Prevalence of Neuroleptic-Induced Movement Disorders in Chronic Schizophrenia Inpatients
Abstract
OBJECTIVE: Since most of the world’s schizophrenia patients are treated with conventional antipsychotics, the authors evaluated various methods for establishing the prevalence of neuroleptic-induced movement disorders in these patients. METHOD: DSM-IV criteria and established score thresholds on a movement disorder rating scale were used to identify cases of neuroleptic-induced movement disorder in a representative Estonian patient sample of 99 chronic institutionalized schizophrenia patients, 18–65 years old, treated with conventional neuroleptics (79.8%) or clozapine (20.2%). RESULTS: Neuroleptic-induced movement disorders according to DSM-IV criteria were found in 61.6% of the group: 31.3% had neuroleptic-induced akathisia, 23.2% had neuroleptic-induced parkinsonism, and 32.3% had neuroleptic-induced tardive dyskinesia. Prevalence rates for akathisia and tardive dyskinesia were similar when either DSM-IV criteria or rating scale scores were used, but the prevalence rate for parkinsonism was much lower per DSM-IV criteria than according to rating scale score. CONCLUSIONS: Nearly two-thirds of chronic schizophrenia patients suffered from a neuroleptic-induced movement disorder. Globally, extrapyramidal adverse effects still impose a huge burden on the majority of neuroleptic-treated individuals with schizophrenia. The discrepancy between the standard identification methods for neuroleptic-induced movement disorder indicate the need for further research.
Neuroleptic-induced movement disorders constitute a worldwide problem in the treatment of schizophrenia because of the limited affordability of atypical antipsychotic drugs and because even the atypical antipsychotics can cause extrapyramidal symptoms (1, 2). Reported prevalences for neuroleptic-induced movement disorders are 29%–74% (3–6): 9%–35% for neuroleptic-induced akathisia (2–4, 6–9), 9%–36% for neuroleptic-induced parkinsonism (2–6, 9, 10), and 18%–46% for neuroleptic-induced tardive dyskinesia (2–6, 10–12).
The adverse motor effects have been identified with clinical rating scales primarily aimed at assessing symptom severity. The generalizability of previous results is reduced by the variety of rating scales, variability of threshold values used, and heterogeneity of study populations. To our knowledge, no previous studies have evaluated the prevalence of neuroleptic-induced movement disorders by using DSM-IV research criteria. According to the DSM-IV Task Force, these criteria should be tested and possibly refined in future studies.
Our aim was to assess the prevalence of neuroleptic-induced movement disorders in an Estonian institutionalized population by using various diagnostic criteria.
Method
We recruited 99 institutionalized adult patients with chronic schizophrenia from a state nursing home in central Estonia with 354 inhabitants, 172 of whom were diagnosed with schizophrenia. Inclusion criteria were DSM-IV diagnosis of schizophrenia or schizoaffective disorder, stable antipsychotic medication regimen (for at least 1 month), and age of 18–65 years. Diagnoses were made by a psychiatrist (S.J.) according to a semistructured interview that used DSM-IV criteria for schizophrenia and available medical records. Sixty-eight schizophrenia patients failed to meet inclusion criteria: 63 were older than 65, and five were not receiving antipsychotic medication. One patient was excluded because of severe somatic and neurological illness; there were four refusals.
Written informed consent was obtained from the subjects, and the study was approved by local ethics committee. Data were collected from Oct. 10, 2001, to March 27, 2002.
An experienced clinician (S.J.) assessed all subjects to identify neuroleptic-induced movement disorders (akathisia, parkinson-ism, or tardive dyskinesia) in accordance with DSM-IV. The temporal connection between a neuroleptic-induced movement disorder and a neuroleptic medication was established retrospectively by interview and medical records. The same clinician (S.J.) evaluated prevalence and severity of neuroleptic-induced movement disorders using the Barnes Rating Scale for Drug-Induced Akathisia (13), the Simpson-Angus Rating Scale (14), and the Abnormal Involuntary Movement Scale (AIMS) (15). The threshold value for akathisia was a Barnes scale total score of 2 or more (scale range=0–5) (13); for parkinsonism, the threshold value was a Simpson-Angus Rating Scale mean global score of 0.3 or more (scale range=0–4) (14). Cases of tardive dyskinesia were defined by the AIMS according to Schooler-Kane criteria, which require at least moderate dyskinetic movements in one body area or mild dyskinetic movements in two body areas (15, 16).
Student’s independent sample t tests and chi-square tests were used for comparison of the subjects with and without neuroleptic-induced movement disorders. The software used in analyses was SPSS 11.0 (17).
Results
Of the 99 participants, 45 (45.5%) were male, and 54 (54.5%) were female. The mean age was 49.7 years (SD=9.5). The mean length of continuous treatment in the hospital or nursing home was 13.6 years (SD=9.0). Seventy-nine (79.8%) patients were receiving conventional antipsychotics, and 20 (20.2%) were receiving clozapine (one was receiving clozapine combined with sulpiride). Sixteen (16.2%) patients were receiving combinations of typical antipsychotics (either predominantly low-dose [N=10] or predominantly high-dose [N=6] neuroleptic regimens), and 63 (63.6%) were receiving monotherapy (haloperidol: N=29; zuclopenthixol: N=28; perphenazine, chlorpromazine, or thioridazine: N=6). No new atypical antipsychotics were used. The mean daily chlorpromazine equivalent dose (18) was 328 mg (SD=221). Seven (7.1%) patients received benzodiazepines, 13 (13.1%) received tricyclic antidepressants, 15 (15.2%) received anticonvulsants, and one (1.1%) received lithium. Fourteen (14.2%) patients received the anticholinergic drug trihexyphenidyl. Sixty-four (64.7%) patients were smokers.
The total prevalence of neuroleptic-induced movement disorders according to DSM-IV criteria was 61.6%: 31.3% had neuroleptic-induced akathisia, 23.2% had neuroleptic-induced parkinsonism, and 32.3% had neuroleptic-induced tardive dyskinesia (see Figure 1 for comorbidity). The prevalence of neuroleptic-induced movement disorders in the patients receiving clozapine was significantly lower than in those receiving conventional antipsychotics (35.0% versus 68.4%, respectively) (χ2=7.51, df=1, p=0.006). The mean age of those with a neuroleptic-induced movement disorder (51.5 years [SD=8.5]) was significantly higher than that of patients with no neuroleptic-induced movement disorder (46.9 years [SD=10.4]) (t=–2.28, df=97, p<0.03). Among the neuroleptic-induced movement disorder subgroups, a significant difference in mean age was found only in the neuroleptic-induced parkinsonism group (53.7 [SD=7.3] versus 48.5 years [SD=9.8]; t=–2.77, df=59, p=0.008). Chi-square tests revealed no differences between patients with a neuroleptic-induced movement disorder and those without with respect to sex, smoking status, and use of antidepressants, anticonvulsants, benzodiazepines, or anticholinergic drugs. Student’s independent sample t tests also revealed no differences in length of institutionalization or antipsychotic dosage in chlorpromazine equivalents between patients with and those without a neuroleptic-induced movement disorder.
The prevalence of neuroleptic-induced akathisia according to global score on the Barnes scale was 27.3% (27 patients), which was 92% consistent with the prevalence according to DSM-IV criteria (i.e., of 99 patients classified as having or not having akathisia per the Barnes scale, the DSM-IV criteria similarly identified 91; sensitivity=93% [N=25 of 27 similarly classified as having akathisia], specificity=92% [N=66 of 72 similarly classified as not having akathisia]). The prevalence of neuroleptic-induced parkinsonism according to mean score on the Simpson-Angus Rating Scale was 72.7% (72 patients), which was 50.5% consistent with the prevalence according to DSM-IV criteria (i.e., of 99 patients classified as having or not having parkinsonism per the Simpson-Angus Rating Scale, the DSM-IV criteria similarly identified 50; sensitivity=32% [N=23 of 72 similarly classified as having parkinsonism], specificity=100% [N=27 of 27 similarly classified as not having parkinsonism]). The prevalence of neuroleptic-induced tardive dyskinesia according to Schooler-Kane criteria for the AIMS was 31.3% (31 patients), which was 99.0% consistent with the prevalence according to DSM-IV criteria (i.e., of 99 patients classified as having or not having tardive dyskinesia per the AIMS, the DSM-IV criteria similarly identified 98; sensitivity=100% [N=31 of 31 similarly classified as having tardive dyskinesia], specificity=99% [N=67 of 68 similarly classified as not having tardive dyskinesia]).
Discussion
We examined the prevalence of three DSM-IV-defined neuroleptic-induced movement disorders (akathisia, parkinsonism, and tardive dyskinesia) in a group of chronic schizophrenia inpatients receiving treatment that represented standard treatment in transitional and developing economies, where most of the world’s psychiatric patients live. Since atypical antipsychotics are unavailable, cheaper means of reducing neuroleptic-induced movement disorders, such as lowering antipsychotic dosage and using clozapine or anticholinergics, are employed. The clinical practice in the study population was first to lower the antipsychotic dose and then, if necessary, to add anticholinergics.
To our knowledge, this is the first prevalence study to use DSM-IV criteria for identification of neuroleptic-induced movement disorders and one of the few studies that estimates the three neuroleptic-induced movement disorders simultaneously. We found that nearly two-thirds of the patients suffer from a neuroleptic-induced movement disorder despite the relatively low antipsychotic doses and the use of anticholinergics. Since the mean antipsychotic dose in the study population was relatively low, we suggest that the prevalence of neuroleptic-induced movement disorders probably is higher with commonly used antipsychotic doses.
The sample was gathered from the largest nursing home in Estonia; approximately 13% of all institutionalized psychiatric patients in Estonia are treated there. The sample is representative of schizophrenia patients in the nursing home, since 91% of all patients 18–65 years old participated. The medication of the sample represents typical medication available in state institutions for schizophrenia patients in Estonia (19).
The prevalence rates of neuroleptic-induced movement disorders defined by DSM-IV criteria compared with those obtained by rating scales were similar for akathisia and tardive dyskinesia but very different for parkinsonism. The Simpson-Angus Rating Scale-based case definition for neuroleptic-induced parkinsonism (threshold of >0.3) yielded a prevalence of more than 70%, whereas the DSM-IV-defined prevalence was less than 30%. This may be caused for example by overrepresentation of rigidity items in Simpson-Angus Rating Scale (2) or by too low of a Simpson-Angus Rating Scale score threshold value in this population, or alternatively, by low sensitivity of DSM-IV neuroleptic-induced parkinsonism criteria. The differences in sensitivity between Simpson-Angus Rating Scale and DSM-IV case definition of neuroleptic-induced parkinsonism raise the question of whether Simpson-Angus Rating Scale item selection should be reestablished, score threshold redefined, or whether refinements of neuroleptic-induced parkinsonism criteria in upcoming versions of DSM should be undertaken. Receiver operating characteristic curve analysis using the Simpson-Angus Rating Scale and DSM-IV criteria yielded an optimal threshold value of 0.92 instead of 0.3 for the Simpson-Angus Rating Scale.
The main limitations of this study are connected to the nature of neuroleptic-induced movement disorder: 1) co-occurrence of spontaneous movement disorders, commonly detected in schizophrenic populations (20), could not be excluded, and 2) the DSM-IV diagnoses of neuroleptic-induced movement disorder in this study, as in clinical settings in general, are partially based on retrospective information.
In conclusion, nearly two-thirds of institutionalized schizophrenia patients were shown to suffer from adverse effects of conventional antipsychotics. Since the costs of atypical antipsychotics are too high for most patients of the world, other ways of coping with neuroleptic-induced movement disorder must be explored.
Received Jan. 15, 2003; revision received July 1, 2003; accepted July 10, 2003. From Voisiku Nursing Home, Poltsamaa County, Estonia. Address reprint requests to Dr. Holi, Department of Psychiatry, Lapinlahti Hospital, P.O. Box 320, 00029 HUS, Finland. Supported by the Finnish Medical Society (Finska Läkaresällskapet) and the Eli Lilly (Suisse) S.A. Estonian Affiliate.
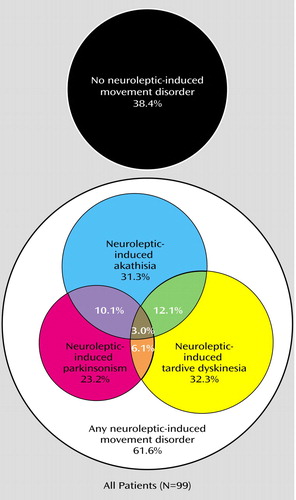
Figure 1. Neuroleptic-Induced Movement Disorders in 99 Estonian Inpatients With Chronic Schizophrenia Treated With Conventional Antipsychotics or Clozapine
1. Tarsy D, Baldessarini RJ, Tarazi FI: Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002; 16:23–24Crossref, Medline, Google Scholar
2. Cunningham Owens DG: A Guide to the Extrapyramidal Side-Effects of Antipsychotic Drugs. Cambridge, UK, Cambridge University Press, 1999Google Scholar
3. Muscettola G, Barbato G, Pampallona S, Cashello M, Bollini P: Extrapyramidal syndromes in neuroleptic-treated patients: prevalence, risk factors, and association with tardive dyskinesia. J Clin Psychopharmacol 1999; 19:203–208Crossref, Medline, Google Scholar
4. Van Harten PN, Matroos GE, Hoek HW, Kahn RS: The prevalence of tardive dystonia, tardive dyskinesia, parkinsonism and akathisia: the Curacao Extrapyramidal Syndromes Study, I. Schizophr Res 1996; 19:195–203Crossref, Medline, Google Scholar
5. Modestin J, Stephan PL, Erni T, Umari T: Prevalence of extrapyramidal syndromes in psychiatric inpatients and the relationship of clozapine treatment to tardive dyskinesia. Schizophr Res 2000; 42:223–230Crossref, Medline, Google Scholar
6. McCreadie RG, Robertson LJ, Wiles DH: The Nithsdale Schizophrenia Surveys. IX: akathisia, parkinsonism, tardive dyskinesia and plasma neuroleptic levels. Br J Psychiatry 1992; 160:793–799Crossref, Medline, Google Scholar
7. Halstead S, Barnes T, Speller J: Akathisia: prevalence and associated dysphoria in an in-patient population with chronic schizophrenia. Br J Psychiatry 1994; 164:177–183Crossref, Medline, Google Scholar
8. Sachdev P: The epidemiology of drug-induced akathisia. Schizophr Bull 1995; 21:431–461Crossref, Medline, Google Scholar
9. Casey DE: Extrapyramidal syndromes: epidemiology, pathophysiology and the diagnostic dilemma. CNS Drugs 1996; 5:1–12Crossref, Google Scholar
10. McCreadie RG: The Nithsdale Schizophrenia Survey: II: abnormal movements. Br J Psychiatry 1982; 140:587–590Crossref, Medline, Google Scholar
11. Jeste DV, Wyatt RJ: Changing epidemiology of tardive dyskinesia: an overview. Am J Psychiatry 1981; 138:297–309Link, Google Scholar
12. Kane JM, Smith JM: Tardive dyskinesia: prevalence and risk factors 1959 to 1979. Arch Gen Psychiatry 1982; 39:473–481Crossref, Medline, Google Scholar
13. Barnes TRE: A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
14. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
15. Munetz M, Benjamin S: How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry 1988; 39:1172–1177Abstract, Google Scholar
16. Schooler NR, Kane JM: Research diagnosis for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
17. SPSS 11.0 for Windows. Chicago, SPSS, 2001Google Scholar
18. Bazire S: Psychotropic Drug Directory. Dinton, UK, Quay Books, 2000Google Scholar
19. Jaanson P: Skisofreenia haiglaväline ravi. Uurimus küsimustiku põhjal (Description of the treatment of schizophrenia spectrum psychosis outpatients using a questionnaire). Eesti Arst 2002; 81:333–337Google Scholar
20. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar



