Semantic Dysfunction in Women With Schizotypal Personality Disorder
Abstract
OBJECTIVE: This study examined whether early or late processes in semantic networks were abnormal in women with a diagnosis of schizotypal personality disorder. The N400 component of the EEG event-related potentials was used as a probe of semantic processes. METHOD: Word pairs were presented with short and long stimulus-onset asynchronies to investigate, respectively, early and late semantic processes in 16 women with schizotypal personality disorder and 15 normal female comparison subjects. Event-related potentials were recorded in response to the last words in a pair. RESULTS: With the short stimulus-onset asynchrony, the N400 amplitude was less negative in the schizotypal personality disorder group than in the normal comparison group. No group differences were found with the long stimulus-onset asynchrony. CONCLUSIONS: The finding of a less negative than normal N400 amplitude with the short stimulus-onset asynchrony in women with schizotypal personality disorder supports the hypothesis that persons with this disorder evince an overactivation of semantic networks. The absence of group differences with the long stimulus-onset asynchrony, which is primarily sensitive to processes involved in context integration, suggests that in this group of schizotypal personality disorder subjects, additional demands on working memory may be necessary to bring out the semantic dysfunction.
Language dysfunction in schizophrenia has been regarded a cardinal symptom of this disorder for almost a century (1). Clinical reports abound with examples of speech samples characterized by loose associations, lack of sensitivity to context, defective use of pronouns (2–4), or the inability to carry on a conversation bound by a theme (5). More recently, it has been proposed that language abnormalities in schizophrenia are mediated by dysfunctional processes in semantic memory. Specifically, it has been suggested that processes of activation and/or context utilization may be abnormal.
Although several studies have investigated semantic processes in schizophrenia, few studies have evaluated schizotypal personality disorder. Persons with a diagnosis of either schizotypal personality disorder or schizophrenia share the same genetic diathesis (6, 7). However, persons with schizotypal personality disorder do not have the potentially confounding history of psychosis or hospitalization. Furthermore, studies of cognitive functions in subjects with schizotypal personality disorder have reported impairments similar to but less severe than those observed in schizophrenic subjects (8–11).
Semantic memory can be thought of in terms of its structure and the processes that operate on it. Recent formulations postulate that the structure of semantic memory is instantiated by a network of semantic features, such that words close in meaning, such as “water,” “fish,” and “ocean,” are well connected with each other, and words of distant meanings, such as “water,” “pen,” and “free,” are not well connected (12, 13). The activation of one of the words in the network “spreads” to related items: for example, the activation of “water” will partially activate “fish” and “ocean” but not “pen” or “free” (14). The spread of activation is limited either by a process of decay or by inhibition, thereby not permitting the activation to propagate across large portions of the network. It is generally postulated that the initial spread of activation is automatic and dominates the first 500 msec of word processing. Beyond this time window, controlled processes come into play. They include expectancy (15, 16), which involves generating word sets before the appearance of a target, and semantic matching, which involves contextually based word choices.
Semantic priming has been used to assess processes in semantic networks. In a semantic priming paradigm, a subject sees word pairs and decides whether a target letter-string is a real word or a nonword, or pronounces a target word. If the first (prime) and second (target) word in a pair are related, e.g., “water” and “fish,” shorter reaction times and/or fewer errors are observed in response to the target (“fish”) relative to when the two words are not related, e.g., “water” and “pen.” In behavioral experiments, these savings in reaction times are called priming. Experimental designs that use short stimulus-onset asynchronies and a low proportion of related to unrelated word pairs are believed to assess early processes of activation, while designs that use longer stimulus-onset asynchronies are believed to assess controlled processes.
Much of the debate about semantic dysfunction in schizophrenia focuses on whether the loci of dysfunction are in the automatic spreading of activation or in the later stage of controlled processes. The hypothesis of dysfunction in automatic activation within semantic networks assumes that the initial activation is faster acting and/or spreads too far, which results in loose associations and derailed thinking (17–20). The hypothesis of disturbed controlled processes assumes that semantic dysfunction manifests itself later, when the processes of integrating a prior semantic context come into play (21–27). This inability to maintain the prior context or task-relevant information could produce speech marked by loose and bizarre associations. Thus, similar clinical manifestations, e.g., loose associations and derailed thinking processes, are explained by two opposing theoretical frameworks.
Some of the difficulties in resolving the debate lie in methodological differences across studies, including the use of different stimulus-onset asynchronies, differences in the proportion of related and unrelated primes, and the use of lexical-decision rather than word-pronunciation tasks. Another important variable is the subject population chosen. Inclusion of medicated, chronically ill schizophrenic subjects may influence the results (18, 25), e.g., by complicating correct computations of reaction times for the schizophrenic and comparison groups.
Finally, all of the studies discussed in this section used behavioral measures as their dependent variables. Inevitably, a reaction time or an accuracy measure is a final outcome variable in that it is the summation over all processes that occur between the presentation of the stimulus and a response. Accordingly, in the present study we have turned to the use of EEG event-related potentials to provide a more direct measure of semantic processing dysfunction in female subjects with schizotypal personality disorder.
Measurement of event-related potentials allows one to observe and quantify changes in electrophysiological response to a stimulus (e.g., a word) as they unfold over time from the millisecond a target word is presented to a subject. The N400 event-related potential has been associated with semantic processes. It is sensitive to the ease of gaining access to semantic/lexical memory networks and as such is a good index of different types of priming (28, 29), i.e., the more difficult it is to relate a word to the previous context, the larger the N400 amplitude. For example, in word-pair paradigms, N400 is elicited by both related and unrelated words, and the degree of negativity (i.e., the difficulty of fitting a word into context) distinguishes between the related-word and unrelated-word targets conditions.
It is believed that several semantic processes may impinge on the N400 latency and amplitude. For example, the N400 effect in schizophrenia crucially depends on the stimulus-onset asynchrony used in the study. With a short stimulus-onset asynchrony, the N400 reflects, while not being a direct index of, initial processes of activation. With a long stimulus-onset asynchrony, the N400 reflects processes of context integration. Thus, in the debate between hypotheses focused on late, inefficient use of context, as opposed to early overactivation, the following predictions can be made: 1) the hypothesis of inefficient use of context in a clinical population predicts a more negative N400 in sentence processing and long stimulus-onset asynchrony word-pair paradigms; 2) the hypothesis of early overactivation in a clinical population predicts a less negative N400 in short stimulus-onset asynchrony word-pair paradigms; 3) if both of these hypotheses are correct, the N400 will be either less or more negative in a clinical group, relative to normal comparison subjects, depending on the paradigm, i.e., the semantic processes explored in the study.
To our knowledge, most studies that have used event-related potentials to examine language processing in schizophrenia have included both sentence and long stimulus-onset asynchrony word-pair paradigms, i.e., probed late, context integration processes. In most of these studies, more negative N400 amplitude has been found in schizophrenic subjects relative to normal comparison subjects, especially for related-word targets, where minimal N400 amplitude is usually found in normal comparison subjects (11, 30, 31). Similar, but less severe, impairment in processing final words in sentences has been found in subjects with schizotypal personality disorder (32). Thus, these results lend support for the hypothesis that both persons with schizophrenia and those with schizotypal personality disorder make less efficient use of context.
In the present study we used a lexical-decision paradigm to test semantic processing in subjects with schizotypal personality disorder and in normal comparison subjects in two conditions: short (450 msec from onset to onset) and long (1000 msec from onset to onset) stimulus-onset asynchrony. With the long stimulus-onset asynchrony, the offset-to-onset between a prime and target was 800 msec, identical to the interstimulus interval used in the previous study of sentence processing in schizotypal personality disorder (32).
We tested the following two hypotheses: 1) initial processes within semantic networks in the schizotypal personality disorder group will be characterized by overactivation as evinced by a less negative N400 amplitude with the short stimulus-onset asynchrony (since less effort will be needed to make a semantic link between the two concepts), and 2) no group differences will emerge with the long stimulus-onset asynchrony. We assumed that in women with schizotypal personality disorder, normal context integration processes will exist in the absence of additional demands on working memory. We think that working memory demands imposed by processing sentences rather than by word context are necessary to cause an impairment in semantic processing in women with schizotypal personality disorder (32).
Method
Subjects
The study subjects were 16 women who met the full criteria for DSM-IV schizotypal personality disorder according to the Structured Clinical Interview for DSM-IV—Patient Edition (33) and 15 female comparison subjects, matched for age, IQ, and parental socioeconomic status. All subjects were right-handed and had English as their first language. The subjects were invited to participate in the study and received a complete description of the study. Those who decided to participate signed an informed consent form. All subjects were given the Structured Clinical Interview for DSM-IV Personality Disorders (34) to determine their clinical status. Diagnoses were made by a psychologist (M.V.) or a psychiatrist (C.C.D., M.F.). Interrater reliability for diagnosis, computed by using the kappa statistic at regular time intervals in the study, was good (kappa>0.89).
The exclusion and inclusion criteria for all subjects were age between 18 and 55 years and no history of 1) ECT; 2) neurological illness or head injury, defined as a loss of consciousness and or structural sequelae after head trauma; 3) DSM-IV substance dependence in the past 5 years or DSM-IV substance abuse in the last year; 4) alcohol use in the last 24 hours; 5) drug use in the last year; 6) use of any medication that might affect cognitive function or EEG event-related potentials, such as steroids and barbiturates; and 7) use of any psychoactive medication; or 8) hearing, vision, or upper body impairment that might interfere with obtaining reliable test scores. In addition, for comparison subjects, the presence of personality disorders (axis II) in themselves or their first-degree relatives was an exclusion criterion.
The mean ages of the study groups were 33.2 years (SD=11.4) for the comparison group and 31.2 (SD=10.1) for the schizotypal personality disorder group. The mean IQ was 119.8 (SD=8.4) in the comparison group and 114.6 (SD=12.2) in the schizotypal personality disorder group. The mean socioeconomic status, as measured by the Hollingshead Index of Social Position, was 4.3 (SD=0.5) for the comparison subjects and 3.6 (SD=0.7) for the schizotypal personality disorder subjects; parental socioeconomic status was 4.5 (SD=0.6) for the comparison subjects and 4.0 (SD=0.9) for the schizotypal personality disorder subjects. The two groups differed significantly in the subjects’ socioeconomic status (t=3.37, df=24, p<0.002), which likely reflects illness-related social decline of the subjects with schizotypal personality disorder.
Stimuli and Procedures
Stimuli were two sets of 120 word pairs (35), each set consisting of four categories with 30 word pairs in each. In each word pair, the first word (the prime word) was followed by a target word that was 1) related (36) (mean log frequency=1.63 [SD=0.77]), 2) unrelated (36) (mean log frequency=1.72 [SD=0.62]), 3) a legal English nonword (a letter string that conforms to the grammatical and phonological rules of English but is not an actual English word, such as “frak”), or 4) an illegal nonword (a letter string that does not conform to those rules, such as “fkra”). All words were presented visually in the middle of the computer screen at a 4° visual angle, with the subjects seated at a distance of 60 cm from the screen. One set of word pairs was presented with a long stimulus-onset asynchrony (200 msec prime and target exposure time, 800 msec from prime offset to target onset) and the second set was presented with a short stimulus-onset asynchrony (200 msec prime and target exposure time, 250 msec from offset to onset). All word pairs were presented in a pseudorandom fashion to prevent repetition of the same type of stimulus. The two asynchronies were randomly presented across subjects, with each word list appearing both with the short and long stimulus-onset asynchrony so that event-related potential responses to comparable stimuli could be studied across subjects.
The subject made a decision if the target word was a word or a nonword by pressing a response button. Half of the subjects pressed a button with their right hand for words and with their left hand for nonwords, and the other half pressed a button with their left hand for words and with their right hand for nonwords. The task was self-paced, i.e., the next word-pair appeared on the screen after the subject made the response to the target of the previous word pair. Responses emitted 2 seconds after the appearance of the target word were treated as misses.
EEG Acquisition
EEG was acquired with a 32-electrode montage by using a Neuroscan electrocap (El Paso, Tex.). Six additional electrodes were placed at the two earlobes, right and left temple, and supra- and infraorbital sites on the left side to monitor for horizontal and lateral eye movements. The impedance was kept at or below 5 kΩ at all electrode locations and checked at the beginning of every EEG. The EEG was recorded over 924 msec after the target onset, with a 100-msec prestimulus baseline, digitized at the rate of 256 points, with filter settings at DC to 40 Hz. The left ear was used as reference, and the EEG was referenced to linked ears offline. The EEG was edited offline with the Semlitsch correction algorithm to remove contamination from eye movements. Single sweeps were rejected if the amplitude at any of the electrodes exceeded ±75 mV. Separate average waveforms were constructed for each target stimulus type: related, unrelated, legal nonword, and illegal nonword with the two stimulus-onset asynchronies for all subjects individually. Group grand averages were made on the basis of individual subjects’ averages.
Event-Related Potential Data
Measurement windows for the event-related potentials of interest were established on the basis of inspection of the individual waveforms and grand averages for a given asynchrony. To best characterize the group differences, which on visual inspection seemed to span the whole event-related potential epoch, three measurement windows were selected: 1) N100-P200: 90–250 msec poststimulus, 2) N400: 300–400 msec poststimulus, and 3) P600: 450–650 msec poststimulus. The N100-P200 and P600 event-related potentials were measured as a mean area under the curve, while N400 was measured both as mean area under the curve and as a peak latency to examine group latency differences.
Statistical Analyses
N100-P200
Because of the scalp distribution of the N100-P200 event-related potential (11), two electrode chains were selected for analysis by using mixed model analyses of variance (ANOVAs): 1) a frontomidline chain (Fz, Cz) and 2) a frontolateral chain (F3/4, C3/4, FTC1/2). Diagnosis was a between-subject variable for both electrode chains (two levels: schizotypal personality disorder and normal comparison). For the frontomidline chain, the within-group variables were condition (two levels: related- and unrelated-word targets) and electrode (two levels: Fz and Cz). For the frontolateral chain, the within-subject variables were condition (two levels: related- and unrelated-word targets), electrode (three levels: F3/4, C3/4, and FTC1/2), and hemisphere (right and left).
N400
Because of the distribution of group voltage differences across the scalp for the N400 event-related potential (11) (Figure 1), three electrode chains were selected for analyses: 1) midline chain: Fz, Cz, and Pz; 2) frontolateral chain: F3/4, C3/4, and FTC1/2; and 3) lateral-parietal chain: P3/4, TCP1/2, and CP1/2. Since prior work has demonstrated the usefulness of the N400 group comparisons in nonsubtracted conditions (11, 37), we directly compared the N400 amplitude in the two groups by using waveforms for responses to related-word and to unrelated-word targets. To ascertain that the group effects depended on the stimulus-onset asynchrony, we started with the overall multivariate analysis of variance (MANOVA) with stimulus-onset asynchrony as a factor. In addition, to demonstrate that the paradigm used in the study elicited the expected N400 effect in the normal subjects, we also conducted an overall MANOVA by using data from the normal subjects. Since we predicted that group differences would be evinced with the short but not with the long stimulus-onset asynchrony, we did separate ANOVA analyses for the two types of stimulus-onset asynchronies. We used mixed model ANOVAs (SPSS 10.0, SPSS, Inc., Chicago). For the midline chain, group (two levels: normal and schizotypal personality disorder) was a between-subject variable and condition (two levels: related- and unrelated-word targets) and electrode (three levels) were within-subject variables. For the frontolateral and lateral-parietal chains, group was a between-subject variable, and hemisphere (two levels: left and right), condition (two levels: related- and unrelated-word targets), and electrode (three levels) were within-subject variables. Greenhouse-Geisser correction was used where necessary (i.e., for all significant interaction effects obtained for k + 1 measurements, k=2).
P600
The ANOVA model used was identical to that used for the N400 component. The same electrode chains were used for the P600 and N400 amplitudes.
Results
N100-P200
No main effects were found for the frontomidline chain or the frontolateral chain. The analysis of the interaction between group and hemisphere did not reach significance.
N400
The overall MANOVA included data for the two stimulus-onset asynchronies and all electrodes placed in the region-of-interest approach (in the midline, frontal, and parietal regions). The overall MANOVA with stimulus-onset asynchrony added as a factor showed a three-way interaction of stimulus-onset asynchrony, condition, and group (F=4.28, df=2, 24, p<0.03), suggesting that group effects in this study depended on the stimulus-onset asynchrony and the word target condition. This finding was further substantiated by the region-of-interest analyses.
N400 effects in normal comparison subjects
The MANOVAs with factors of stimulus-onset asynchrony (long and short stimulus-onset asynchrony), condition (related- and unrelated-word targets), and electrode (with the number of electrodes dependent on the electrode chain analyzed) found a main effect of condition for all three electrode chains: frontomidline (F=18.46, df=1, 12, p<0.001), frontolateral (F=9.06, df=1, 13, p<0.01), and lateral-parietal (F=10.4, df=1, 12, p<0.007). No other main effects or interactions were significant.
N400 short stimulus-onset asynchrony
For the frontomidline electrode chain, there was a main effect of condition (F=15.4, df=1, 28, p<0.001), with a more negative N400 amplitude in the related-word condition than in the unrelated-word condition (Table 1). There was no main effect of group; and a three-way interaction of group by condition by electrode did not reach significance (F=3.49, df=2, 56, p<0.07). As Figure 2 shows, N400 amplitude at Fz in the related-word condition was less negative in the schizotypal personality disorder group than in the comparison group.
For the frontolateral electrode chain, there was a main effect of condition (F=6.24, df=1, 30, p<0.02), with more negative N400 amplitude in the unrelated-word than in the related-word condition. There was a significant three-way interaction of group, condition, and hemisphere (F=4.78, df=1, 30, p<0.04). To follow up on this interaction, we conducted mixed model ANOVAs separately for the related-word and unrelated-word conditions. The ANOVAs included group as a between-subject variable and hemisphere (left versus right) and electrode (three levels) as within-subject variables. For the related-word condition, there was a significant group-by-hemisphere interaction (F=4.51, df=1, 30, p<0.05). In the follow-up t tests, there was a group effect for the left frontal sites (t=–2.0, df=28.9, p<0.03, one-tailed) but not for the right frontal sites (t=–0.93, df=28.9, p<0.18, one-tailed), with a less negative N400 amplitude in the schizotypal personality disorder group than in the comparison group (Figure 2). No significant effects were found for the unrelated-word condition (Figure 3).
At the lateral-parietal electrode chain, there was a main effect of condition (F=7.96, df=1, 29, p<0.009), with more negative N400 in the unrelated-word than in the related-word condition. The three-way interaction between group, condition, and hemisphere did not reach significance (F=3.86, df=1, 29, p<0.06). Less negative N400 amplitude was observed in the left hemisphere electrode sites in the related-word condition in the schizotypal personality disorder group than in the normal comparison group.
N400 long stimulus-onset asynchrony
No group differences or significant interactions were found for the long stimulus-onset asynchrony for the midline or the two lateral electrode chains (Table 2, Figure 1).
N400 latency
For the short stimulus-onset asynchrony, no differences in latency between groups were observed for any of the electrode chains.
For the long stimulus-onset asynchrony, no group differences were observed for the midline or lateral-parietal electrode chains. A group-by-electrode interaction was found for the frontolateral electrode chain (F=2.89, df=5, 26, p<0.04). Longer latencies were observed in the schizotypal personality disorder group than in the normal comparison group. Unidirectional t tests showed significant differences between groups in latency at F3 (t=–3.25, df=30, p<0.0001), FTC1 (t=–2.67, df=30, p<0.006), and F4 (t=–2.39, df=30, p<0.02) in the related-word condition.
P600
Short stimulus-onset asynchrony
For amplitude with the short stimulus-onset asynchrony, a three-way interaction between group, condition, and electrode was found for the midline electrode chain (F=3.86, df=2, 52, p=0.051). There was a tendency for the P600 at Fz to be more positive in normal comparison subjects in the unrelated-word condition; however, follow-up t tests did not confirm the group separation at any of the electrodes. There were no group differences for the frontolateral and the lateral-parietal electrode chains.
For amplitude with the long stimulus-onset asynchrony, no group differences in amplitude were observed for the midline or lateral-parietal electrode chains (Table 2). A group-by-condition-by electrode interaction was observed for the frontolateral electrode chain (F=3.62, df=2, 58, p<0.04). In the follow-up t tests, the groups did not show significant differences at any electrode location in both the related-and the unrelated-word conditions.
Summary of Results
Differences in amplitude between groups were found in the N400 latency range in the related-word condition with the short stimulus-onset asynchrony but not with the long stimulus-onset asynchrony. The differences with the short stimulus-onset asynchrony were most prominent over the left frontal area and consisted of a less negative N400 amplitude in the schizotypal personality disorder group than in the normal comparison group. No group differences existed for the P600 waveform. As for the N100-P200 complex, the interaction between group and hemisphere did not reach statistical significance.
Discussion
In this study of lexical decision, a less negative N400 amplitude was found for related words with the short stimulus-onset asynchrony in the schizotypal personality disorder group than in the normal comparison group. Even though less negative N400 amplitudes with the short stimulus-onset asynchrony were observed across the entire scalp in the schizotypal personality disorder group, statistically significant group differences emerged only for the left frontal area. The less negative N400 amplitude with the short stimulus-onset asynchrony suggests greater ease of gaining access to words within semantic memory in the schizotypal personality disorder group. This finding is consistent with a dysfunction in early semantic processing brought about by overactivation (11, 37, 38).
At the same time, no group differences in the N400 amplitude were found with the long stimulus-onset asynchrony. An earlier study of sentence processing that used an identical offset-to-onset time interval (32) found a more negative N400 amplitude in the schizotypal personality disorder group, where computing the context put substantial demands on working memory. Thus, the finding of no group differences with the long stimulus-onset asynchrony, in conjunction with the results of the previous study, suggests that even though context integration is involved both in single-word and in sentence processing, these processes are different. Sentential context integration requiring additional working memory and/or attentional resources may be necessary to bring out semantic dysfunction in this group of subjects.
These results supported both of our hypotheses: 1) the presence of semantic overactivation in schizotypal personality disorder at the initial stages of processing and 2) the absence of abnormalities at later stages of processing in an experimental paradigm without heavy demands on working memory.
To our knowledge, this is the first study to report event-related potential evidence of dysfunction in early semantic processes in subjects with schizotypal personality disorder and, in particular, in women with this disorder. A few studies of event-related potentials examining cognitive function in female schizophrenic patients have suggested a smaller degree of impairment in women than in men (39, 40). It is thus likely that the localized group difference found in the women with schizotypal personality disorder in this study may reflect a milder form of impairment in this group. Acquiring data on event-related potentials in men with schizotypal personality disorder will help clarify if the degree of dysfunction is gender mediated.
The evidence for abnormal early semantic processes found in this study is also relevant for the understanding of semantic dysfunction in schizophrenia. First, the findings suggest that, in addition to abnormal late processes in schizophrenia (demonstrated in previous studies), early processes may be compromised. Second, the findings suggest that these abnormal processes also exist in persons with schizotypal personality disorder, who do not have the clinical symptoms of schizophrenia. Event-related potential data for first-episode patients with schizophrenia would further expand our understanding of language dysfunction in that disorder.
 |
 |
Received Feb. 12, 2001; revisions received Oct. 19, 2001, and April 25, 2002; accepted May 7, 2002. From the Department of Psychiatry, Harvard Medical School, Boston; the Neuroscience Laboratory, VA Boston Healthcare System, Brockton Campus; Massachusetts Mental Health Center, Boston; and the Department of Radiology, Brigham and Women’s Hospital, Boston. Address reprint requests to Dr. McCarley or Dr. Niznikiewicz, Psychiatry 116A, VA Boston Healthcare System, Brockton Campus, 940 Belmont St., Brockton, MA 02301; [email protected] or [email protected] (e-mail). Supported by the National Alliance for Research on Schizophrenia and Depression (Dr. Niznikiewicz, Dr. Frumin), NIMH grants MH-52807 and MH-40799 to Dr. McCarley, NIMH grants MH-00746 and MH-01110 to Dr. Shenton, a Department of Veterans Affairs Career Development Award to Dr. Dickey, a Department of Veterans Affairs Psychiatry Research/Neuroscience Fellowship to Dr. Frumin, and the Department of Veterans Affairs Center for Basic and Clinical Neuroscience Studies of Schizophrenia (Dr. McCarley).
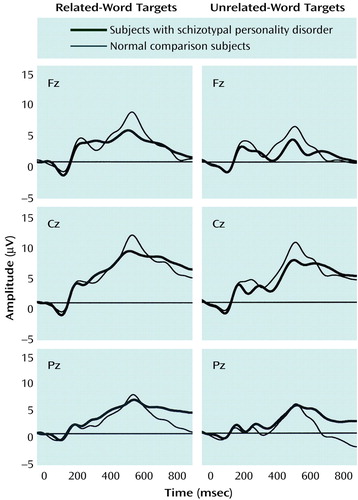
Figure 1. Grand Average EEG Waveforms of 15 Normal Female Comparison Subjects and 16 Female Subjects With Schizotypal Personality Disorder During Presentation of Related- and Unrelated-Word Targets With Long Stimulus-Onset Asynchrony
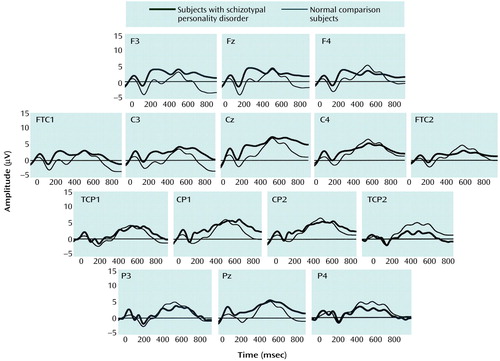
Figure 2. Grand Average EEG Waveforms of 15 Normal Female Comparison Subjects and 16 Female Subjects With Schizotypal Personality Disorder During Presentation of Related-Word Targets With Short Stimulus-Onset Asynchrony

Figure 3Grand Average EEG Waveforms of 15 Normal Female Comparison Subjects and 16 Female Subjects With Schizotypal Personality Disorder During Presentation of Unrelated-Word Targets With Short Stimulus-Onset Asynchrony
1. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
2. Andreasen NC: Thought, language, and communication disorders, I: clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry 1979; 36:1315-1321Crossref, Medline, Google Scholar
3. Rochester SR, Martin JR: Crazy Talk: A Study of the Discourse of Schizophrenic Speakers. New York, Plenum, 1979Google Scholar
4. Shenton ME, Solovay MR, Holzman PS: Comparative studies of thought disorder, II: schizoaffective disorder. Arch Gen Psychiatry 1987; 44:21-30Crossref, Medline, Google Scholar
5. Harvey PD: Speech competence in manic and schizophrenic psychoses: the association between clinically rated thought disorder and cohesion and reference performance. J Abnorm Psychol 1993; 92:368-377Crossref, Google Scholar
6. Kety SS, Rosenthal D, Wender PH, Schulsinger F: The types and prevalence of mental illness in the biological and adoptive families of adopted schizophrenics. J Psychiatr Res 1968; 6:345-362Crossref, Google Scholar
7. Kendler KS, McGuire M, Gruenberg AM, Walsh D: Schizotypal symptoms and signs in the Roscommon Family Study: their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry 1995; 52:296-303Crossref, Medline, Google Scholar
8. Condray R, Steinhauer SR: Schizotypal personality disorder in individuals with and without schizophrenic relatives: similarities and contrasts in neurocognitive and clinical functioning. Schizophr Res 1992; 7:33-41Crossref, Medline, Google Scholar
9. Trestman RL, Keefe RSE, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Siever L: Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res 1995; 59:127-136Crossref, Medline, Google Scholar
10. Voglmaier MM, Seidman LJ, Salisbury DF, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder. Biol Psychiatry 1997; 41:530-540Crossref, Medline, Google Scholar
11. Niznikiewicz MA, O’Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW: ERP assessment of visual and auditory language processing in schizophrenia. J Abnorm Psychol 1997; 1:85-94Crossref, Google Scholar
12. Collins AM, Loftus E: A spreading activation theory of semantic priming. Psychol Rev 1975; 82:407-428Crossref, Google Scholar
13. Neely JH: Semantic priming effects in visual word recognition: a selective review of current findings and theories, in Basic Processes in Reading: Visual Word Recognition. Edited by Besner D, Humphreys GW. Hillsdale, NJ, Lawrence Erlbaum Associates, 1991, pp 264-336Google Scholar
14. Anderson JR: The Architecture of Cognition. Cambridge, Mass, Harvard University Press, 1983Google Scholar
15. Posner M, Snyder C: Facilitation and inhibition in the processing of signals, in Attention and Performance, 5th ed. Edited by Rabbitt P, Dornic S. New York, Academic Press, 1975, pp 669-682Google Scholar
16. den Heyer K, Briand K, Dannenbring GL: Strategic factors in a lexical-decision task: evidence for automatic and attention-driven processes. Mem Cognit 1983; 11:374-381Crossref, Medline, Google Scholar
17. Spitzer M, Braun U, Maier S, Hermle L, Maher BA: Indirect semantic priming in schizophrenic patients. Schizophr Res 1993; 11:71-80Crossref, Medline, Google Scholar
18. Manschreck T, Maher B, Milavetz JJ, Ames D, Wesstein CC, Schneyer M: Semantic priming in thought disordered schizophrenic patients. Schizophr Res 1988; 1:61-66Crossref, Medline, Google Scholar
19. Henik A, Nissimov E, Priel B, Umansky R: Effects of cognitive load on semantic priming in patients with schizophrenia. J Abnorm Psychol 1995; 104:576-584Crossref, Medline, Google Scholar
20. Moritz S, Andresen B, Domin F, Martin E, Probsthein E, Kretschmer G, Krausz M, Naber D, Spitzer M: Increased automatic spreading activation in healthy subjects with elevated scores in a scale assessing schizophrenic language disturbances. Psychol Med 1999; 29:161-170Crossref, Medline, Google Scholar
21. Vinogradov S, Ober BA, Shenaut GK: Semantic priming of work pronunciation and lexical decision in schizophrenia. Schizophr Res 1992; 8:171-181Crossref, Medline, Google Scholar
22. Chapin K, Vann LE, Lycaki H, Josef N, Meyendorff E: Investigation of the associative network in schizophrenia using the semantic priming paradigm. Schizophr Res 1989; 2:355-360Crossref, Medline, Google Scholar
23. Blum NA, Freides D: Investigating thought disorder in schizophrenia with the lexical decision task. Schizophr Res 1995; 16:217-224Crossref, Medline, Google Scholar
24. Passerieux C, Segui J, Besche C, Chevalier JF, Widlöcher D, Hardy-Bayle MC: Heterogeneity in cognitive functioning of schizophrenic patients evaluated by a lexical decision task. Psychol Med 1997; 27:1295-1302Crossref, Medline, Google Scholar
25. Barch DM, Cohen JD, Servan-Schreiber D, Steingard SS, Steinhauer S, van Kammen D: Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol 1995; 105:592-601Crossref, Google Scholar
26. Barch DM, Carter CS, Perlstein W, Baird J, Cohen JD, Schooler N: Increased Stroop facilitation effects in schizophrenia are not due to increased automatic spreading activation. Schizophr Res 1999; 39:51-64Crossref, Medline, Google Scholar
27. Cohen JD, Barch DM, Carter C, Servan-Schreiber D: Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol 1999; 108:120-133Crossref, Medline, Google Scholar
28. Holcomb PJ: Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology 1993; 30:47-60Crossref, Medline, Google Scholar
29. Kutas M, Hillyard SA: An electrophysiological probe of incidental semantic association. J Cognitive Sci 1989; 1:38-49Google Scholar
30. Grillon C, Ameli R, Glazer WM: N400 and semantic categorization in schizophrenia. Biol Psychiatry 1991; 29:467-480Crossref, Medline, Google Scholar
31. Mitchell PF, Andrews S, Fox A, Catts PB, McConghy N: Active and passive attention in schizophrenia: an ERP study of information processing in a linguistic task. J Abnorm Psychol 1991; 32:101-124Google Scholar
32. Niznikiewicz MA, Voglmaier M, Shenton ME, Seidman LJ, Dickey CC, Rhoads R, Teh E, McCarley RW: Electrophysiological correlates of language processing in schizotypal personality disorder. Am J Psychiatry 1999; 156:1052-1058Abstract, Google Scholar
33. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P). Washington, DC, American Psychiatric Press, 1995Google Scholar
34. First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L: Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. Washington, DC, American Psychiatric Press, 1997Google Scholar
35. Holcomb PJ, Neville HJ: Auditory and visual semantic priming in lexical decision: a comparison using evoked potentials. Lang Cognit Processes 1990; 5:281-312Crossref, Google Scholar
36. Kucera H, Francis WN: Computational Analysis of Present Day American English. Providence, RI, Brown University Press, 1982Google Scholar
37. Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW: Word recall in schizophrenia: a connectionist model. Am J Psychiatry 1998; 155:1685-1690Link, Google Scholar
38. Goldman-Rakic PS: Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory, in Psychopathology and the Brain. Edited by Carroll BJ, Barrett JE. New York, Raven Press, 1991, pp 1-23Google Scholar
39. Hetrick WP, Sandman CA, Bunney WE Jr, Jin Y, Potkin SG, White MH: Gender differences in gating of the auditory evoked potential in normal subjects. Biol Psychiatry 1996; 39:51-58Crossref, Medline, Google Scholar
40. Gur RE, Petty RG, Turetsky BI, Gur RC: Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res 1996; 21:1-12Crossref, Medline, Google Scholar



