each assessment interval.
All categorical variables (other than abstinence status) were analyzed with chi-square tests. Normally distributed continuous variables were analyzed with Student’s t tests. Nonparametric tests (Mann-Whitney U and Spearman’s correlation) were performed on nonnormally distributed variables.
Results
Delivery of Study Treatments
Smoking-cessation medications
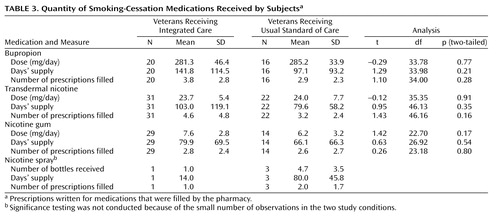
Table 2 presents the proportion of subjects receiving integrated care and usual standard of care who obtained prescriptions for study medications that were filled by the pharmacy. The subjects randomly assigned to integrated care were significantly more likely than subjects receiving usual standard of care to receive prescriptions for transdermal nicotine and nicotine gum, but the differences between study conditions were not significant for other smoking-cessation medications. Subjects in integrated care also received more types of smoking-cessation medications (i.e., gum, patch, spray, or bupropion) than subjects in usual standard of care (integrated care: mean=2.5, SD=0.7; usual standard of care: mean=1.7, SD=1.0) (z=3.21, p<0.01, two-tailed). Ninety-four percent of the subjects in integrated care filled prescriptions for two or more types of smoking-cessation medications over the course of the study compared to 64% of the subjects in usual standard of care (χ2=7.3, df=1, p<0.01, two-tailed). Table 3 shows that subjects in both conditions who received smoking-cessation medications were given doses and a course of treatment consistent with U.S. Public Health Service practice guidelines (14).
Behavioral counseling
All subjects randomly assigned to integrated care participated in at least one behavioral counseling smoking-cessation session, and 88% of the subjects in usual standard of care had at least one treatment contact. Subjects in integrated care received an average of 5.15 (SD=1.2) manual-driven protocol smoking-cessation sessions, compared to an average of 2.6 (SD=2.1) sessions for subjects in usual standard of care (z=5.35, p<0.0001, two-tailed). Additional provider-initiated follow-up contacts (mean=3.9, SD=3.9) were delivered to integrated care subjects, involving assessment of smoking status and reapplication of interventions deemed appropriate by the case manager. The number of smoking-cessation sessions received by all subjects was significantly correlated with the number of assessment occasions (four possible) when they were abstinent from smoking (rs=0.35, p<0.004, two-tailed).
Smoking-Cessation Outcomes
Subject compliance with follow-up assessments was 83% across all four assessment intervals (83% at month 2, 81% at month 4, 81% at month 6, and 84% at month 9). Medical records and/or retrospective subject interview data were available to determine smoking status for 96% of the remaining observations. Four percent of the observations were missing and were handled by recording subjects as presumptive smokers. The two study conditions did not differ significantly in the number of missing observations (p>0.05).
Figure 1 presents smoking abstinence data for each assessment interval and the results of the generalized estimating equation analysis. At each assessment interval, the odds of not smoking at that interval were over five times greater for the subjects in integrated care than the subjects in usual standard of care (odds ratio=5.23, 95% confidence interval=1.76–15.54, p<0.002, two-tailed). The proportion of subjects who achieved abstinence at one or more assessment intervals was greater for the subjects in integrated care (integrated care=52% versus usual standard of care=25%) (χ2=4.82, df=1, p<0.02, two-tailed). The subjects in integrated care were also abstinent at more follow-up assessment periods (four possible) compared to the subjects in usual standard of care (mean=1.1, SD=1.4, for integrated care versus mean=0.4, SD=0.8, for usual standard of care) (z=2.38, p<0.02, two-tailed). The repeated 7-day point prevalence abstinence rate was 12% for integrated care and 3% for the usual standard of care, a statistically nonsignificant difference (χ2=1.66, df=1, p=0.20, two-tailed).
Quality Assurance Outcomes
For the group as a whole, scores on the PTSD Checklist and the Beck Depression Inventory gathered at 6 and 9 months did not change significantly from baseline (all p>0.05). Change scores were not significantly different for abstainers versus continued smokers at either assessment interval (all p>0.05).
Satisfaction With Treatment
The subjects in integrated care were significantly more satisfied with the amount of smoking-cessation treatment they received compared to the subjects in usual standard of care (mean=3.9, SD=0.3, for integrated care versus mean=3.5, SD=0.7, for the usual standard of care) (z=3.21, p<0.001, two-tailed). Ratings for the quality of treatment were also significantly higher for the integrated care condition (mean=3.7, SD=0.5, for integrated care versus mean=3.4, SD=0.6, for the usual standard of care) (z=2.08, p<0.04, two-tailed).
Discussion
This study demonstrated the feasibility of training mental health providers to integrate guideline-based smoking-cessation treatment into mental health care for veterans with PTSD. PTSD clinic prescribers readily incorporated the delivery of tobacco-cessation medications into their clinical practice. Case managers also documented a high rate of adherence to prescribed behavioral counseling interventions, although the absence of verification through independent ratings of session content limits the validity of this finding. The integrated model of smoking-cessation treatment tested here was more effective for PTSD patients than for care provided by VA smoking-cessation specialists, as measured by point-prevalence abstinence. The difference between study conditions on the repeated 7-day point prevalence abstinence measure (12% for integrated care, 3% for the usual standard of care) was not significant, owing to the limited statistical power of the study. Since completing this randomized trial, PTSD clinic staff have delivered integrated care to 107 additional patients, indicating sustainability of the intervention in routine clinical practice. (For these patients, carbon monoxide-verified [≤10 ppm], repeated 7-day point prevalence abstinence quit rates measured at 4, 6, and 9 months after treatment onset were 15%.)
Subjects in integrated care participated in a greater number of smoking-cessation counseling sessions than subjects in usual standard of care and were significantly more likely to receive transdermal nicotine and nicotine gum. They were also more likely to receive combination tobacco-cessation pharmacotherapy, which has been shown to improve smoking quit rates over use of monotherapy (14). These findings suggest that integrated care was a more effective vehicle than the usual standard of care for delivering cessation treatments of sufficient intensity. Integrated care may have been more successful at engaging subjects in cessation treatment, as indicated by higher satisfaction ratings for integrated care than usual standard of care and encouraging medication compliance. Additionally, the continuous therapeutic relationship afforded by integrated care, as opposed to the episodic care provided by the usual standard of care, may have improved access to ongoing monitoring and provider-initiated relapse management. Other research has shown a “dose-response” relationship between the number of smoking-cessation contacts and smoking outcomes (14, 33, 35), as well as higher quit rates for extended versus brief tobacco use treatments (45). In fact, some treatment trials suggest that cessation may be related more to the number of counseling sessions received than specific therapeutic methods used for smokers with a positive history of depression (32–35). Successful smoking-cessation treatment for veterans with PTSD may similarly require a greater number of contacts over an extended time.
Guideline-recommended smoking-cessation treatments yielded point prevalence quit rates of 15% to 25% in U.S. Public Health Service meta-analyses of 6,000 studies involving follow-up assessments of at least 5 months (12). Individuals with mental disorders were typically excluded from smoking-cessation clinical trials reviewed by the U.S. Public Health Service, as active psychiatric comorbidities complicate cessation efforts and are associated with significantly reduced quit rates (5, 46–49). The fact that point prevalence quit rates observed in this study fell within the range of those reported in meta-analyses of treatment trials is encouraging, given that our subjects were chronically mentally ill and experienced moderate to severe symptoms of PTSD and depression. Research is clearly needed that improves upon the repeated 7-day point prevalence abstinence quit rate (12%) of the integrated-care condition. Reapplication of integrated care over a longer period in order to promote “recycling” is one possible strategy to test. Another is to increase the intensity of initial integrated care interventions in an effort to prevent early relapse to smoking. Application of integrated care on a systemwide basis, even with a prolonged smoking quit rate of 12%, still has a potential for producing 10,000 quitters from within the population of PTSD patients seen at the VA alone (estimates derived from VA databases [11, 50, 51]).
There exist remarkably few smoking-cessation clinical trials involving individuals with current mental disorders that provide a standard of comparison for the present study. A number of studies have been conducted with smokers having a history of past major depression (32–36, 52, 53). However, these studies do not provide the appropriate standard of comparison for the present study, and the preponderance of evidence from these trials does not substantiate the hypothesis that history-positive smokers are less likely to quit than history-negative smokers (54). Clinical trials involving smokers with current mental disorders who meet the minimum recommended standard for abstinence at follow-up assessments (≥6 months [37, 55]) have focused on schizophrenia and alcohol dependence. (Single point prevalence abstinence rates at the terminal 6-month follow-up assessment for studies of smokers with schizophrenia: 11% [56], 12% [57], 7%–17% [58], and 19% [59]; single point prevalence abstinence rates at the terminal 12-month assessment for studies of alcohol-dependent patients: 12% [60] and 13%–19% [61].) Quit rates from these studies of clinical samples are roughly equivalent to, if not slightly lower than, the single-point prevalence quit rates for integrated care reported here. The only study (61) reporting prolonged abstinence (repeated 7-day point prevalence abstinence at follow-up months 1, 3, 6, and 12) found quit rates of 10% and 12% for two active tobacco-cessation treatments involving recovering alcoholics. These results are comparable to the repeated 7-day point prevalence abstinence quit rate of 12% in the present study.
The rate of smoking abstinence for integrated care subjects at study termination was less than half that observed at the 2-month assessment. Several possible mechanisms that may contribute to smoking relapse in PTSD patients deserve further exploration. Specifically, pre- and postcessation negative affective states (24, 32, 33, 44, 46, 62) and increased symptoms of depression immediately following a quit attempt (63) increase relapse susceptibility and may have suppressed sustained quit rates in this study. Heightened withdrawal sensitivity has predicted relapse in smokers with a history of depression in some studies (64, 65) and may possibly explain continued smoking in PTSD patients as well.
Stopping smoking in this treatment trial was not associated with worsening symptoms of PTSD or depression measured at 6 and 9 months after treatment onset. Our results are more consistent with studies showing that mood disturbances do not result from stopping smoking (32, 46, 66) than with studies showing that they do (25–27, 67) among individuals with a history of depression. However, comparing our results with those of others is attenuated because of the marked differences in study groups (i.e., current PTSD versus a history of major depression). Three other possible explanations for this finding also deserve consideration. First, transient symptom exacerbations related to postcessation nicotine withdrawal may have occurred shortly after subjects’ cessation attempts but were undetected because outcomes were only measured at four discrete time points in our study. Second, the fact that 86% of the subjects in the present research were receiving antidepressant medications (other than bupropion for smoking cessation) for PTSD may have prevented potential deterioration effects. This possibility is supported by evidence showing that sertraline reduces nicotine withdrawal symptoms (53) and that nortriptyline alleviates postcessation negative affect (35) in smokers with a history of major depression. Third, subjects who experienced worsening psychiatric symptoms upon trying to quit smoking may have simply resumed smoking in order to manage these symptoms. Our data do not support this latter explanation since symptoms of PTSD and depression did not change from baseline to the 6- and 9-month assessment for subjects who continued to smoke compared to those who quit. However, the nonsignificant difference between subjects who quit and those who did not on symptom measures may reflect low statistical power owing to the small number of quitters involved in comparisons at months 6 and 9.
Interpretation of this study’s findings may be limited by several methodological shortcomings. This investigation did not follow the convention of measuring outcomes from a clearly demarcated quit date or the end of the intervention period because smoking-cessation treatment was conceptualized as a continuous process involving reapplication of treatment and multiple quit attempts (31). The comparability of findings with other research that measures outcomes from an initial quit date may therefore be limited. Biomarkers of longer-term nonsmoking status were not used but should ideally have supplemented expired carbon monoxide in order to verify subject self-reports. Continuous abstinence was also not assessed because it is not verifiable with standard biological assays. However, continuous abstinence data based on subject self-report alone would have been informative because subject reports of smoking status are generally valid (68). Proportions of subjects who received prescriptions filled by the pharmacy were reported, but a specific measure of medication adherence was not included. Thus, it is impossible to determine to what degree, if any, medication adherence may have affected outcomes. PTSD patients with a current substance abuse disorder were included in the study, but those with current substance dependence were not. Thus, our findings may not generalize to smokers with PTSD and comorbid substance dependence disorders. Finally, a much larger study group is clearly required in order to conclude definitively that integrated care is more effective than the usual standard of care for smoking cessation in other clinical settings.
This service delivery study was conducted in the spirit of a clinical effectiveness trial in order to promote generalization to “real world” health care settings (69). To this end, treatments were represented as they are likely to be actually practiced, and broad subject inclusion criteria were used to ensure that the study group reflected the population of VA PTSD clinic patients. Despite threats to internal validity inherent in such trials (69), this study provided a favorable test of the feasibility and outcome of APA recommendations (49) to implement smoking-cessation treatment in practice-based settings for patients with current mental disorders. Additional studies of integrated models of smoking-cessation treatment for mentally ill patients are warranted, given the extraordinarily high prevalence of heavy smoking in these individuals (41% [5] to 50% [70]).
 |
 |
 |
Presented in part at the International Society for Traumatic Stress Studies, New Orleans, Dec. 6–9, 2001. From the Northwest Network Mental Illness Research, Education, and Clinical Center, the Center for Excellence in Substance Abuse Treatment and Education, and the Biostatistics Unit of the Health Services Research and Development Center of Excellence, Department of Veterans Affairs, Seattle, Wash.; and the University of Washington School of Medicine, the Department of Psychiatry and Behavioral Sciences, and the School of Nursing, Seattle. Address correspondence and reprint requests to Dr. McFall, VA Puget Sound Health Care System, PTSD Programs (S-116 MHC), 1660 S. Columbian Way, Seattle, WA 98108; [email protected] (e-mail). Supported by the Department of Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center, the Center for Excellence in Substance Abuse Treatment and Education, and a grant from the University of Washington Alcohol and Drug Abuse Institute.
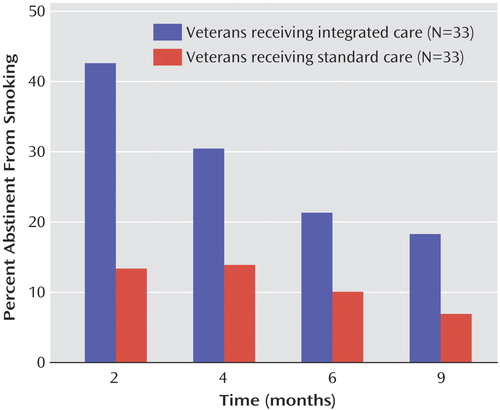
Figure 1. Point Prevalence Smoking Abstinence Rates for Veterans With Posttraumatic Stress Disorder in Integrated Care Versus the Usual Standard of Carea
aSignificant effect of group (odds ratio=5.23, 95% CI=1.76–15.54, p<0.001, two-tailed).
1. Narrow WE, Rae DS, Robins LN, Regier DA: Revised prevalence estimates of mental disorders in the United States. Arch Gen Psychiatry 2002; 59:115–123Crossref, Medline, Google Scholar
2. Breslau N: Epidemiology of trauma and posttraumatic stress disorder, in Psychological Trauma. Edited by Yehuda R. Washington, DC, American Psychiatric Press, 1998, pp 1–29Google Scholar
3. Hankin CS, Spiro A III, Miller DR, Kazis L: Mental disorders and mental health treatment among US Department of Veterans Affairs outpatients: the Veterans Health Study. Am J Psychiatry 1999; 156:1924–1930Abstract, Google Scholar
4. Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA: Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med 2004; 164:394–400Crossref, Medline, Google Scholar
5. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH: Smoking and mental illness: a population-based prevalence study. JAMA 2000; 284:2606–2610Crossref, Medline, Google Scholar
6. Cigarette smoking among adults—United States, 2000. MMWR Morb Mortal Wkly Rep 2002; 51:642–645Medline, Google Scholar
7. Breslau N, Davis GC, Schultz LR: Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry 2003; 60:289–294Crossref, Medline, Google Scholar
8. Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Davidson JR, Fairbank JA: Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict Behav 1997; 22:637–647Crossref, Medline, Google Scholar
9. Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA: Smoking in Vietnam combat veterans with post-traumatic stress disorder. J Trauma Stress 1995; 8:461–472Crossref, Medline, Google Scholar
10. Shalev A, Bleich A, Ursano RJ: Posttraumatic stress disorder: somatic comorbidity and effort tolerance. Psychosomatics 1990; 31:197–203Crossref, Medline, Google Scholar
11. Miller DR, Kalman D, Ren XS, Lee AF, Niu Z, Kazis L: Health Behaviors of Veterans in the VHA: Tobacco Use:1999 Large Health Survey of VHA Enrollees. Washington, DC, Department of Veterans Affairs, Veterans Health Administration, Office of Quality and Performance,2001, p 5Google Scholar
12. Fiore MC, Hatsukami DK, Baker TB: Effective tobacco dependence treatment. JAMA 2002; 288:1768–1772Crossref, Medline, Google Scholar
13. Beckham JC, Lytle BL, Vrana SR, Hertzberg MA, Feldman ME, Shipley RH: Smoking withdrawal symptoms in response to a trauma-related stressor among Vietnam combat veterans with posttraumatic stress disorder. Addict Behav 1996; 21:93–101Crossref, Medline, Google Scholar
14. US Department of Health and Human Service: Treating Tobacco Use and Dependence: Clinical Practice Guideline. Rockville, Md, US Public Health Service, 2000Google Scholar
15. Thorndike AN, Rigotti NA, Stafford RS, Singer DE: National patterns in the treatment of smokers by physicians. JAMA 1998; 279:604–608Crossref, Medline, Google Scholar
16. Thorndike AN, Stafford RS, Rigotti NA: US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001; 3:85–91Crossref, Medline, Google Scholar
17. Goldstein MG, Niaura R, Willey-Lessne C, DePue J, Eaton C, Rakowski W, Dube C: Physicians counseling smokers: a population-based survey of patients’ perceptions of health care provider-delivered smoking cessation interventions. Arch Intern Med 1997; 157:1313–1319Crossref, Medline, Google Scholar
18. Ockene JK: Primary care-based smoking interventions. Nicotine Tob Res 1999; 1(suppl 2):S189-S193Google Scholar
19. Thompson RS, Michnich ME, Friedlander L, Gilson B, Grothanus LC, Storer B: Effectiveness of smoking cessation interventions integrated into primary care practice. Med Care 1988; 26:62–76Crossref, Medline, Google Scholar
20. Sherman SE, Yano EM, Lanto AB, Simon B, Rubenstein LV: Veterans who smoke: do they want to quit and are we helping them? in Proceedings of the VA Health Services Research and Development 19th Annual Meeting. Washington, DC, Department of Veterans Affairs, 2001, pp 67–68Google Scholar
21. Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR: Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull 1998; 24:589–608Crossref, Medline, Google Scholar
22. Hellerstein DJ, Rosenthal RN, Miner CR: Integrating services for schizophrenia and substance abuse. Psychiatr Q 2001; 72:291–306Crossref, Medline, Google Scholar
23. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
24. Covey LS, Glassman AH, Stetner F: Depression and depressive symptoms in smoking cessation. Compr Psychiatry 1990; 31:350–354Crossref, Medline, Google Scholar
25. Covey LS, Glassman AH, Stetner F: Major depression following smoking cessation. Am J Psychiatry 1997; 154:263–265Link, Google Scholar
26. Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss JF, Cooper TB: Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther 1993; 54:670–679Crossref, Medline, Google Scholar
27. Glassman AH, Covey LS, Stetner F, Rivelli S: Smoking cessation and the course of major depression: a follow-up study. Lancet 2001; 357:1929–1932Crossref, Medline, Google Scholar
28. Forbes D, Creamer M, Biddle D: The validity of the PTSD Checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther 2001; 39:977–986Crossref, Medline, Google Scholar
29. Beck AT, Steer RA, Garbin MG: Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77–100Crossref, Google Scholar
30. Curry SJ, McBride CM: Relapse prevention for smoking cessation: review and evaluation of concepts and interventions. Annu Rev Public Health 1994; 15:345–366Crossref, Medline, Google Scholar
31. Abrams DB, Niaura R: Planning evidence-based treatment of tobacco dependence, in The Tobacco Dependence Treatment Handbook. Edited by Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. New York, Guilford, pp 1–26Google Scholar
32. Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Goldstein MG, Burgess ES, Miller IW: Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol 2001; 69:471–480Crossref, Medline, Google Scholar
33. Hall SM, Munoz RF, Reus VI: Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol 1994; 62:141–146Crossref, Medline, Google Scholar
34. Hall SM, Munoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, Hartz DT: Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo-controlled study. J Consult Clin Psychol 1996; 64:1003–1009Crossref, Medline, Google Scholar
35. Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E: Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 1998; 55:683–690Crossref, Medline, Google Scholar
36. Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude-Griffin R: Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry 2002; 59:930–936Crossref, Medline, Google Scholar
37. Hughes JR, Keely JP, Niaura R, Ossip-Klein DJ, Richmond RL, Swan GE: Measures of abstinence from tobacco in clinical trials: issues and recommendations. Nicotine Tob Res 2003; 5:13–25; correction, 5:603Crossref, Medline, Google Scholar
38. Velicer WF, Prochaska JO: A comparison of four self-report smoking cessation outcome measures. Addict Behav 2004; 29:51–60Crossref, Medline, Google Scholar
39. Keely JP, Hughes JR, Carpenter MJ: A method to convert prolonged abstinence and point prevalence quit rates, in Proceedings of the Seventh Annual Meeting of the Society for Research on Nicotine and Tobacco. Middleton, Wis, SRNT, 2001, p 73Google Scholar
40. Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D, Tsoh JY, Niaura R: Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcomes. Nicotine Tob Res 2001; 3:193–202Crossref, Medline, Google Scholar
41. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K: The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Crossref, Medline, Google Scholar
42. Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL: Depression and the dynamics of smoking. JAMA 1990; 264:1541–1545Crossref, Medline, Google Scholar
43. Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R: Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav 2001; 15:13–17Crossref, Medline, Google Scholar
44. Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, Baile WF: The effects of depressed mood on smoking cessation: mediation by postcessation self-efficacy. J Consult Clin Psychol 2003; 71:292–301Crossref, Medline, Google Scholar
45. Hall SM, Humfleet GM, Reus VI, Munoz RF, Cullen J: Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry 2004; 161:2100–2107Link, Google Scholar
46. Kinnunen T, Doherty K, Militello FS, Garvey AJ: Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol 1996; 64:791–798Crossref, Medline, Google Scholar
47. Rigotti NA: Treatment of tobacco use and dependence. N Engl J Med 2002; 346:506–512Crossref, Medline, Google Scholar
48. Niaura R, Abrams DB: Smoking cessation: progress, priorities, and prospects. J Consult Clin Psychol 2002; 70:494–509Crossref, Medline, Google Scholar
49. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Nicotine Dependence. Am J Psychiatry 1996; 153(Oct suppl)Google Scholar
50. Fontana A, Rosenheck R: Specialized Outpatient PTSD Programs (SOPPs) Workload: Fiscal Year 2002. West Haven, Conn, VA Connecticut Health Care System, VHA Northeast Program Evaluation Center, 2002Google Scholar
51. Flora C: Readjustment Counseling Service: Activity Reporting System for FY02. Washington, DC, Department of Veterans Affairs, Readjustment Counseling Service, 2002Google Scholar
52. Covey LS, Hughes D, Glassman AH, Blazer D, George LK: Ever smoking, quitting, and psychiatric disorders: evidence from the Durham Epidemiological Catchment Area. Tob Control 1994; 3:222–227Crossref, Google Scholar
53. Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K: A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. Am J Psychiatry 2002; 159:1731–1737Link, Google Scholar
54. Hitsman B, Borrelli B, McChargue DE, Spring B, Niaura R: History of depression and smoking cessation outcome: a meta-analysis. J Consult Clin Psychol 2003; 71:657–663Crossref, Medline, Google Scholar
55. Pierce JP, Gilpin EA: A minimum 6-month prolonged abstinence should be required for evaluating smoking cessation trials. Nicotine Tob Res 2003; 5:151–153Crossref, Medline, Google Scholar
56. Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC: A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob Res 2001; 3:397–403Crossref, Medline, Google Scholar
57. Addington J, el Guebaly N, Campbell W, Hodgins DC, Addington D: Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998; 155:974–976Link, Google Scholar
58. George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, Rounsaville BJ, Kosten TR: Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000; 157:1835–1842Link, Google Scholar
59. George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, Kosten TR: A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry 2002; 52:53–61Crossref, Medline, Google Scholar
60. Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Morse RM, Palmen MA, Bruce BK: Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res 1994; 18:867–872Crossref, Medline, Google Scholar
61. Burling TA, Burling AS, Latini D: A controlled smoking cessation trial for substance-dependent inpatients. J Consult Clin Psychol 2001; 69:295–304Crossref, Medline, Google Scholar
62. Hall SM, Munoz RF, Reus VI, Sees KL: Nicotine, negative affect and depression. J Consult Clin Psychol 1993; 61:761–767Crossref, Medline, Google Scholar
63. Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, Miller IW: Patterns of change in depressive symptoms during smoking cessation: who’s at risk for relapse? J Consult Clin Psychol 2002; 70:356–361Crossref, Medline, Google Scholar
64. Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS: Heavy smokers, smoking cessation, and clonidine. JAMA 1988; 259:2863–2866Crossref, Medline, Google Scholar
65. Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J: Smoking, smoking cessation, and major depression. JAMA 1990; 264:1546–1549Crossref, Medline, Google Scholar
66. Tsoh JY, Humfleet GL, Muñoz RF, Reus VI, Hartz DT, Hall SM: Development of major depression after treatment for smoking cessation. Am J Psychiatry 2000; 157:368–374; correction, 157:1359Link, Google Scholar
67. Ginsberg D, Hall SM, Reus VI, Munoz RF: Mood and depression diagnosis in smoking cessation. Exp Clin Psychopharmacol 1995; 3:389–395Crossref, Google Scholar
68. Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S: The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994; 84:1086–1093Crossref, Medline, Google Scholar
69. Glasgow RE, Lichtenstein E, Marcus AC: Why don’t we see more translation of health promotion research to practice? rethinking the efficacy-to-effectiveness transition. Am J Public Health 2003; 93:1261–1267Crossref, Medline, Google Scholar
70. Substance Abuse and Mental Health Services Administration, Office of Applied Studies: National Household Survey on Drug Abuse 2000. http://www.DrugAbuseStatistics.samhsa.govGoogle Scholar



