Protective Association of Genetic Variation in Alcohol Dehydrogenase With Alcohol Dependence in Native American Mission Indians
Abstract
OBJECTIVE: Two alcohol dehydrogenase genes (ADH2 and ADH3 on chromosome 4) and one aldehyde dehydrogenase gene (ALDH2 on chromosome 12) exhibit functional polymorphisms. The goal of this study was to determine whether any associations exist between the ADH2, ADH3, and ALDH2 polymorphisms and alcohol dependence in a group of Native Americans. An additional goal was to determine if any associations exist between these polymorphisms and the endophenotype, maximum number of drinks ever consumed in a 24-hour period. METHOD: Mission Indian adults (N=340) were recruited for participation from reservations in southern California. Each participant completed an interview with the Semi-Structured Assessment for the Genetics of Alcoholism. A blood sample was collected from each participant for genotyping at the ALDH2, ADH2, and ADH3 loci. RESULTS: Sixty percent of all participants (72% of men and 53% of women) met lifetime DSM-III-R criteria for alcohol dependence. A significant difference in the ADH2 allele distributions was found between alcohol-dependent and non-alcohol-dependent participants. Those with alcohol dependence were significantly less likely to have the ADH2*3 allele (odds ratio=0.28) and significantly more likely to have the ADH2*1 allele (odds ratio=2.00) than those who were not alcohol dependent. Individuals with ADH2*3 reported a lower number of maximum drinks ever consumed in a 24-hour period, compared to those without this allele. CONCLUSIONS: These results are consistent with genetic linkage studies showing protective associations for alcohol dependence and related behavior on chromosome 4 and suggest that ADH2 polymorphisms may account for these findings. These results also highlight the utility of evaluating protective factors in populations with high rates of alcohol dependence.
Alcohol abuse and dependence (alcoholism) are multifactorial, polygenic disorders involving complex gene-gene and gene-environment interactions. To date, the genes with the strongest associations with alcoholism are those that encode the major enzymes involved in alcohol metabolism, alcohol dehydrogenase and aldehyde dehydrogenase. Two alcohol dehydrogenase genes (ADH2 and ADH3 on chromosome 4) and one aldehyde dehydrogenase gene (ALDH2 on chromosome 12) exhibit functional polymorphisms that influence the rates of conversion of alcohol to acetaldehyde and acetaldehyde to acetate (1, 2). In humans, three polymorphisms related to alcohol conversion have been identified for ADH2 (*1, *2, *3), two for ADH3 (*1, *2), and two for ALDH2 (*1, *2).
The ALDH2*2 polymorphism, which is prevalent in Asian populations but extremely rare in non-Asians, has the strongest protective association with alcohol dependence. Asians who are homozygous for ALDH2*2 have almost zero risk, whereas heterozygotes are about one-third as likely to be alcoholic, compared to those without this allele (3–13). According to the main hypothesis about the mechanism underlying this association, the isoenzyme encoded by the ALDH2*2 allele leads to impaired conversion of acetaldehyde to acetate, causing elevated levels of acetaldehyde (14–19), greater sensitivity to alcohol (16, 20, 21), and lower levels of alcohol consumption (10, 22–25).
The ADH2*2 polymorphism has also has been associated with lower rates of alcoholism. This relationship has been found in Asians, after the analyses controlled for the presence of ALDH2*2 (4–13, 26, 27), as well as in Caucasians (28, 29). ADH2*2 is highly prevalent among Asians and infrequent in most Caucasians except for individuals of Jewish descent (30, 31). A meta-analysis indicated that individuals with ADH2*2 are about one-third as likely to be alcoholic, compared to those without this allele (32). A less common polymorphism of ADH2, the ADH2*3 allele, is prevalent in African Americans (33, 34) and has also been identified, although in low prevalence, among South Africans of mixed ancestry (35) and Native American Mission Indians of mixed but no known African ancestry (36). ADH2*3 has been associated with a negative family history of alcoholism in a pilot study of African Americans, but no associations for alcohol abuse and dependence were found, most likely because of the small number of subjects in the study and the study’s low statistical power (37).
The ADH3*1 polymorphism is prevalent in Asians, Caucasians, and African Americans. A few studies have reported that ADH3*1 might also be associated with lower risk for alcohol dependence (6, 7, 11). Recent investigations, however, have found that the observed differences in the allele distribution of ADH3 between alcoholics and comparison subjects can be accounted for by a linkage disequilibrium between ADH3*1 and ADH2*2 (4, 28, 38). The ADH2 and ADH3 genes are located in tandem on chromosome 4 (39, 40), and polymorphisms at these two loci do not occur independently. Thus, the relationships of ADH2*2 and ADH3*1 with lower risk for alcohol dependence appear to be associated.
On the basis of the kinetic properties of the alcohol dehydrogenase gene polymorphisms (1, 2), the hypothesized mechanism underlying their associations with alcoholism has been that isoenzymes encoded by the ADH2*2, ADH2*3, and ADH3*1 alleles should lead to faster metabolism of alcohol (31, 41, 42) and therefore a more rapid production of acetaldehyde compared with isoenzymes encoded by ADH2*1 and ADH3*2. This in turn should lead to increased alcohol sensitivity and lower levels of alcohol consumption in individuals with the ADH2*2, ADH2*3, or ADH3*1 alleles (22, 25, 29–31, 43).
The present report is part of a larger study assessing risk factors for alcoholism in Mission Indians, a population selected because of their high rate of alcohol-related problems. The goal of this investigation was to determine if any associations existed between the ALDH2, ADH2, and ADH3 polymorphisms and alcohol dependence in this tribe of Native Americans. An additional goal was to determine if these gene polymorphisms relate to the maximum number of drinks ever consumed in a 24-hour period. This endophenotype is associated with a diagnosis of alcohol dependence and was chosen for analysis because a genome screen for this variable found evidence of linkage on chromosome 4 in the area of the alcohol dehydrogenase gene cluster (44).
Method
A total of 340 (213 female participants) Mission Indian adults between the ages of 18 and 73 years who were of mixed heritage but were at least one-sixteenth Native American were recruited from reservations in southern California. An accurate assessment of each participant’s ancestry was not available; however, it was possible to categorize a subgroup (N=246, 72%) according to whether their heritage was <50% (N=123) or ≥50% (N=123) Native American.
Participants were recruited by means of flyers and by word-of-mouth within the Mission Indian community, which consists of approximately 3,000 individuals living on six geographically contiguous reservations. After complete description of the study to subjects, written informed consent was obtained. Each participant completed an interview with the Semi-Structured Assessment for the Genetics of Alcoholism (45–47), which was used to make a lifetime diagnosis of alcohol dependence according to DSM-III-R criteria and to assess the maximum number of drinks ever consumed in a 24-hour period. A drink was defined as approximately 9 g of absolute alcohol (e.g., 12 ounces of beer, 4 ounces of wine, or a single shot of hard alcohol), and a list of alcohol equivalents was provided to each participant.
In addition, a blood sample was collected from each participant. Samples were sent to Indiana University for genotyping at the ALDH2, ADH2, and ADH3 loci by using polymerase chain reaction of DNA and allele-specific oligonucleotide probes (48, 49). Logistic regression analysis and case-control odds ratio calculations were used to examine associations between ADH2 and ADH3 allele distributions and alcohol dependence. Multiple regression analysis was used to examine associations between ADH2 and ADH3 alleles and the continuous variable, maximum number of drinks. For the logistic regression and multiple regression analyses, gender was first entered as a covariate. Three alleles (ADH2*2, ADH2*3, and ADH3*1) were coded 0, 1, or 2, respectively, and next entered simultaneously into each analysis. The reference group, therefore, consisted of subjects with the ADH2*1/*1 and ADH3*2/*2 genotypes. These codings assume linear relationships between each allele and alcohol-related behavior, which is supported by previous association studies of these genes and their hypothesized mechanisms of action, although other models are also possible (4, 50).
Results
A total of 203 participants (60%) met the criteria for a lifetime DSM-III-R diagnosis of alcohol dependence. Men were significantly more likely to be alcohol dependent (N=91, 72%) than women (N=112, 53%) (χ2=12.03, df=1, p≤0.001).
ALDH2 genotyping indicated that all participants had the ALDH2*1/*1 genotype, thus the ALDH2*1 allele frequency was 1.0. ADH2 genotyping indicated that 299 subjects had the ADH2*1/*1 genotype, 20 had ADH2*1/*2, two had ADH2*2/*2, 17 had ADH2*1/*3, and two had ADH2*2/*3. Thus, the ADH2*1 allele frequency was 0.93, the ADH2*2 allele frequency was 0.04, and the ADH2*3 allele frequency was 0.03. ADH3 genotyping indicated that 157 subjects had the ADH3*1/*1 genotype, 131 had ADH3*1/*2, and 52 had ADH3*2/*2. Thus, the ADH3*1 allele frequency was 0.65 and the ADH3*2 allele frequency was 0.35. Men and women did not differ significantly in their allele distributions of ADH2 (χ2=0.09, df=2, p=0.96) or ADH3 (χ2=0.93, df=1, p=0.34).
After covarying for gender (beta=–0.85, df=1, p≤0.0001), logistic regression analysis revealed a significant association between ADH2*3 and alcohol dependence (beta=–1.23, df=1, p<0.02), but ADH2*2 (beta=–0.28, df=1, p=0.48) and ADH3*1 (beta=–0.17, df=1, p=0.30) were not significantly associated with alcohol dependence. Case-control odds ratio calculations, which controlled for gender, indicated that participants with alcohol dependence were significantly less likely to have the ADH2*3 allele (odds ratio=0.28, 95% confidence interval [CI]=0.10–0.77, p<0.02) and significantly more likely to have the ADH2*1 allele (odds ratio=2.00, 95% CI=1.10–3.65, p<0.03), compared to those who were not alcohol dependent. No significant differences were found between alcohol-dependent and non-alcohol-dependent participants in the allele distributions of ADH2*2 (odds ratio=0.70, 95% CI=0.33–1.49, p=0.35), ADH3*1 (odds ratio=0.81, 95% CI=0.60–1.10, p=0.17), or ADH3*2 (odds ratio=1.24, 95% CI=0.91–1.70, p=0.17), after control for gender. Table 1 shows the ADH2 and ADH3 genotype and allele distributions grouped according to lifetime diagnosis of alcohol dependence.
For the variable maximum number of drinks ever consumed in a 24-hour period, data were missing for two non-alcohol-dependent male subjects, one with the ADH2*1/*1 and ADH3*1/*2 genotypes and one with the ADH2*2/*2 and ADH3*1/*1 genotypes, and these subjects were excluded from the analyses. The remaining 338 participants reported consuming an average maximum of 26.9 drinks (SD=23.2, range=1 to 192). Analyses revealed that this variable was positively skewed. A natural logarithm (log) transformation was used to normalize the distribution for all analyses, but values from untransformed data are presented. Consistent with previous research (44), men reported a significantly higher maximum number of drinks (mean=34.1, SD=26.9, N=125) than women (mean=22.7, SD=19.6, N=213) (F=20.03, df=1, 336, p≤0.0001), and participants with a diagnosis of alcohol dependence reported a significantly higher maximum number of drinks (mean=34.0, SD=24.6, N=203) than those without alcohol dependence (mean=16.2, SD=15.8, N=135) (F=125.86, df=1, 336, p≤0.0001).
After covarying for gender (F=19.26, df=1, 333, p≤0.0001), multiple regression analysis revealed a significant association between ADH2*3 and the log of maximum number of drinks (F=5.48, df=1, 331, p=0.003), but ADH2*2 (F=0.18, df=1, 333, p=0.59) and ADH3*1 (F=0.17, df=1, 333, p=0.60) were not significantly associated with the log of maximum number of drinks. Figure 1 displays the maximum number of drinks ever consumed in a 24-hour period by subjects grouped according to ADH2 genotype. Participants with an ADH2*3 allele reported a lower maximum number of drinks (mean=15.4, SD=10.2, N=19), compared to those without an ADH2*3 allele (mean=27.6, SD=23.6, N=319).
Because of the potential for population stratification, additional analyses were conducted on data for the subgroup of participants who were categorized as having either <50% (N=123) or ≥50% (N=123) Native American heritage. No significant associations were found between Native American heritage and the allele distributions of ADH2 (χ2=3.27, df=2, p=0.20) or ADH3 (χ2=0.01, df=1, p=0.92). Mission Indians with ≥50% Native American heritage were significantly more likely to be alcohol dependent than those with <50% Native American heritage (70% versus 54%; χ2=6.89, df=1, p=0.009). No significant association was found between Native American heritage and the log of the maximum number of drinks ever consumed (F=0.29, df=1, 242, p=0.59); the mean maximum number of drinks was 29.9 (SD=25.2) for those with ≥50% Native American heritage and 28.2 (SD=25.2) for those with <50% Native American heritage.
Discussion
The Mission Indians evaluated in this study had a high rate of alcohol dependence in both men (72%) and women (53%), which is consistent with investigations of other Native American tribes (51–53). This study also replicated previous work from our laboratory that found a low prevalence of ADH2*2 and ADH2*3, two alleles that have been associated with alcohol-related behavior in other populations. In a prior report on 95 Mission Indian men with at least one-eighth Native American ancestry, the prevalence of ADH2*2 was 0.01 and the prevalence of ADH2*3 was 0.06 (36), compared with prevalences of 0.04 and 0.03, respectively, for these alleles in the current study’s larger group of participants with a greater degree of admixture.
A protective association of ADH2*3 with alcohol dependence was also found in this study. Mission Indians with an ADH2*3 allele were about one-third less likely than those without this allele to have a lifetime diagnosis of alcohol dependence (odds ratio=0.28). This reduced risk for alcohol dependence is approximately equal to that associated with having either one ALDH2*2 or one ADH2*2 allele in previous studies of other ethnic groups (3–13, 26–29, 32). In addition, individuals with ADH2*3 reported a maximum number of drinks ever consumed in a 24-hour period that was almost half that reported by those without this allele. As well as being related to a diagnosis of alcohol dependence, this endophenotype has been found to have a heritability of approximately 50% in a study of adult Australian twins (A.C. Heath, personal communication, 2002) and was related to the ALDH2*2 allele in a study of Asian Americans (54). The present findings suggest that the investigation of genetic associations with the presence or absence of disorders can be enhanced by also evaluating related behaviors measured as continuous variables, where relationships may be more easily detected.
Moreover, these results are consistent with genetic linkage studies showing protective associations for alcohol-related behavior on chromosome 4. Genome scans of family members from the Collaborative Study on the Genetics of Alcoholism found evidence suggestive of protection against alcohol dependence (55), as well as a lower number of maximum drinks ever consumed in a 24-hour period (44), on the area of chromosome 4 that includes the ADH2 and ADH3 genes. ADH3 genotyping indicated that the maximum number of drinks was not associated with variation at this locus (44), but ADH2 polymorphisms were not evaluated. A similar association with alcohol dependence on chromosome 4 was reported from a genome screen of Native Americans (56). Results from the present study suggest ADH2 polymorphisms may account for these findings.
ADH2*2, ADH3*1, and ADH3*2 were not associated with alcohol-related behavior in this study. Although Mission Indians with the ADH2*2 allele were less likely to be alcohol dependent and reported a lower maximum number of drinks in a 24-hour period (Figure 1) than individuals with the ADH2*1/*1 genotype, the differences were not statistically significant. This null finding may be related to the low prevalence of the ADH2*2 allele in this population, although a similar ADH2*2 prevalence is found in non-Jewish Caucasians, for whom a protective association with alcohol dependence has been observed (28, 29).
Studies of children with fetal alcohol syndrome have found protective associations of ADH2*2 in South Africans of mixed ancestry (35) and of ADH2*3 in African Americans (34, 43, 57). It has been hypothesized that faster alcohol metabolism leading to a more rapid production of acetaldehyde, increased sensitivity to alcohol, and lower levels of alcohol consumption is the mechanism by which these alleles protect against both fetal alcohol syndrome and alcohol dependence. ADH2*3 has been associated with faster alcohol metabolism in African Americans (33) and a nonsignificant trend was observed in a small group of Mission Indian men (42), but other studies have not found significant associations between ADH2*2 and alcohol metabolism (19, 58) or alcohol sensitivity (59). In addition, there is no direct evidence that faster alcohol metabolism leads to greater production of acetaldehyde. Results from this study support the hypothesis that the protective association between ADH2*3 and alcohol dependence is mediated, in part, by lower levels of alcohol consumption. Additional research is needed to determine if ADH2*3 is also associated with elevated levels of acetaldehyde or greater sensitivity to alcohol.
It is also possible that the association of ADH2*3 with alcohol-related behavior in this group of subjects may not be a genuine causal effect, but may be related to either linkage disequilibrium with a nearby locus or population stratification because of racial admixture. Although an attempt was made to address concerns about heterogeneity, complete data on all participants’ Native American heritage were not available. Therefore, it will be important to determine the generalizability of the present findings for other Native American and non–Native American populations, particularly for African Americans, among whom ADH2*3 is prevalent.
In conclusion, this study provides evidence that the ADH2*3 allele is associated with lower rates of alcohol dependence and lower rates of heavy drinking. These findings also highlight the utility of evaluating protective factors in populations with high rates of alcohol dependence. Although the prevalence of ADH2*3 was low in this group of Mission Indians, the high prevalence of alcohol dependence and large variability in drinking behavior in this Native American tribe made it possible to detect significant associations between this genetic polymorphism and alcohol-related behavior.
 |
Received Feb. 20, 2002; revisions received May 31 and Sept. 3, 2002; accepted Sept. 10, 2002. From the Department of Neuropharmacology, Scripps Research Institute, San Diego; the Department of Psychiatry, University of California, San Diego; the Psychology Service, Veterans Affairs San Diego Healthcare System; and the Departments of Medicine and Pharmacology, Indiana University, Indianapolis. Address reprint requests to Dr. Wall, Psychology Service (116B), Veterans Affairs San Diego Healthcare System, 3350 La Jolla Village Dr., San Diego, CA 92161; [email protected] (e-mail). Supported in part by NIH grants AA-10201, AA-00269, AA-07611, and RR-00833 and by the NIH National Center on Minority Health and Health Disparities. The authors thank Erica Bisson, Consuelo Garcia-Andrade, David Gilder, Lilach Harris, Philip Lau, Susan Lopez, Evelyn Phillips, Judith Shester, Melanie Vallone, and Vincent Wong for assistance with this study.
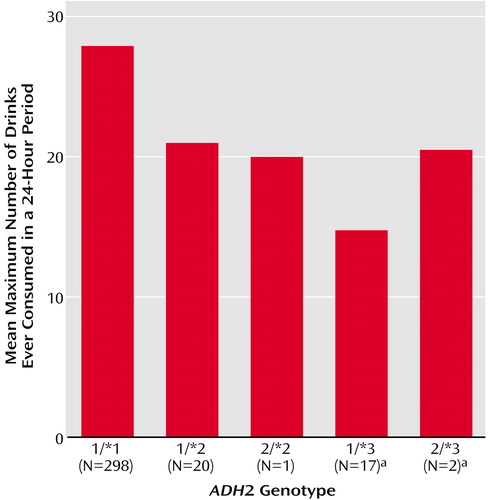
Figure 1. Maximum Number of Drinks Ever Consumed in a 24-Hour Period for 338 Native American Mission Indians Grouped According to ADH2 Genotype
aSignificant difference between subjects with (N=19) and without (N=319) the ADH2*3 allele (F=5.45, df=1, 331, p=0.003), after control for gender.
1. Bosron WF, Ehrig T, Li T-K: Genetic factors in alcohol metabolism and alcoholism. Semin Liver Dis 1993; 13:126-135Crossref, Medline, Google Scholar
2. Crabb DW, Dipple KM, Thomasson HR: Alcohol sensitivity, alcohol metabolism, risk for alcoholism, and the role of alcohol and aldehyde dehydrogenase genotypes. J Lab Clin Med 1993; 122:234-240Medline, Google Scholar
3. Chen Y-C, Lu R-B, Peng G-S, Wang M-F, Wang H-K, Ko H-C, Chang Y-C, Lu J-J, Li T-K, Yin S-J: Alcohol metabolism and cardiovascular response in an alcoholic patient homozygous for the ALDH2*2 variant gene allele. Alcohol Clin Exp Res 1999; 23:1853-1860Crossref, Medline, Google Scholar
4. Chen C-C, Lu R-B, Chen Y-C, Wang M-F, Chang Y-C, Li T-K, Yin S-J: Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet 1999; 65:795-807Crossref, Medline, Google Scholar
5. Higuchi S, Matsushita S, Murayama M, Takagi S, Hayashida M: Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry 1995; 152:1219-1221Link, Google Scholar
6. Thomasson HR, Edenberg HJ, Crabb DW, Mai X-L, Jerome RE, Li T-K, Wang S-P, Lin Y-T, Lu R-B, Yin S-J: Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet 1991; 48:667-681Google Scholar
7. Chen WJ, Loh EW, Hsu Y-PP, Chen C-C, Yu J-M, Cheng ATA: Alcohol-metabolizing genes and alcoholism among Taiwanese Han men: independent effect of ADH2, ADH3, and ALDH2. Br J Psychiatry 1996; 168:762-767Crossref, Medline, Google Scholar
8. Iwahashi K: Heterozygous for ALDH2 in alcohol dependence: relationship between the ALDH2 genotype and personality disorder in alcohol-dependent patients with the flushing syndrome (letter). Biol Psychiatry 1995; 37:137Crossref, Medline, Google Scholar
9. Maezawa Y, Yamauchi M, Toda G, Suzuki H, Sakurai S: Alcohol-metabolizing enzyme polymorphisms and alcoholism in Japan. Alcohol Clin Exp Res 1995; 19:951-954Crossref, Medline, Google Scholar
10. Muramatsu T, Wang Z-C, Fang Y-R, Hu K-B, Hequin Y, Yamada K, Higuchi S, Harada S, Kono H: Alcohol and aldehyde dehydrogenase genotypes and drinking behavior in Chinese living in Shanghai. Hum Genet 1995; 96:151-154Crossref, Medline, Google Scholar
11. Nakamura K, Iwahashi K, Matsuo Y, Miyatake R, Ichikawa Y, Suwaki H: Characteristics of Japanese alcoholics with the atypical aldehyde dehydrogenase 2*2: a comparison of the genotypes of ALDH2, ADH2, ADH3, and cytochrome P-4502E1 between alcoholics and nonalcoholics. Alcohol Clin Exp Res 1996; 20:52-55Crossref, Medline, Google Scholar
12. Shen Y-C, Fan J-H, Edenberg HJ, Li T-K, Cui Y-H, Wang Y-F, Tian C-H, Zhou C-G, Zhou R-L, Wang J, Zhao Z-L, Xia G-Y: Polymorphism of ADH and ALDH genes among four ethnic groups in China and effects upon the risk for alcoholism. Alcohol Clin Exp Res 1997; 21:1272-1277Crossref, Medline, Google Scholar
13. Tanaka F, Shiratori Y, Yokosuka O, Imazeki F, Tsukada Y, Omata M: High incidence of ADH2*1/ALDH2*1 genes among Japanese dependents and patients with alcoholic liver disease. Hepatology 1996; 23:234-239Crossref, Medline, Google Scholar
14. Enomoto N, Takase S, Yasuhara M, Takada A: Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes. Alcohol Clin Exp Res 1991; 15:141-144Crossref, Medline, Google Scholar
15. Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T, Harada S: Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcohol 1994; 29:707-710Medline, Google Scholar
16. Peng G-S, Wang M-F, Chen CY, Luu S-Y, Chau H-C, Li T-K, Yin S-J: Involvement of acetaldehyde for full protection against alcoholism by homozygosity of the variant allele of mitochondrial aldehyde dehydrogenase gene in Asians. Pharmacogenetics 1999; 9:463-476Medline, Google Scholar
17. Takeshita T, Morimoto K: Accumulation of hemoglobin-associated acetaldehyde with habitual drinking in the atypical ALDH2 genotype. Alcohol Clin Exp Res 2000; 24:1-7Medline, Google Scholar
18. Wall TL, Peterson CM, Peterson KP, Johnson ML, Thomasson HR, Cole M, Ehlers CL: Alcohol metabolism in Asian-American men with genetic polymorphisms of aldehyde dehydrogenase. Ann Intern Med 1997; 127:376-379Crossref, Medline, Google Scholar
19. Yoshihara E, Ameno K, Nakamura K, Ameno M, Itoh S, Ijiri I, Iwahasi K: The effects of the ALDH2*1/2, CYP2E2E1 C1/C2 and C/D genotypes on blood ethanol elimination. Drug Chem Toxicol 2000; 23:371-379Crossref, Medline, Google Scholar
20. Wall TL, Thomasson HR, Schuckit MA, Ehlers CL: Subjective feelings of alcohol intoxication in Asians with genetic variations of ALDH2 alleles. Alcohol Clin Exp Res 1992; 16:991-995Crossref, Medline, Google Scholar
21. Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL: Genetic risk for alcoholism relates to level of response to alcohol in Asian American men and women. J Stud Alcohol 2002; 63:74-82Medline, Google Scholar
22. Higuchi S, Matsushita S, Muramatsu T, Murayama M, Hayashida M: Alcohol and aldehyde dehydrogenase genotypes and drinking behavior in Japanese. Alcohol Clin Exp Res 1996; 20:493-497Crossref, Medline, Google Scholar
23. Sun F, Tsuritani I, Honda R, Ma Z-M, Yamada Y: Association of genetic polymorphisms of alcohol metabolizing enzymes with excessive alcohol consumption in Japanese men. Hum Genet 1999; 105:295-300Crossref, Medline, Google Scholar
24. Takeshita T, Morimoto K: Self-reported alcohol-associated symptoms and drinking behavior in three ALDH2 genotypes among Japanese university students. Alcohol Clin Exp Res 1999; 23:1065-1069Crossref, Medline, Google Scholar
25. Takeshita T, Morimoto K, Mao XQ, Hashimoto T, Furuyama J: Characterization of the three genotypes of low Km aldehyde dehydrogenase in a Japanese population. Hum Genet 1994; 94:217-223Medline, Google Scholar
26. Chen WJ, Loh EW, Hsu Y-PP, Cheng ATA: Alcohol dehydrogenase and aldehyde dehydrogenase and alcoholism among Taiwanese aborigines. Biol Psychiatry 1997; 41:703-709Crossref, Medline, Google Scholar
27. Thomasson HR, Crabb DW, Edenberg HJ, Li T-K, Hwu H-G, Chen C-C, Yeh E-K, Yin S-J: Low frequency of the ADH2*2 allele among Atayal natives of Taiwan with alcohol use disorders. Alcohol Clin Exp Res 1994; 18:640-643Crossref, Medline, Google Scholar
28. Borras E, Coutelle C, Rosell A, Fernandez-Muixi F, Broch M, Crosas B, Hjelmqvist L, Lorenzo A, Gutierrez C, Santos M, Szczepanek M, Heilig M, Quattrocchi P, Farres J, Vidal F, Richart C, Mach T, Bogdal J, Jornvall H, Seitz HK, Couzigou P, Pares X: Genetic polymorphisms of alcohol dehydrogenase in Europeans: the ADH2*2 allele decreases the risk for alcoholism and is associated with ADH3*1. Hepatology 2000; 31:984-989Crossref, Medline, Google Scholar
29. Whitfield JB, Nightingale BN, Bucholz KK, Madden PAF, Heath AC, Martin NG: ADH genotypes and alcohol use and dependence in Europeans. Alcohol Clin Exp Res 1998; 22:1463-1469Crossref, Medline, Google Scholar
30. Neumark YD, Friedlander Y, Thomasson HR, Li T-K: Association of the ADH2*2 allele with reduced ethanol consumption in Jewish men in Israel. J Stud Alcohol 1998; 59:133-139Crossref, Medline, Google Scholar
31. Shea SH, Wall TL, Carr LG, Li T-K: ADH2 and alcohol-related phenotypes in Ashkenazic Jewish American college students. Behav Genet 2001; 31:231-239Crossref, Medline, Google Scholar
32. Whitfield JB: Meta-analysis of the effects of alcohol dehydrogenase genotype on alcohol dependence. Alcohol Alcohol 1997; 32:613-619Crossref, Medline, Google Scholar
33. Thomasson HR, Beard JD, Li T-K: ADH2 gene polymorphisms are determinants of alcohol pharmacokinetics. Alcohol Clin Exp Res 1995; 19:1494-1499Crossref, Medline, Google Scholar
34. McCarver DG, Thomasson HR, Martier SS, Sokol RJ, Li T-K: Alcohol dehydrogenase-2*3 allele protects against alcohol-related birth defects among African Americans. J Pharmacol Exp Ther 1997; 283:1095-1101Medline, Google Scholar
35. Viljoen DL, Carr LG, Foroud TM, Brooke L, Ramsay M, Li T-K: Alcohol dehydrogenase-2*2 allele is associated with decreased prevalence of fetal alcohol syndrome in the mixed-ancestry population of the Western Cape Province, South Africa. Alcohol Clin Exp Res 2001; 25:1719-1722Crossref, Medline, Google Scholar
36. Wall TL, Garcia-Andrade C, Thomasson HR, Carr LG, Ehlers CL: Alcohol dehydrogenase polymorphisms in Native Americans: identification of the ADH2*3 allele. Alcohol Alcohol 1997; 32:129-132Crossref, Medline, Google Scholar
37. Ehlers CL, Gilder DA, Harris L, Carr L: Association of the ADH2*3 allele with a negative family history of alcoholism in African American young adults. Alcohol Clin Exp Res 2001; 25:1773-1777Crossref, Medline, Google Scholar
38. Osier M, Pakstis AJ, Kidd JR, Lee J-F, Yin S-J, Ko H-C, Edenberg HJ, Lu R-B, Kidd KK: Linkage disequilibrium at the ADH2 and ADH3 loci and risk for alcoholism. Am J Hum Genet 1999; 64:1147-1157Crossref, Medline, Google Scholar
39. Tsukahara M, Yoshida A: Chromosomal assignment of the alcohol dehydrogenase cluster to human chromosome 4q21-23 by in situ hybridization. Genomics 1989; 4:218-220Crossref, Medline, Google Scholar
40. Yasunami M, Kikuchi I, Sarapata D, Yoshida A: The human class I alcohol dehydrogenase gene cluster: three genes are tandemly organized in an 80-kb-long segment of the genome. Genomics 1990; 7:152-158Crossref, Medline, Google Scholar
41. Thomasson HR, Crabb DW, Edenberg HJ, Li T-K: Alcohol and aldehyde dehydrogenase polymorphisms and alcoholism. Behav Genet 1993; 23:131-136Crossref, Medline, Google Scholar
42. Wall TL, Garcia-Andrade C, Thomasson HR, Cole M, Ehlers CL: Alcohol elimination in Native American Mission Indians: an investigation of interindividual variation. Alcohol Clin Exp Res 1996; 20:1438-1442Crossref, Medline, Google Scholar
43. Jacobson SW, Chiodo L, Jester J, Carr L, Sokol R, Jacobson J, Li T-K: Protective effects of ADH2*3 in African American infants exposed to prenatally to alcohol (abstract). Alcohol Clin Exp Res 2000; 24(suppl 5):28AGoogle Scholar
44. Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, Foroud T, Li T-K, Begleiter H, Reich T, Rice JP: A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet 2000; 96:632-637Crossref, Medline, Google Scholar
45. Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA: A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 1994; 55:149-158Crossref, Medline, Google Scholar
46. Bucholz KK, Hesselbrock VM, Shayka JJ, Nurnberger JI, Schuckit MA, Reich T: Reliability of individual diagnostic criterion for psychoactive substance dependence and the impact on diagnosis. J Stud Alcohol 1995; 56:500-505Crossref, Medline, Google Scholar
47. Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V: A validity study of the SSAGA: a comparison with the SCAN. Addiction 1999; 94:1361-1370Crossref, Medline, Google Scholar
48. Crabb DW, Edenberg HJ, Bosron WF, Li T-K: Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity: the inactive ALDH2(2) allele is dominant. J Clin Invest 1989; 83:314-316Crossref, Medline, Google Scholar
49. Xu Y, Carr LG, Bosron WF, Li T-K, Edenberg HJ: Genotyping of human alcohol dehydrogenase at the ADH2 and ADH3 loci following DNA sequence amplification. Genomics 1988; 2:209-214Crossref, Medline, Google Scholar
50. Waldman IW, Robinson BF, Rhee SH: A logistic regression extension of the transmission disequilibrium test for continuous traits: application to linkage disequilibrium between alcoholism and candidate genes DRD2 and ADH3. Genet Epidemiol 1999; 17:S379-S384Google Scholar
51. Gill K, Elk ME, Deitrich RA: A description of alcohol/drug use and family history of alcoholism among urban American Indians. Am Indian Alsk Native Ment Health Res 1997; 8:41-52Crossref, Medline, Google Scholar
52. Kunitz SJ, Gabriel KR, Levy JE, Henderson E, Lampert K, McCloskey J, Quintero G, Russell S, Vince A: Alcohol dependence and conduct disorder among Navajo Indians. J Stud Alcohol 1999; 60:159-167Crossref, Medline, Google Scholar
53. Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D: Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcohol Clin Exp Res 1998; 22:518-523Crossref, Medline, Google Scholar
54. Wall TL, Shea SH, Chan KK, Carr LG: A genetic association with the development of alcohol and other substance use behavior in Asian Americans. J Abnorm Psychol 2001; 110:173-178Crossref, Medline, Google Scholar
55. Reich T, Edenberg HJ, Goate A, William JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li T-K, Conneally PM, Nurnberger JI, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H: Genome-wide search for genes affecting risk for alcohol dependence. Am J Med Genet Neuropsychiatr Genet 1998; 81:206-215Crossref, Google Scholar
56. Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D: Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet Neuropsychiatr Genet 1998; 81:216-221Crossref, Medline, Google Scholar
57. McCarver DG: ADH2 and CYP2E1 genetic polymorphisms: risk factors for alcohol-related birth defects. Drug Metab Dispos 2001; 29:562-565Medline, Google Scholar
58. Whitfield JB, Zhu G, Duffy DL, Birley AJ, Madden PAF, Heath AC, Martin NG: Variation in alcohol pharmacokinetics as a risk factor for alcohol dependence. Alcohol Clin Exp Res 2001; 25:1257-1263Crossref, Medline, Google Scholar
59. Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG: Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med 1999; 29:1069-1081Crossref, Medline, Google Scholar



