Intact Implicit Learning in Schizophrenia
Abstract
OBJECTIVE: Schizophrenia impairs performance on explicit, but not implicit, memory tasks, indicating that conscious awareness at retrieval is a critical determinant of impaired memory. The authors investigated implicit learning, i.e., knowledge acquisition in the absence of conscious awareness, in patients with schizophrenia. METHOD: An artificial grammar learning task was used to assess implicit learning in 48 patients with schizophrenia and 24 healthy comparison subjects. The subjects were first presented with letter strings that were generated according to the rules of a finite-state grammar paradigm. They were then required to indicate whether new letter strings were “grammatical,” depending on whether or not the strings corresponded to the rules. IQ, working memory, explicit memory, verbal fluency, and speed of processing were also assessed. RESULTS: Patients performed significantly worse than the comparison subjects on cognitive tasks that assessed episodic memory, verbal fluency, working memory, and speed of processing. In contrast, patients classified as being correct more grammatical than nongrammatical letter strings, and the magnitude of the difference was similar to that observed in healthy comparison subjects. CONCLUSIONS: Implicit learning, as assessed with an artificial grammar learning task, is intact in patients with schizophrenia. Conscious awareness might be a critical determinant of memory impairment both at encoding and at retrieval.
Schizophrenia impairs many cognitive functions, such as perception, memory, language, attention, and executive functions (1). In the face of this wide range of impairments in patients with schizophrenia, the issue must be raised of why everything does not go astray. For instance, why are some patients still able to adapt to everyday situations? Why are some of them able to work? Beyond the fact that patients are more or less cognitively impaired, one plausible explanation is that, within a particular cognitive domain, some processes are impaired whereas others are not. This can be best exemplified by memory, one of the most severely impaired functions (2), which is likely to explain difficulties encountered by these patients in everyday life (3). The critical determinant of impaired memory is conscious awareness at retrieval (4–6). Schizophrenia impairs performance on explicit memory tasks, such as recall and recognition, in which subjects are required to retrieve information from memory consciously. In contrast, it does not impair performance on implicit memory tasks, such as stem completion and procedural memory tasks, for which subjects are not required to retrieve material consciously (4). Thus, a patient may display inappropriate behavior when action must be guided consciously by explicit memory processes but appropriate behavior when action is driven more automatically by implicit memory processes.
While implicit memory refers to the unconscious retrieval of knowledge, implicit learning refers to its unconscious acquisition: subjects have no conscious awareness that they are learning, and the acquired knowledge is inaccessible to awareness (7). It is surprising that implicit learning has not yet been explored in studies of schizophrenia, since it plays a major role in everyday life situations, and most of our acquired knowledge is probably learned implicitly. It has been argued that generating emotional reactions, forming impressions about people, drawing inferences, and many other high-level cognitive operations are supported by implicit learning (8). Implicit learning also plays a major role during development and the constitution of the self (9). The prototypical example is that of a child who is learning his or her native tongue. The child hears many exemplars of the language and is progressively able to abstract its grammatical rules. This abstract knowledge makes it possible for the child to understand and produce sentences he or she has never heard before. Learning is implicit because it is not intentional and conscious, and the acquired grammatical rules are mainly inaccessible to conscious awareness.
The paradigm most often used for experimental investigations of implicit learning is that of an artificial grammar learning task. Subjects first are presented with letter strings generated by a finite-state grammar paradigm. At the test phase, they are informed that the letter strings have been composed on the basis of a complex system of rules. They are then required to indicate whether new letter strings are “grammatical,” depending on whether or not the strings correspond to the rules. Most experiments show that subjects, while being unable to report the rules of the grammar, can classify correctly about 60%–80% of the strings (10, 11). Thus, these sturdies provide strong evidence that subjects learn something of the task. Several interpretations have been put forward to account for what and how subjects learn. According to the abstractionist account, learning is implicit and is supported by the progressive abstraction of the rules underlying the formation of letter strings (12). This account has been challenged with the proposal that learning is based not on the acquisition of abstract rules but on knowledge related to individual instances of the artificial grammar task. Subjects might learn bigrams or trigrams of the presented strings (i.e., chunks of two or three consecutive letters), memory for these parts being sufficient to explain that performance is above chance. According to one proposal, this knowledge is implicit, depending on the associative chunk strength, that is, the frequency with which chunks appear in the letter strings (13). In parallel with this chunk-frequency knowledge, chunk novelty (bigrams or trigrams seen during the test phase that were not presented during the learning phase) could also be a determining factor, since the novelty of the chunk might be sufficient to induce subjects to correctly classify the letter string as nongrammatical. According to another proposal, the knowledge acquired is explicit, resulting from the conscious application of explicit memory strategies to letter strings. This might be the case when some particularly salient features are present in the letter strings. However, evidence that patients with organic amnesia, who suffer from severely defective explicit memory, perform normally in artificial grammar learning tasks indicates that explicit learning plays only a marginal role, if any, in artificial grammar learning tasks (14, 15). Taken together, these results indicate that, depending on the specific constraints of the tasks, what is learned in these tasks is implicit knowledge based on abstract grammatical rules and on specific aspects of letter strings (16).
This study aimed at investigating implicit learning in patients with schizophrenia by using an artificial grammar learning task. Chunk strength, chunk novelty, and salient aspects of letter strings were controlled for so that knowledge based on specific letter strings played no significant role in implicit learning. The hypothesis that conscious awareness is a critical determinant of memory impairment not only at retrieval but also at encoding predicted that implicit learning, which does not require conscious awareness, would be intact.
Method
Subjects
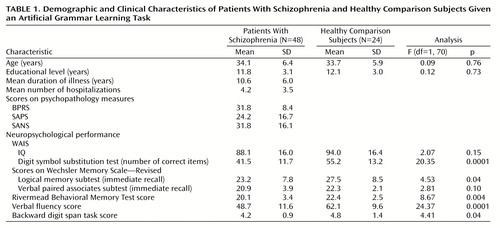
Forty-eight outpatients (17 women and 31 men) whose native language was French were recruited from the Department of Psychiatry of the Strasbourg University Hospital. They were clinically stable and fulfilled DSM-IV criteria for schizophrenia (paranoid: N=25; residual: N=6; disorganized: N=11; undifferentiated: N=6), as determined by consensus of the current treating psychiatrist and two senior psychiatrists from the research team. Forty-four patients had been receiving long-term neuroleptic treatment (mean dose=305.3 mg/day [SD=301.4] in chlorpromazine equivalents). Twenty-three patients also had been receiving antiparkinsonian treatment (trihexyphenidyl hydrochloride, mean dose=8.3 mg/day [SD=5.2]), five had been receiving antidepressant treatment (paroxetine [N=3], fluoxetine [N=1], or clomipramine [N=1]), and two were taking lithium carbonate. Patients with histories of traumatic brain injury, epilepsy, alcohol or substance abuse, or other neurological problems were excluded.
The comparison group consisted of 24 subjects (nine women and 15 men) recruited through local advertisements. The two groups did not differ significantly in gender composition, age, or education (Table 1). Comparison subjects had no history of alcoholism, drug abuse, or neurological illness. They were not receiving any medications. The protocol was approved by the regional committee of ethics. All subjects provided written informed consent.
Episodic memory performance was assessed with the logical memory and verbal paired associates subtests of the Wechsler Memory Scale—Revised (17) and the Rivermead Behavioral Memory Test (18). Working memory was assessed with the backward digit span task, and verbal fluency was measured through a category generation task. Complementary tasks were carried out to assess general cognitive functions. IQ was assessed with a short form of the WAIS-R, and speed of processing was measured with the digit symbol substitution test from the WAIS-R. On all aspects of neuropsychological performance except for IQ and score on the verbal paired associates subtest, the patients performed significantly worse than the comparison subjects (Table 1).
Materials
The finite-state grammar paradigm (Figure 1) was adapted from Mathews et al. (10) and included six different letters (F, V, M, T, R, and X) distributed in 14 positions between eight nodes; the structure also included one entry and two exits. Letter strings were formed by starting at one entry and navigating from one node to another, following the direction indicated by the arrows. A letter was generated at each node transition and a letter string terminated when an exit was reached. The finite-state grammar paradigm used in the present study was constructed in such a way that it did not produce any meaningful strings (e.g., TV). The grammar paradigm could generate a total of 63 grammatical strings of four to seven letters, the latter being the maximum string length used in this study. In the learning phase, 51 of the possible grammatical letter strings were presented. The 12 remaining grammatical strings were kept for the test phase, where they were presented along with 12 nongrammatical letter strings. These nongrammatical strings were generated by violating the rule system at one or two positions within the letter string. These rule violations could occur in any position within the letter string, except in the first and last position.
Our first purpose in the construction of the nongrammatical letter strings was to make them impossible to distinguish from the grammatical strings simply on the basis of any superficial bigram or trigram knowledge formed during the learning phase (15, 16). Indeed, to demonstrate that the subjects’ ability to classify the letter strings rested on their knowledge of some higher-level regularities gained during the learning phase, it was necessary to ensure that this ability could not simply be accounted for by a knowledge of the relative frequency of bigram and trigram chunks in the grammatical strings. Therefore, we constructed the material for the test phase in such a way that grammatical and nongrammatical letter strings were similar according to different measures of chunk strength. To determine chunk strength, we first calculated the frequency of every bigram and trigram chunk that appeared in the 51 grammatical letter strings presented during the learning phase (for example, the string FVFT includes the bigrams FV-VF-FT and the trigrams FVF-VFT). We then calculated the mean chunk frequencies for each letter string presented during the test phase to obtain its global associative chunk strength. Grammatical and nongrammatical strings did not differ according to 12 chunk strength measures (see reference 19 for details).
Procedure
The task was presented to the subjects on a personal computer.
During the learning phase, each of the 51 letter strings appeared for 3 seconds, one at a time, in a rectangle (14.5 cm × 6 cm) at the center of the screen. As soon as the letter string disappeared, the subject had to reproduce it aloud. If the recall was correct, the next letter string was presented; if not, the item was shown again in the same condition. Three trials were allowed for each letter string, with one point given to the subject for an accurate answer on the first trial, two points if an accurate performance needed two trials, and three points when the subject answered correctly after three trials. The subject was given four points if he or she was unable to reproduce the letter string after three presentations. After a maximum of three presentations, the examiner went on to the next string. The 51 letter strings were presented in a predetermined random order for half of the participants and in the inverse order for the other half. The following instructions were verbally given to subjects: “This is an immediate memory test. On the screen, you will see letter strings composed of the letters F, V, M, R, T, and X. These strings are of variable length. You must read aloud the string that I’ll show you. Once it disappears, try to reproduce this string aloud. The purpose is to reproduce correctly each string at the first trial.”
For the classification task, participants were asked to indicate whether new strings were grammatically correct or not. No feedback was given as to the correctness of their judgments. The 24 letter strings were presented in the same way as in the learning phase, except that there was no presentation time limit. The series of test items was presented twice in the same predetermined random order. Participants were told: “The consonant strings that you memorized a few minutes ago were generated according to a set of complex rules. Now, you are going to classify 24 new letter strings. Some of these strings are constructed according to the same rules as the previous strings, while others contain errors with regard to these rules. Note that these rules are so complex that it is impossible to unravel them. To decide whether a letter string is correct or not, just rely on your intuition, on your feeling. If you think that a letter string is correct, then answer ‘yes,’ and if you consider it wrong, simply say ‘no.’”
Statistical Analyses
The percentages of strings classified as grammatical or nongrammatical were subjected to an analysis of variance (ANOVA) with group (patients versus comparison subjects) as a between-subject variable and grammaticality (grammatical versus nongrammatical) as a repeated measure factor. Pearson’s product-moment correlations between performance on the artificial grammar learning task (for both the learning and test phases) and performance on each of the neuropsychological tests were calculated for the schizophrenic patients. Bonferroni correction was applied to take into account the 10 correlations computed for each variable; the significance level was set at p<0.005.
Results
During the learning phase, patients with schizophrenia needed significantly more presentations of the letter strings (mean=84.02, SD=19.16) than did the comparison subjects (mean=72.96, SD=22.26) (t=2.19, df=70, p<0.04).
The patients with schizophrenia correctly classified 66.4% of the letter strings as grammatical while incorrectly classifying 57.3% of the nongrammatical letter strings as grammatical. The corresponding rates for the comparison subjects were 74.8% and 60.8%. There was a significant effect of grammaticality (F=34.79, df=1, 70, p<0.00001), indicating that the subjects could discriminate between grammatical and nongrammatical items. However, there was no significant group effect (F=2.12, df=1, 70, p=0.15) or group-by-grammaticality interaction (F=1.39, df=1, 70, p=0.24), showing that the grammaticality effect was sFigure 2).
Therefore, both the patients with schizophrenia and the comparison subjects reached similar performance levels in the classification task. However, the patients benefited from having more presentations during the learning phase than the comparison subjects did. To investigate whether performance in the classification task was still intact when the number of learning trials was equivalent for both groups, we discarded from the analyses patients who needed more presentations than the mean of the comparison subjects plus one standard deviation (N=13). We then compared the classification performance of the remaining 35 patients to that of the comparison subjects. The results of the ANOVA confirmed the previous analysis: a significant effect of grammaticality (F=41.13, df=1, 57, p<0.0001) but no group effect (F=1.99, df=1, 57, p=0.16) or group-by-grammaticality interaction (F=0.19, df=1, 57, p=0.66). Similar results were obtained when the seven patients who were receiving antidepressants or lithium were discarded from the analyses.
As expected, patients’ performance in the learning phase of the task was significantly and negatively correlated with performance on the backward digit span task (r=–0.47, df=70, p<0.0001). There were no significant correlations between learning or test phase grammar task performance and any other measures (all r’s <0.28).
Discussion
As predicted, implicit learning, as assessed with an artificial grammar learning task, was intact in patients with schizophrenia. Patients classified as correct more grammatical than nongrammatical letter strings, and the magnitude of the difference was similar to that observed in comparison subjects. This finding was obtained in patients who exhibited cognitive impairment (i.e., performed poorly on cognitive tasks assessing episodic memory, verbal fluency, working memory, and speed of processing). It was also obtained with a relatively high number of patients. The only difference observed in the grammar learning task concerned the number of trials required to correctly recall the letter strings during the learning phase, in that patients needed significantly more presentations than did comparison subjects. This difference was probably due to the impaired working memory exhibited by patients, as suggested by a significant negative correlation between the number of trials required to learn the letter strings and patients’ backward digit span task performance. It raised the possibility that patients reached performance levels in the classification task that were similar to those of the comparison subjects not because of intact implicit learning but because they benefited from more learning trials. However, this possibility might be ruled out, since the classification task performance of a subgroup of patients with a number of presentations equivalent to that of the comparison subjects was still normal.
The question must be raised of what and how patients did learn. Chunk strength, chunk novelty, and salient features of letter strings were controlled for in order to minimize, or even eliminate, the role played by knowledge related to specific letter strings. This was successful, since classification task performance was intact when patients with schizophrenia exhibited an impairment of explicit memory. Moreover, in agreement with previous findings in organic amnesia (14, 20), performance in the implicit and explicit tasks were not significantly correlated, further supporting the notion that implicit and explicit learning processes are independent. Therefore, the study provides strong evidence that patients with schizophrenia gained knowledge that was based on the progressive abstraction of the artificial grammar rules. However, it remains to be established whether patients with schizophrenia are able to transfer abstract rules from one set of symbols to another. In transfer studies, test items are constructed with a different set of symbols than what is used in the learning set while the grammatical rules remain the same (10, 21, 22). Evidence of transfer would provide further evidence that the implicit acquisition of abstract rules is intact in patients with schizophrenia.
Our results are likely to have clinical implications. They suggest that the basic abilities that make it possible to learn implicitly in various everyday life situations and during development are not grossly impaired by schizophrenia. This might explain why, despite the wide range of cognitive impairments that have been reported, everything does not go astray in patients with schizophrenia. Moreover, our results extend to knowledge acquisition the finding that conscious awareness at retrieval is a critical determinant of memory performance. They suggest that it is not the complexity per se of the to-be-acquired knowledge that is the limiting rate factor, since, would this be the case, the acquisition of the complex knowledge underlying artificial grammar learning would not have been possible. Rather, the limiting factor seems to be the explicit nature of the situation, i.e., that the acquisition or retrieval of information requires conscious awareness. Finally, our results have implications for cognitive remediation and for the development of strategies that rely on intact processes to compensate for impaired processes. Since patients are still able to acquire complex knowledge, provided that acquisition is implicit, interventions targeted at implicit learning processes should make it possible for patients to acquire new knowledge relevant to everyday life such as that involved in social situations or professional skills.
 |
Received Aug. 8, 2000; revision received Dec. 12, 2000; accepted Dec. 19, 2000. From INSERM Unité 405, Clinique Psychiatrique, Hôpitaux Universitaires de Strasbourg; and the Faculté de Psychologie, Service de Neuropsychologie, Université de Liège, Liège, Belgium. Address reprint requests to Dr. Danion, INSERM Unité 405, Clinique Psychiatrique, Hôpitaux Universitaires de Strasbourg, 1 place de l’Hôpital – BP 426, 67091 Strasbourg Cedex, France.
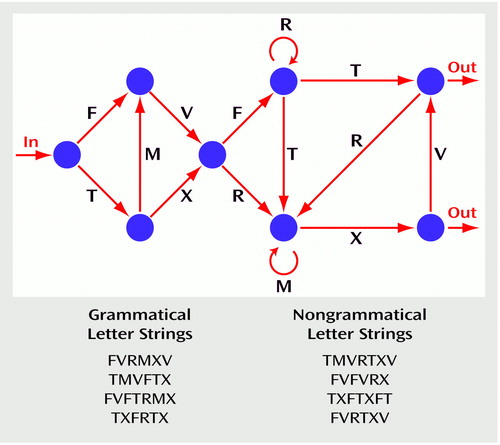
Figure 1. Finite-State Grammar Paradigma Used to Measure Implicit Learning Ability in 48 Patients With Schizophrenia and 24 Healthy Comparison Subjects
aMarkovian rule system adapted from Mathews et al. (10).
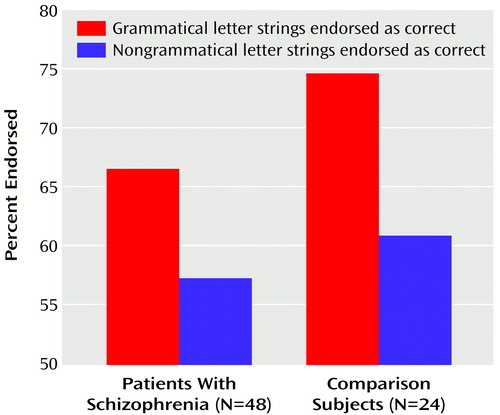
Figure 2. Percentage of Grammatical and Nongrammatical Letter Strings Endorsed as Correct by Patients With Schizophrenia and Healthy Comparison Subjects Given an Artificial Grammar Learning Task
1. Heinrichs WR, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
2. Aleman A, Hijman R, de Haan EHF, Kahn RS: Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 1999; 156:1358–1366Google Scholar
3. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
4. Gras-Vincendon A, Danion JM, Grangé D, Bilik M, Willard-Schroeder D, Sichel JP, Singer L: Explicit memory, repetition priming and cognitive skill learning in schizophrenia. Schizophr Res 1994; 13:117–126Crossref, Medline, Google Scholar
5. Huron C, Danion JM, Giacomoni F, Grange D, Robert P, Rizzo L: Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry 1995; 152:1737–1742Google Scholar
6. Danion JM, Rizzo L, Bruant A: Severe impairment of recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:639–644Crossref, Medline, Google Scholar
7. Reber AS: Implicit Learning and Tacit Knowledge: An Essay on the Cognitive Unconscious. New York, Oxford University Press, 1993Google Scholar
8. Lewicki P, Hill T, Czyzewska M: Nonconscious acquisition of information. Am Psychol 1992; 47:796–801Crossref, Medline, Google Scholar
9. Conway MA, Pleydell-Pearce CW: The construction of autobiographical memories in the Self-Memory System. Psychological Rev 2000; 107:261–288Crossref, Medline, Google Scholar
10. Mathews RC, Buss RR, Stanley WB, Blanchard-Fields F, Cho JR, Druhan B: Role of implicit and explicit processes in learning from examples: a synergistic effect. J Exp Psychol Learn Mem Cogn 1989; 15:1083–1100Google Scholar
11. Reber AS, Lewis S: Implicit learning: an analysis of the form and structure of a body of tacit knowledge. Cognition 1977; 5:333–361Crossref, Google Scholar
12. Reber AS: Implicit learning and tacit knowledge. J Exp Psychol Gen 1989; 118:219–235Crossref, Google Scholar
13. Servan-Schreiber E, Anderson JR: Learning artificial grammars with competitive chunking. J Exp Psychol Learn Mem Cogn 1990; 16:592–608Crossref, Google Scholar
14. Knowlton BJ, Ramus SJ, Squire LR: Intact artificial grammar learning in amnesia: dissociation of classification learning and explicit memory for specific instances. Psychol Sci 1992; 3:172–179Crossref, Google Scholar
15. Knowlton BJ, Squire LR: The information acquired during artificial grammar learning. J Exp Psychol Learn Mem Cogn 1994; 20:79–91Crossref, Medline, Google Scholar
16. Meulemans T, Van der Linden M: Associative chunk strength in artificial grammar learning. J Exp Psychol Learn Mem Cogn 1997; 23:1007–1028Google Scholar
17. Wechsler D: Echelle clinique de mémoire de Wechsler-révisée: Manuel. Paris, Centre de Psychologie Appliquée, 1991Google Scholar
18. Wilson BA, Lockburn J, Baddeley A: The Rivermead Behavioral Memory Test. Reading, UK, Thames Valley Test Co, National Rehabilitation Services, Gaylord, 1985Google Scholar
19. Peigneux P, Meulemans T, Van der Linden M, Salmon E, Petit H: Exploration of implicit artificial grammar learning in Parkinson’s disease. Acta Neurol Belg 1999; 99:107–117Medline, Google Scholar
20. Knowlton BJ, Squire LR: Artificial grammar learning depends on implicit acquisition of both abstract and exemplar-specific information. J Exp Psychol Learn Mem Cogn 1996; 22:169–181Crossref, Medline, Google Scholar
21. Altmann GTM, Dienes Z, Goode A: Modality independence of implicitly learned grammatical knowledge. J Exp Psychol Learn Mem Cogn 1995; 21:899–912Crossref, Google Scholar
22. Gomez RL: Transfer and complexity in artificial grammar learning. Cogn Psychol 1997; 33:154–207Crossref, Medline, Google Scholar



