Anatomic Brain Abnormalities in Monozygotic Twins Discordant for Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: To examine brain-behavior relationships in attention deficit hyperactivity disorder (ADHD), the authors obtained magnetic resonance imaging (MRI) scans of monozygotic twins discordant for ADHD. METHOD: National recruitment was followed by in-person assessment. MRI scans were measured algorithmically for nine pairs of monozygotic twins discordant for ADHD. RESULTS: The affected twins had significantly smaller caudate volumes (mean difference=–0.56 ml, CI=–0.92 to –0.21) than their unaffected co-twins. CONCLUSIONS: These results provide further support for striatal models of ADHD pathophysiology.
Beyond informing estimates of heritability, studies of monozygotic twins discordant for a given disorder can illuminate relationships between brain structures and functions and can identify effects of environmental factors and interactions, since genetic factors are controlled. Following the approach of Hyde et al. (1), we recruited monozygotic twins discordant for attention deficit hyperactivity disorder (ADHD) for an anatomic brain magnetic resonance imaging (MRI) study. Since monozygotic twins are genetically identical, we anticipated that this study would highlight brain regions linked to ADHD that are particularly susceptible to environmental factors.
Method
Twin pairs discordant for ADHD, as defined by DSM-IV, were recruited nationally as described elsewhere in detail (2). Exclusion criteria were a full-scale IQ less than 80, medical or neurological disorders determined from examination or history, and other primary axis I psychiatric disorders. The study was conducted at the Child Psychiatry Branch of the National Institute of Mental Health (NIMH) in Bethesda, Md., between 1996 and 2001. The NIMH institutional review board approved the research protocol, and written informed consent and assent were obtained from parents and children, respectively.
All subjects were studied on the same 1.5-T GE Signa scanner (GE Medical Systems, Milwaukee). T1-weighted images with contiguous 1.5-mm axial and 2.0-mm coronal slices were obtained by using three-dimensional spoiled gradient recalled echo in the steady state with echo time=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=256×192, number of excitations=1, and field of view=24 cm. Head placement was standardized as previously described (3). T2-weighted images were obtained for evaluation by a clinical neuroradiologist. Quantification of MRI images was performed by means of a highly reliable fully automated process that determines gray and white matter volumes for the frontal, temporal, parietal, and occipital lobes as well as caudate and cerebellum volumes, as described elsewhere (4). The minimum and maximum Talairach (x, y, z) coordinates that defined the left caudate were –20, –28, –12 and –3, 23, 27, respectively; the corresponding values for the right caudate were 5, –24, –10 and 20, 23, 26. Although the algorithm provided volumes for the right and left caudate, these did not accord well with values determined by hand tracing; the intraclass correlation coefficient (ICC) was <0.40. By contrast, the total caudate volumes were highly concordant with values derived by hand tracing of the caudate head plus body (N=17, ICC=0.91).
Paired t tests contrasting the affected and unaffected co-twins and Pearson correlations representing twin-twin differences in brain volumes and symptom severity ratings were performed by using SPSS 10.0 for Windows (5). Two-tailed significance levels were used, and the criterion for significance was p≤0.05.
Results
The study began with 25 twin pairs, who completed 3-day in-person assessments that included anatomic MRI scans. Eleven pairs were excluded because the putatively unaffected co-twin had six or more ADHD symptoms of inattention or hyperactivity/impulsivity (without necessarily meeting the impairment criteria); four pairs were excluded for comorbid conditions, including Tourette’s disorder, obsessive-compulsive disorder, and bipolar disorder; and one pair was excluded because of motion artifact. The resulting nine discordant twin pairs included one set of girls. The average age was 11.0 years (SD=3.2, range=5.6–15.6). All of the affected male twins had been previously treated with stimulants, and one of the unaffected twins had received a 1-month empiric trial of methylphenidate; both of the girls were untreated. The duration of stimulant treatment ranged from 1 to 36 months (mean=20.8, SD=10.7).
One affected subject was found to have an unsuspected focal abnormality in the left caudate, left putamen, and adjacent white matter, consistent with a cerebrovascular accident at some unknown time (Figure 1). We evaluated this subject and his twin brother at age 15. Symptoms suggestive of ADHD were first noted at age 3. Structured psychiatric interviews, psychoeducational testing, and parent and teacher ratings confirmed combined-type ADHD, and he had a full-scale WISC-III score of 87. The same assessment of his co-twin revealed no psychiatric or neurologic diagnoses and a full-scale IQ of 95. Both twins were born at 38–39 weeks by uneventful cesarean section. Both twins received physical therapy and speech therapy from ages 3 to 5 years. Their developmental milestones were mildly delayed and included walking at 17 months and use of only simple words until age 3. Their family history revealed possible ADHD in their father and maternal grandfather but no history of treated or formally diagnosed cases.
Overall, the affected monozygotic twins differed significantly from their unaffected co-twins in total caudate volume (mean difference=–0.56 ml, 95% CI=–0.92 to –0.21) (t=3.67, df=8, p=0.006) even when the previously described twin pair was excluded from the analysis (t=3.50, df=7, p=0.01) (Figure 2). Twin-twin differences in caudate volume did not correlate significantly with differences in the teacher or parent Conners rating of hyperactivity severity, with differences in birth weight, or with lifetime duration of stimulant exposure. None of the other twin-twin brain differences (frontal, parietal, temporal, occipital gray and white matter, cerebellum) approached significance (p>0.14).
Discussion
Discordant monozygotic twins provide optimal subjects for elucidating environmental influences on brain development in patients with neuropsychiatric conditions (6). Our findings provide additional support for current models of the pathophysiology of ADHD, which implicate prefrontal-striatal circuitry. Unilateral caudate infarcts have been associated with agitation, hyperactivity, disinhibition, and inattention (7, 8). The size and location of the lesion in one boy with ADHD and the absence of ADHD symptoms in his co-twin suggest that this abnormality contributed to his ADHD. These data are consistent with findings that lesions of the caudate and putamen after closed-head injury are statistically associated with the emergence of secondary symptoms of ADHD (9) and that focal stroke lesions of the putamen in childhood are associated at a trend level (p=0.10) with ADHD symptoms (10).
Since most of the twins referred to us turned out to be concordant for ADHD and were thus excluded from this study (2), we were unable to recruit a group of discordant twins who had not been previously treated with medications. Previously we failed to detect significant effects of medication on regional brain volumes in 49 previously unmedicated singletons with ADHD compared with 103 medicated patients and 139 comparison subjects (4). Although we cannot rule out medication effects in the present study group, we conclude that the association between smaller caudate volume and ADHD is most consistent with a selective vulnerability of the striatum (11, 12) to adverse prenatal environmental factors, such as hypoxia. However, our small group limits our ability to make inferences regarding specific causative factors or to attach much weight to negative findings. Elsewhere (2) we reported that fathers of twins discordant for ADHD rated themselves as significantly lower on the Wender Utah Rating Scale of childhood ADHD symptoms than did fathers of singletons affected with ADHD and that the rate of breech presentation was significantly higher in affected twins than in affected singletons. Those data suggest that discordant twins represent an enriched sample of nongenetic phenocopies that can also inform our understanding of the various causal pathways that can lead to ADHD. Finally, these cases suggest that in the unlikely event of monozygotic twins discordant for ADHD or related conditions in which environmental influences are anticipated, imaging with MRI may yield clinically relevant and scientifically useful information. In the vast majority of ADHD cases, however, neuroimaging is not clinically indicated.
Received Aug. 6, 2002; revision received March 11, 2003; accepted March 19, 2003. From the Child Psychiatry Branch, NIMH, Bethesda, Md. Address reprint requests to Dr. Castellanos, Child Study Center, New York University School of Medicine, Rm. 204, 577 First Ave., New York, NY 10016; [email protected] (e-mail). The authors thank Children and Adults With Attention-Deficit/Hyperactivity Disorder, the National Mothers of Twins Clubs, and Judy Silberg, Ph.D., from the Virginia Twin-Family Study of Adolescent Behavioral Development for help in recruitment for this study, and they thank the children and families who participated.
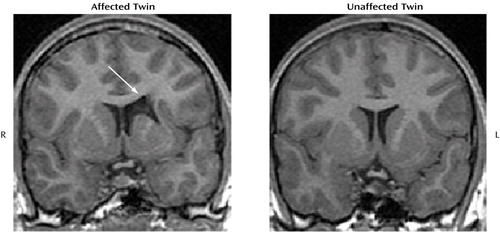
Figure 1. Coronal T1-Weighted MRI Brain Images of a 15-Year-Old Boy With ADHD and a Brain Lesion and His Unaffected Monozygotic Twina
aThe focal abnormality was in the left caudate, putamen, and adjacent white matter (see arrow).
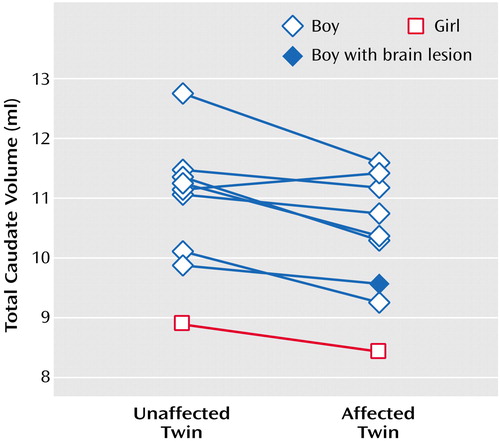
Figure 2. Total Caudate Volumes of Monozygotic Twins Discordant for ADHDa
aBoth of the female twins were unmedicated.
1. Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR: Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twins. Neurology 1995; 45:1176–1182Crossref, Medline, Google Scholar
2. Sharp WS, Gottesman RF, Greenstein DK, Ebens CL, Rapoport JL, Castellanos FX: Monozygotic twins discordant for attention-deficit/hyperactivity disorder: ascertainment and clinical characteristics. J Am Acad Child Adolesc Psychiatry 2003; 42:93–97Crossref, Medline, Google Scholar
3. Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal J, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL: Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:289–295Crossref, Medline, Google Scholar
4. Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL: Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002; 288:1740–1748Crossref, Medline, Google Scholar
5. SPSS I: SPSS Base 10.0 User’s Guide. Chicago, SPSS, 1999Google Scholar
6. Weinberger DR, Zigun JR, Bartley AJ, Jones DW, Torrey EF: Anatomical abnormalities in the brains of monozygotic twins discordant and concordant for schizophrenia. Clin Neuropharmacol 1992; 15(suppl 1, part A):122A-123AGoogle Scholar
7. Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, Gorelick PB, Helgason C, Hier DB: Caudate infarcts. Arch Neurol 1990; 47:133–143Crossref, Medline, Google Scholar
8. Mendez MF, Adams NL, Lewandowski KS: Neurobehavioral changes associated with caudate lesions. Neurology 1989; 39:349–354Crossref, Medline, Google Scholar
9. Herskovits EH, Megalooikonomou V, Davatzikos C, Chen A, Bryan RN, Gerring JP: Is the spatial distribution of brain lesions associated with closed-head injury predictive of subsequent development of attention-deficit/hyperactivity disorder? analysis with brain-image database. Radiology 1999; 213:389–394Crossref, Medline, Google Scholar
10. Max JE, Fox PT, Lancaster JL, Kochunov P, Mathews K, Manes FF, Robertson BA, Arndt S, Robin DA, Lansing AE: Putamen lesions and the development of attention-deficit/hyperactivity symptomatology. J Am Acad Child Adolesc Psychiatry 2002; 41:563–571Crossref, Medline, Google Scholar
11. Wallays C, Feve A, Boudghene F, Fenelon G, Guillard A, Bigot JM: [Hypoxic cerebral lesions: X-ray computed tomography and MRI aspects: apropos of 20 cases: selective vulnerability of the striatopallidum]. J Neuroradiol 1995; 22:77–85 (French)Medline, Google Scholar
12. Binienda Z, Holson RR, Chen FX, Oriaku E, Kim CS, Flynn TJ, Slikker W Jr, Paule MG, Feuers RJ, Ferguson SA: Effects of ischemia-hypoxia induced by interruption of uterine blood flow on fetal rat liver and brain enzyme activities and offspring behavior. Int J Dev Neurosci 1996; 14:399–408Crossref, Medline, Google Scholar



