Brain Regional α-[11C]Methyl-l-Tryptophan Trapping in Impulsive Subjects With Borderline Personality Disorder
Abstract
OBJECTIVE: Neurotransmission of serotonin (or 5-hydroxytryptamine [5-HT]) is thought to be disturbed in patients exhibiting impulsive behaviors. However, until recently it has not been possible to test this hypothesis in the brains of living humans. METHOD: Unidirectional trapping of the 5-HT precursor analog α-[11C]methyl-l-tryptophan (α-[11C]MTrp) has been proposed as an index of 5-HT synthesis capacity. The authors measured brain regional α-[11C]MTrp trapping with positron emission tomography in medication-free subjects with borderline personality disorder (N=13) and a healthy comparison group (N=11). Impulsivity was assessed by using a laboratory measure of behavioral disinhibition, go/no-go commission errors. RESULTS: Compared to healthy men, the men with borderline personality disorder had significantly lower α-[11C]MTrp trapping in corticostriatal sites, including the medial frontal gyrus, anterior cingulate gyrus, superior temporal gyrus, and corpus striatum. In the women with borderline personality disorder, significantly lower α-[11C]MTrp trapping was seen in fewer regions, but in both men and women, negative correlations with impulsivity scores were identified in the medial frontal gyrus, anterior cingulate gyrus, temporal gyrus, and striatum. CONCLUSIONS: Low 5-HT synthesis capacity in corticostriatal pathways may contribute to the development of impulsive behaviors in persons with borderline personality disorder.
Patients with borderline personality disorder are characterized by impulsive aggressive behaviors, repeated self-injury, suicidal behavior, affective lability, and disturbed, chaotic relationships. Across diagnostic categories, these behaviors have been associated with indices of low serotonin (or 5-hydroxytryptamine [5-HT]) neurotransmission: low CSF concentrations of 5-hydroxyindoleacetic acid (1–3), blunted prolactin responses to 5-HT agonists (4), and disturbances to markers on platelets and in plasma (5, 6). Acute tryptophan depletion, a procedure that transiently decreases 5-HT neurotransmission, has been reported to increase impulsive (7) and aggressive (8, 9) behaviors. Together, these studies support the hypothesis that low serotonergic tone plays an etiological role in the pathophysiology of behavioral disinhibition/impulsivity. However, an important caveat to most of these studies is that they relied on peripheral indices of 5-HT function, which at best represent crude measures of neurotransmission in the brain.
Recent developments in functional neuroimaging have enabled researchers to reassess the 5-HT hypothesis of impulsivity by more directly measuring aspects of 5-HT neurotransmission in the brains of living humans. One such method is positron emission tomography (PET) with the tracer α-[11C]methyl-l-tryptophan (α-[11C]MTrp). α-MTrp is a synthetic analog of the 5-HT precursor l-tryptophan. The methyl group prevents the tracer’s incorporation into protein (10). Autoradiography studies indicate that α-MTrp is taken up by 5-HT neurons (11), where it is trapped in the 5-HT synthesis precursor pool and/or metabolized into α-methylserotonin (11, 12). It has been proposed that the rate of this irreversible trapping of α-[11C]MTrp (K*) provides an index of 5-HT synthesis capacity (12).
In the present study, we measured impulsivity and α-[11C]MTrp trapping in medication-free subjects with borderline personality disorder and a healthy comparison group. Impulsivity was defined as the number of commission errors on a go/no-go task. Performances on the go/no-go task were used to map brain 5-HT pathways possibly involved in the expression of behavioral disinhibition/impulsivity.
Method
Subjects
The primary entry criteria for the subjects with borderline personality disorder were satisfaction of the criteria for borderline personality disorder of both DSM-IV and the Diagnostic Interview for Borderline Patients—Revised (DIB-R) (13) (for the latter, we used the recommended cutoff of at least eight out of 10), no current major depression or substance abuse, never having used the putative 5-HT neurotoxins 3,4-methylenedioxymethamphetamine (MDMA) or 3,4-methylenedioxyamphetamine (MDA), and being right-handed. On the go/no-go task, all but one of the subjects with borderline personality disorder scored at least 1 standard deviation above the mean of our normative data.
Volunteers were recruited by newspaper advertisements. The advertisement to recruit subjects with borderline personality disorder described four features: moodiness, temper dysregulation, self-injury, and relationship problems. Approximately 331 respondents were assessed on the telephone, 35 were assessed in person, and 17 were accepted into the study, 14 of whom were scanned. All of the borderline personality disorder subjects were interviewed by one of us (M.L.) with the Structured Clinical Interview for DSM-IV (SCID) and the DIB-R. Family pedigrees were constructed, and histories of psychiatric disorders were determined in first-degree relatives by using DSM-IV criteria. For each relative, psychiatric disorders were judged to be absent, possible, probable, or definite.
All 14 subjects reported extensive histories of impulsive, affectively labile behavior and disturbed interpersonal relationships compatible with moderate to severe cluster B personality disorders, and the features were consistent with borderline personality disorder. Each subject met repeatedly with a research psychologist (M.L.); the total contact time ranged from 12 to 20 hours. Diagnoses were assigned on the basis of all available information, including interviews with the subject and, when deemed possible, other family members and previous treating clinicians. Written reports based on the assessments were reviewed independently by two psychiatrists (C.B., J.P.); all three investigators had to agree that the volunteer met the entry criteria. Seven subjects were recontacted at 1-year follow-up, and the persistence of borderline personality disorder symptoms was confirmed; the other seven could not be reached.
All of the subjects with borderline personality disorder were medication free at testing, and the shortest interval since last treatment was 6 months. The PET data from one subject with borderline personality disorder were lost because of technical difficulties.
Healthy comparison subjects were also recruited through advertisement; 92 respondents to that advertisement were assessed on the telephone, 18 were assessed in person, and 13 were accepted into the study, 11 of whom were scanned. All healthy subjects were interviewed by one of us (M.L.) with the nonpatient version of the SCID, and the reports were reviewed by another (C.B.). Acceptance into the study depended on agreement by both investigators. The exclusion criteria included a history of psychiatric disorders in the subject or a first-degree relative, a score on the Beck Depression Inventory higher than 8, and past use of MDMA or MDA.
All subjects were physically healthy, as determined by a physical examination and standard laboratory tests. On the day of the PET scan, all tested negative on a urine drug screen sensitive to cocaine, opiates, phencyclidine, LSD, tetrahydrocannabinol, and amphetamines (triage panel for drugs of abuse). Women were scanned during the follicular phase of the menstrual cycle.
Written informed consent was obtained from all subjects. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the Montreal Neurological Institute.
Impulsivity
Impulsivity was operationally defined as the number of commission errors (responding when one should not) in the punishment-reward condition of a computerized go/no-go task (14). Of the four go/no-go conditions, punishment-reward is unique in that it is necessary to delay responding (pushing a button) to learn the task. High numbers of commission errors might reflect difficulty delaying and inhibiting behavior. In a larger study group (15), punishment-reward was the go/no-go condition that best discriminated subjects with borderline personality disorder from the healthy comparison group.
Tryptophan Measurement
Three 2-ml venous blood samples were drawn from each subject during the PET study. The samples were centrifuged, and the ultrafiltrate was stored at –80°C for measurement of plasma free tryptophan with high-performance liquid chromatography.
PET and MRI
α-[11C]MTrp was prepared as described previously (16). The studies were carried out in the late morning or early afternoon (scan start time, 11:00 a.m. to 3:00 p.m.) after an overnight fast. The day before the PET study, the subjects received low-protein meals to reduce variability in plasma amino acid concentrations attributable to differences in diet. All subjects were scanned with an ECAT HR+ tomograph (CTI/Siemens). Three-dimensional dynamic images were blurred to 8.1 mm full width at half maximum in the transaxial direction by using a Hanning filter. Between 200 and 800 MBq of α-[11C]MTrp were administered intravenously over 2 minutes. All studies consisted of 60-minute dynamic PET scans, starting at the onset of tracer injection. Thirteen blood samples were drawn from an antecubital vein to plot plasma time activity curves. Before tracer injection, transmission scans were performed by using gallium-68 for attenuation correction.
Each subject underwent a magnetic resonance imaging (MRI) scan (160 slices, 1 mm thick) obtained with a 1.5-T Philips Gyroscan. Coregistration of the individual PET and MRI images was performed by using an automatic procedure (17), and the PET images were then transferred into Talairach space (18).
Calculation of α-MTrp Trapping
Input functions were estimated with the venous-sinus-normalized venous plasma radioactivity curve (19). To calculate α-MTrp trapping, distribution volumes (milliliters per gram) were fitted as a function of exposure time (minutes) (20). The unidirectional trapping of the tracer was then determined by using data points obtained from 20 to 60 minutes, during which apparent steady state is achieved (19). Functional images of α-MTrp trapping were then calculated pixel by pixel (21).
Statistical Parametric Mapping Analysis
The functional PET data were analyzed with SPM96 (22). Each functional image in Talairach space was smoothed by using a Gaussian filter (14 mm full width at half maximum) to reduce the effect of individual variability in cortical gyral anatomy. Proportional scaling was applied to remove the effect of global differences on regional values among subjects. Functional images were normalized by the mean global values. Statistical parametric mapping (SPM) comparisons were made at a threshold of 80% of the peak value, the usual cut-off in SPM studies of regional cerebral blood flow (rCBF). The t test was applied pixel by pixel to determine regional differences between groups. The t values were then converted to a normal standard distribution (z values), independent of the degree of freedom of the error, and this distribution constituted the statistical parametric map. The errors associated with the pixel values have been shown to approximate a Gaussian distribution (21). Statistically significant regional differences were identified by using dual criteria. First, the height threshold (u) used to interpret the t test in terms of probability level was set at z=2.58 (p=0.01, two-tailed), with each cluster requiring a peak of z≥3.0. Second, the extent threshold (k) was set as 100 voxels, suitable for the 14-mm full width at half maximum filter and sufficient to remove small noisy clusters, which may reach significance by chance. The distribution of significant clusters (SPM{t}) was depicted by rendering significant pixel clusters onto three-dimensional images of the brain. Since sex-related differences in regional α-[11C]MTrp trapping have been reported (23), men and women were analyzed separately in the group comparisons.
We also identified regions where α-MTrp trapping correlated with the number of punishment-reward commission errors. Using SPM96, we generated pixel-by-pixel correlational maps and identified clusters that met the preceding criteria for statistical significance.
Statistical Analyses
Unless otherwise specified, the data were analyzed with 1) analyses of variance followed by Newman-Keuls post hoc tests or 2) Fisher’s exact tests.
Results
Subject Characteristics
All of the subjects in the borderline personality disorder group met both the DSM-IV and DIB-R criteria for borderline personality disorder as well as the DSM-IV criteria for at least one other psychiatric disorder, typically a personality disorder or past mood or substance use disorder. None met the criteria for current major depression or substance abuse. All but one of the eight female subjects with borderline personality disorder reported a childhood history of physical or sexual abuse, and the rate was significantly higher than that for men (one of five) (Fisher’s exact test, p<0.02).
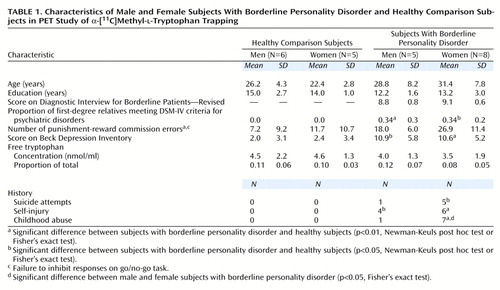
Five of the 13 subjects with borderline personality disorder had had at least one psychiatric hospitalization, most commonly after a suicide attempt. All of the subjects with borderline personality disorder had been medication free for a minimum of 6 months; six had previously received prescriptions for psychotropic medications, although only three had ever taken them; for those three, the intervals since the end of their last treatments were 6, 24, and 214 months, respectively. The subjects with borderline personality disorder had significantly higher scores on the Beck Depression Inventory and Hamilton Depression Rating Scale than the comparison subjects (Newman-Keuls post hoc test, p<0.001), but the scores were still within the subclinical range. Age did not differ between groups when analyzed according to sex (Table 1).
There were neither group nor sex differences in plasma free tryptophan concentration or in the fraction of free tryptophan (free/total) (p>0.30) (Table 1).
Impulsivity: Go/No-Go Commission Errors
Compared to the healthy subjects, the subjects with borderline personality disorder made significantly more commission errors collapsed over all four conditions (F=19.48, df=1, 20, p<0.0003). As reflected by a significant group-by-condition interaction (F=4.71, df=3, 60, p<0.006), the effect varied with the type of commission error. As seen in a larger study group (15), the subjects with borderline personality disorder made significantly more punishment-reward commission errors than did the healthy comparison group (Newman-Keuls post hoc test, p<0.0002) (Table 1).
Global and Regional α-[11C]MTrp Trapping
There was neither a significant effect of group (F=1.39, df=1, 20, p=0.25) nor a group-by-sex interaction (F=0.55, df=1, 20, p=0.46) for global K* values. There was, though, a significant effect of sex (F=4.32, df=1, 20, p=0.05) (men: mean=2.55 ml/g per minute, SD=1.6; women: mean=3.99 ml/g per minute, SD=1.9).
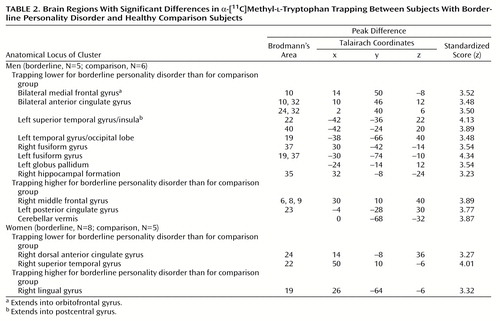
In the men, α-[11C]MTrp trapping (Table 2, Figure 1) was significantly lower for the subjects with borderline personality disorder than for the healthy subjects in the bilateral medial frontal cortex (peak difference in Brodmann’s area 10) extending into the orbitofrontal cortex and bilateral anterior cingulate (Brodmann’s areas 24, 32), the left superior temporal gyrus extending into the parietal lobe (Brodmann’s areas 22, 40), both the right (Brodmann’s area 37) and left (Brodmann’s areas 19, 37) fusiform gyri, the left inferior temporal gyrus extending into the occipital lobe (Brodmann’s area 19), the left corpus striatum, and the right hippocampal formation (Brodmann’s area 35). α-[11C]MTrp trapping was significantly higher in the men with borderline personality disorder than in the comparison men in the left posterior cingulate gyrus (Brodmann’s area 23), the right middle frontal gyrus (Brodmann’s areas 6, 8, 9), and the cerebellar vermis (Table 2, Figure 1). After Bonferroni corrections for multiple comparisons, α-[11C]MTrp trapping remained significantly lower in two clusters: 1) the left medial frontal cortex, rostral anterior cingulate, and orbitofrontal cortex and 2) the right fusiform gyrus.
In the women, group differences in α-[11C]MTrp trapping were also found, although they were not identical to those identified in men. Relative to the healthy women, the women with borderline personality disorder had significantly lower α-[11C]MTrp trapping in the right superior temporal gyrus (Brodmann’s area 22) and right middle cingulate gyrus (Brodmann’s area 24) and significantly higher values in the right lingual gyrus (Brodmann’s area 19) (Table 2, Figure 2).
Correlations Between α-[11C]MTrp Trapping and Impulsivity
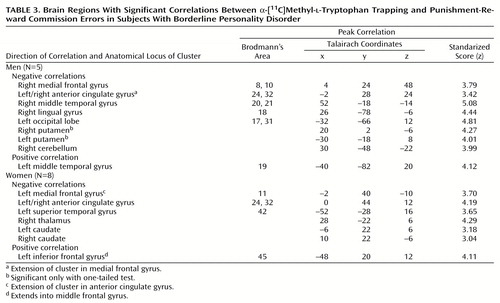
In the women with borderline personality disorder, significant correlations between punishment-reward commission errors and α-[11C]MTrp trapping were seen in many of the same regions where group differences were identified in men. Significant negative correlations were seen in the left medial frontal cortex (Brodmann’s area 11), bilateral anterior cingulate (Brodmann’s areas 24, 32), left superior temporal gyrus (Brodmann’s area 42), right posterior thalamus, and bilateral caudate. A positive correlational cluster was identified in the left inferior frontal gyrus (Brodmann’s area 45) (Table 3).
In the men with borderline personality disorder, punishment-reward commission errors correlated negatively with α-[11C]MTrp trapping in regions strikingly similar to those seen in women: right medial frontal gyrus (Brodmann’s areas 8, 10), bilateral anterior cingulate gyrus (Brodmann’s areas 24, 32), right middle temporal gyrus (Brodmann’s areas 20, 21), right gyrus lingualis (Brodmann’s area 18), and left occipital lobe (Brodmann’s areas 17, 31). A positive correlation with punishment-reward commission errors was found in the left middle temporal gyrus (Brodmann’s area 19). Whereas the women had significant negative correlations in the bilateral striatum, those for the men only approached significance, achieving it only with one-tailed tests (Table 3).
Discussion
To our knowledge, the present study is the first investigation in vivo of an index of presynaptic 5-HT neurotransmission in the brains of unmedicated subjects with borderline personality disorder. The results suggest that 1) as a group, borderline personality disorder subjects exhibit disturbed α-[11C]MTrp trapping in both the cortex and basal ganglia and 2) α-[11C]MTrp trapping correlates in selective brain pathways with a measure of impulsivity.
Compared to healthy subjects, subjects with borderline personality disorder have higher levels of α-[11C]MTrp trapping in some brain regions and less in others. Both men and women with borderline personality disorder exhibited low α-[11C]MTrp trapping in aspects of the superior temporal gyrus (Brodmann’s area 22) and anterior cingulate (Brodmann’s area 24). In the male subjects, low α-[11C]MTrp trapping was also observed in the medial frontal cortex (Brodmann’s area 10) extending into the orbitofrontal cortex, as well as in the corpus striatum. These sites correspond to regions thought to have important roles mediating the planning, initiation, and inhibition of goal-directed behaviors (frontal cortex, orbitofrontal cortex, anterior cingulate gyrus, striatum), working memory (medial frontal cortex, anterior cingulate, and superior temporal gyrus), and affect (temporal cortex, limbic areas).
The regions identified in the present study have also been implicated in previous functional neuroimaging studies of patients with a wide range of clinically impulsive disorders. Glucose metabolism and rCBF appear to be low in the frontal cortex and their subcortical connections, including the prefrontal and premotor cortex, anterior cingulate, thalamus, and caudate-putamen (24, 25). Lifetime histories of impulsive, aggressive acts have been reported to correlate negatively with glucose metabolism in the medial frontal cortex, anterior frontal cortex, orbitofrontal cortex, and temporal cortex (24). This hypocortical activity might be related to low 5-HT neurotransmission. A recent report (26) suggested that in patients with cluster B personality disorders, rCBF responses to a fenfluramine challenge—an indirect measure of serotonergic responsivity—are blunted in the prefrontal, cingulate, and orbitofrontal cortices.
The statistical parametric maps of the differences between the borderline personality disorder and comparison groups differed for men and women. A sex difference in α-[11C]MTrp trapping might be related to gender differences in the behavioral phenotype. As seen in larger study groups, more of the men than women met the criteria for antisocial personality disorder (two of five versus zero of eight), narcissistic personality disorder (two of five versus zero of eight), and a past substance use disorder (five of five versus four of eight), and fewer had a history of binge eating (one of five versus five of eight).
The subjects with borderline personality disorder made significantly more punishment-reward commission errors on the go/no-go task than did the healthy subjects. This finding is consistent with the clinical observation that patients with borderline personality disorder have difficulty inhibiting behavior and/or delaying responses. Among our subjects with borderline personality disorder, punishment-reward commission errors correlated with α-[11C]MTrp trapping. These correlations were observed within or near brain regions that differentiated these subjects from the healthy comparison group. In both the men and women with borderline personality disorder, punishment-reward commission errors were negatively correlated with α-[11C]MTrp trapping in the medial frontal gyrus, anterior cingulate gyrus, temporal cortex, and striatum. These results suggest that 1) the degrees of dysregulated impulse control and α-[11C]MTrp trapping are related and 2) female subjects with borderline personality disorder who have high impulsivity scores have serotonergic disturbances in the same regions as men with borderline personality disorder.
The validity of these findings rests on the following considerations. The α-MTrp method is a new technique, and concerns have been expressed regarding the physiological significance of tracer trapping in the brain. In particular, it has been argued that the α-MTrp method images only transport of tryptophan through the blood-brain barrier (27, 28) and/or its incorporation into the protein precursor pool (29). Some of these concerns have been addressed in detail elsewhere (30, 31). To further characterize the kinetics of α-MTrp and tryptophan in the brain, the brain regional uptake and trapping constants for labeled tryptophan and α-MTrp were recently compared in rats for the two metabolic pathways, the 5-HT metabolic pathway and tryptophan incorporation into proteins (32). The results indicate that, in the 5-HT metabolic pathway, the brain uptake and trapping constants of labeled tryptophan and α-MTrp are significantly correlated (p=0.0007). In comparison, the tissue uptake and trapping constants of α-MTrp do not correlate with the incorporation of tryptophan into proteins or the transport of either tryptophan or α-MTrp across the blood-brain barrier, as assessed by the permeability surface area product (32–34). Moreover, the regional α-[11C]MTrp distribution volume dynamic curves in the brain are consistent with trapping of the tracer. A two-compartment fit of distribution volume data would be expected to show that, over time, the distribution volume would plateau, whereas in the case of a three-compartment fit, the distribution volume would be expected to increase, as a reflection of trapping. As recently noted by others (28), adding a constant for irreversible trapping of the tracer significantly improves fit. This observation, together with the finding that α-MTrp trapping correlates with tryptophan metabolism in the 5-HT pathway, supports the contention that α-[11C]MTrp brain uptake and trapping, termed 5-HT synthesis capacity (12), relates directly to regional 5-HT synthesis.
There are several other issues for consideration. First, the size of the study group was modest (N=13). Second, the subjects met the criteria not only for borderline personality disorder but also, on average, for two to three additional diagnoses, typically other cluster B disorders, past substance abuse, or past mood disorders. The possible relation between abnormal α-[11C]MTrp trapping and these other disorders is unclear. Preliminary results (35) suggest that major depression is associated with disturbances in α-[11C]MTrp trapping that overlap, although are not identical to, those seen in the present study. Third, the female subjects with borderline personality disorder tended to be older than the healthy women, but most were in their 20s and 30s.
In conclusion, the PET/α-[11C]MTrp method is relatively new, and the present results should be seen as exploratory. However, the study suggests that, in at least some patients with borderline personality disorder, α-[11C]MTrp trapping is disturbed in neocortical and limbic structures, and these alterations could contribute to decreasing the threshold for behavioral disinhibition. Neither impulsivity nor disturbances to 5-HT neurotransmission are likely to account for the entire clinical picture of borderline personality disorder, though (36). Instead, the etiology of personality disorders is undoubtedly complex and multifactorial, including various genetic, neurobiological, developmental, psychodynamic, and psychosocial factors, all thought to contribute to the diverse clinical expressions of borderline personality disorder.
 |
 |
 |
Revised version of reports presented at the 36th annual meeting of the American College of Neuropsychopharmacology, Kamuela, Hawaii, Dec. 8–12, 1997, and the 37th annual meeting of the American College for Neuropsychopharmacology, Las Croabas, Puerto Rico, Dec. 14–18, 1998. Received April 3, 2000; revision received Nov. 2, 2000; accepted Nov. 27, 2000. From the Department of Psychiatry and the Department of Neurology and Neurosurgery, McGill University. Address reprint requests to Dr. Benkelfat, Department of Psychiatry, McGill University, Research and Training Building, 1033 Pine Ave. West, Montréal, Québec H3A 1A1, Canada; [email protected] (e-mail). Supported by an operating grant from the Fonds de la Recherche en Santé du Québec to Dr. Benkelfat, a salary award from Fonds de la Recherche en Santé du Québec to Dr. Benkelfat, a Scientist Award from the Medical Research Council of Canada to Dr. Blier, and a Young Investigators Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Leyton. The authors thank Rick Fukusawa, Gary Sauchuk, Dean Jolly, and Mirjana Kovacevic for technical assistance, Dr. Robert Lisbona for help during the PET scans, and Drs. Robert Cohen and Scott Rauch for their comments on previous versions of this article.
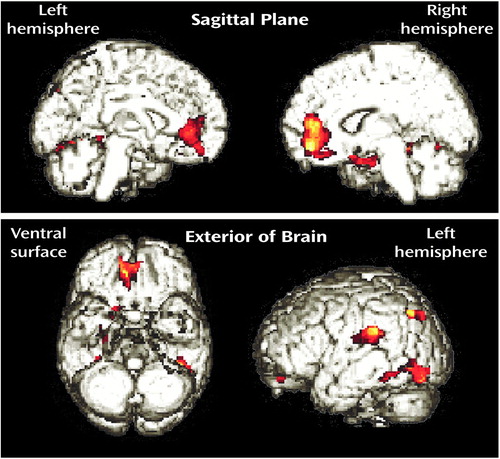
Figure 1. Statistical Parametric Maps (SPM{t}) Showing Brain Regions Where Trapping of α-[11C]Methyl- l-Tryptophan Was Significantly Lower in Men With Borderline Personality Disorder (N=5) Than in Healthy Men (N=6)a
aThe significant clusters are superimposed on rendered MRI images.
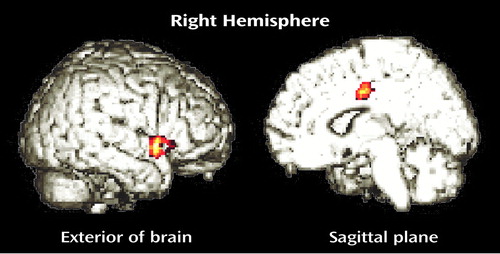
Figure 2. Statistical Parametric Maps (SPM{t}) Showing Brain Regions Where Trapping of α-[11C]Methyl- l-Tryptophan Was Significantly Lower in Women With Borderline Personality Disorder (N=8) Than in Healthy Women (N=5)a
aThe significant clusters are superimposed on rendered MRI images.
1. Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK: Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 1982; 139:741–746Link, Google Scholar
2. Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal fluid 5–hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Google Scholar
3. Åsberg M: Neurotransmitters and suicidal behavior: the evidence from cerebrospinal fluid studies. Ann NY Acad Sci 1997; 836:158–181Crossref, Medline, Google Scholar
4. Coccaro EF: Central serotonin and impulsive aggression. Br J Psychiatry 1989; 155(suppl 8):52–62Google Scholar
5. Coccaro EF, Kavoussi RJ, Sheline YI, Lish JD, Csernansky JG: Impulsive aggression in personality disorder correlates with tritiated paroxetine binding in the platelet. Arch Gen Psychiatry 1996; 53:531–536Crossref, Medline, Google Scholar
6. Mann JJ, McBride PA, Anderson GM, Mieczkowski TA: Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiatry 1992; 32:243–257Crossref, Medline, Google Scholar
7. LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN: Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. Am J Psychiatry 1999; 156:1771–1779Google Scholar
8. Cleare AJ, Bond AJ: The effect of tryptophan depletion and enhancement on subjective and behavioural aggression in normal male subjects. Psychopharmacology (Berl) 1995; 118:72–81Crossref, Medline, Google Scholar
9. Moeller FG, Dougherty DM, Swann AC, Collins D, Davis CM, Cherek DR: Tryptophan depletion and aggressive responding in healthy males. Psychopharmacology (Berl) 1996; 126:97–103Crossref, Medline, Google Scholar
10. Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL: A new method to measure brain serotonin synthesis in vivo, I: theory and basic data for a biological model. J Cereb Blood Flow Metab 1990; 10:1–12Crossref, Medline, Google Scholar
11. Cohen Z, Tsuiki K, Takada A, Beaudet A, Diksic M, Hamel E: In vivo-synthesized radioactively labelled α-methyl serotonin as a selective tracer for visualization of brain serotonin neurons. Synapse 1995; 21:21–28Crossref, Medline, Google Scholar
12. Chugani DC, Muzik O: α-[11C]Methyl-l-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab 2000; 20:2–9Crossref, Medline, Google Scholar
13. Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL: The revised Diagnostic Interview for Borderlines: discriminating BPD from other axis II disorders. J Personal Disord 1989; 3:10–18Crossref, Google Scholar
14. LeMarquand DG, Pihl RO, Young SN, Tremblay RE, Séguin JR, Palmour RM, Benkelfat C: Tryptophan depletion, executive functions, and disinhibition in aggressive, adolescent males. Neuropsychopharmacology 1998; 19:333–341Crossref, Medline, Google Scholar
15. Leyton M, Young SN, Paris J, Pihl RO, Benkelfat C: Suicide attempts and impulsivity (abstract). Biol Psychiatry 1999; 45(suppl 8):146SGoogle Scholar
16. Mzengeza S, Venkatachalam TK, Diksic M: Asymmetric radiosynthesis of α-[11C]methyl-l-tryptophan for PET studies. Nucl Med Biol 1995; 22:303–307Crossref, Medline, Google Scholar
17. Collins DL, Neelin P, Peter TM, Evans AC: Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994; 18:192–205Crossref, Medline, Google Scholar
18. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
19. Nishizawa S, Leyton M, Okazawa H, Benkelfat C, Mzengeza S, Diksic M: Validation of a less invasive method for measurement of serotonin synthesis rate with α-[11C]methyl-tryptophan. J Cereb Blood Flow Metab 1998; 18:1121–1129Google Scholar
20. Patlak CS, Blasberg RG, Fenstermacher JD: Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3:1–7Crossref, Medline, Google Scholar
21. Okazawa H, Diksic M: Image generation of the serotonin synthesis rate using α-methyl-tryptophan and PET. J Comput Assist Tomogr 1998; 22:777–785Crossref, Medline, Google Scholar
22. Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RSJ: The relationship between global and local changes in PET studies. J Cereb Blood Flow Metab 1990; 10:458–466Crossref, Medline, Google Scholar
23. Okazawa H, Leyton M, Benkelfat C, Mzengeza S, Diksic M: Application of statistical mapping analysis to serotonin synthesis images generated in healthy volunteers using PET and α-[11C]methyl-l-tryptophan. J Psychiatry Neurosci 2000; 25:359–370Medline, Google Scholar
24. Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schulz SC, Cohen RM: Positron-emission tomography and personality disorders. Neuropsychopharmacology 1994; 10:21–28Crossref, Medline, Google Scholar
25. Kuruoglu AC, Arikan Z, Vural G, Karatas M, Arac M, Isik E: Single photon emission computerized tomography in chronic alcoholism: antisocial personality disorder may be associated with decreased frontal perfusion. Br J Psychiatry 1996; 169:348–354Crossref, Medline, Google Scholar
26. Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, Sevin E, Nunn M, Mitropoulou V: d,l-Fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 1999; 20:413–423Crossref, Medline, Google Scholar
27. Shoaf SE, Carson R, Hommer D, Williams W, Higley JD, Schmall B, Herscovitch P, Eckelman W, Linnoila M: Brain serotonin synthesis rates in rhesus monkeys determined by [11C]α-methyl-l-tryptophan and positron emission tomography compared to CSF 5-hydroxyindole-3-acetic acid concentrations. Neuropsychopharmacology 1998; 19:345–353Crossref, Medline, Google Scholar
28. Shoaf SE, Carson RE, Hommer D, Williams WA, Higley JD, Schmall B, Herscovitch P, Eckelman WC, Linnoila M: The suitability of [11C]-alpha-methyl-l-tryptophan as a tracer for serotonin synthesis: studies with dual administration of [11C] and [14C] labeled tracer. J Cereb Blood Flow Metab 2000; 20:244–252Crossref, Medline, Google Scholar
29. Gharib A, Balende C, Sarda N, Weissmann D, Plenevaux A, Luxen A, Bobillier P, Pujol JF: Biochemical and autoradiographic measurements of brain serotonin synthesis rate in the freely moving rat: a reexamination of the alpha-methyl-l-tryptophan method. J Neurochem 1999; 72:2593–2600Google Scholar
30. Benkelfat C, Young SN, Okazawa H, Leyton M, Diksic M: The validity of the PET/alpha-[11C]methyl-l-tryptophan method for measuring rates of serotonin synthesis in the human brain. Neuropsychopharmacology 1999; 21:153–157Crossref, Medline, Google Scholar
31. Diksic M, Leyton M, Benkelfat C: Is alpha-methyl-l-tryptophan a good tracer for brain serotonin synthesis measurements, and does the lumped constant vary in different structures of the rat brain? J Neurochem 1999; 73:2621–2624Google Scholar
32. Diksic M, Tohyama Y, Takada A: Brain net unidirectional uptake of alpha-[14c]methyl-L-tryptophan (alpha-MTrp) and its correlation with regional serotonin synthesis, tryptophan incorporation into proteins, and permeability surface area products of tryptophan and alpha-MTrp. Neurochem Res 2000; 25:1537–1546Google Scholar
33. Takada A, Grdisa M, Gjedde A, Yamamoto YL: Rapid steady-state analysis of blood-brain transfer of L-Trp in rat, with special reference to the plasma protein binding. Neurochem Int 1983; 23:351–359Crossref, Google Scholar
34. Fenstermacher JD, Blasberg RG, Patlak C: Methods for quantifying the transport of drugs across brain barrier systems. Pharmacol Ther 1981; 14:P217–P248Google Scholar
35. Rosa P, Diksic M, Okazawa H, Debonnel G, Blier P, Mzengeza S, Kapczinski F, Leyton M, Benkelfat C: Rates of brain 5-HT synthesis in patients with major depression, in Abstracts of Panels and Posters, 38th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, ACNP, 1999, p 261Google Scholar
36. Siever LJ, Davis KL: A psychobiological perspective on the personality disorders. Am J Psychiatry 1991; 148:1647–1658Google Scholar



