Effects of Sustained-Release Bupropion and Supportive Group Therapy on Cigarette Consumption in Patients With Schizophrenia
Abstract
OBJECTIVE: The study examined the efficacy, tolerability, and safety of supportive group psychotherapy and adjunctive sustained-release bupropion for nicotine addiction in patients with schizophrenia. METHOD: Eight patients participated in a 14-week open-label trial. End expired breath carbon monoxide level, symptom levels, neuropsychological performance, and suppression of the P50 event-related potential were measured before and after the 14-week trial. RESULTS: Patients showed a decrease in carbon monoxide levels that was not associated with any worsening in symptom, neuropsychological, or P50 suppression measures. CONCLUSIONS: Use of sustained-release bupropion in combination with supportive group therapy may help patients with schizophrenia decrease their cigarette consumption.
The rate of cigarette smoking among patients with schizophrenia is as much as three times as high as that of the normal population (1), and yet these patients have less access to smoking cessation programs than the normal population (2). Smoking places patients at increased risk for nonpsychiatric medical morbidity and mortality. In addition, smoking places them at greater psychiatric risk, because components in cigarette smoke stimulate hepatic microsomal enzymes, increasing the rate of metabolism of psychotropic medications (3). Until recently, nicotine addiction in patients with schizophrenia has been understudied, and it continues to be undertreated. Although these patients may be less able to comply with traditional smoking cessation programs, some studies have supported their ability to respond to psychoeducational smoking cessation programs that have been modified to meet their needs (2). Other research has supported the use of sustained-release bupropion in the treatment of nicotine addiction in the general population (4).
The main goal of the pilot study reported here was to determine if a combination of supportive group therapy and sustained-release bupropion would be a safe and effective treatment for nicotine addiction in patients with schizophrenia. Nicotine consumption has been hypothesized to modulate auditory hallucinations and attention, and therefore, we also measured concomitant changes in patients’ symptoms, suppression of the P50 event-related potential (5), and cognitive abilities (6).
Method
Nine medically stable outpatients from the Maryland Psychiatric Research Center volunteered for the study. Patients had stable cigarette smoking habits and expressed interest in decreasing their smoking. Patients with neurologic illnesses, a current depressive episode, or active substance abuse and those receiving bupropion were excluded from participation. All patients had a DSM-IV diagnosis of either schizophrenia or schizoaffective disorder and were judged to be clinically stable by their treatment team. All patients provided written informed consent before study entry.
The study design included a 2-week stabilization period to obtain baseline measures and a 14-week treatment phase. During the treatment phase, patients participated in nine sessions of weekly group therapy led by clinic nurses trained in the educational model of the American Cancer Society Fresh Start Program, modified for patients with schizophrenia (2). Subjects were told that the goal was to stop smoking, but they were encouraged to continue to participate even if they were not successful in complete cessation. Adjunctive sustained-release bupropion was started at the third group session, initially 150 mg once a day for 3 days and then 150 mg twice a day for the remainder of the study. Adjunctive nicotine replacement was not prescribed.
The primary measure of cigarette consumption was end expired breath carbon monoxide level (7), since patients’ self-reports of the number of cigarettes smoked per day were suspected to be unreliable. A EC 50 Micro III Smokerlyzer breath carbon monoxide monitor (Bedfont Scientific, Kent, U.K.) was used to measure patients’ end expired breath carbon monoxide weekly throughout the study, immediately before or immediately after each support group, which met at 2:00 p.m. Clinical assessments were obtained during the first week of stabilization, at the beginning of treatment with sustained-release bupropion (week 2), on the smoking quit day (week 4), at the end of the support group (week 8), and at study completion (week 14). Clinical assessments were done with the Brief Psychiatric Rating Scale (BPRS), the Scale for the Assessment of Negative Symptoms (SANS) (8), and the Fagerstrom Test for Nicotine Dependence (9). During the second week of the stabilization period and at study completion (week 14), the patients received a brief neuropsychological test battery consisting of the Rey Auditory Verbal Learning Test (10) and the Brief Visuospatial Memory Test (11) to measure auditory and visual-spatial memory, the Gordon Diagnostic System Continuous Performance Task (12) to measure attention, and the Grooved Pegboard test (13) to measure manual dexterity. Measures of P50 suppression (ratio of response to successive auditory clicks [S2/S1]) (14) were also obtained during the second week of stabilization and at study completion.
T tests were used to assess baseline and end-of-study differences in end expired breath carbon monoxide level, symptom ratings, neuropsychological test performance, and P50 suppression.
Results
Nine subjects entered the study, and eight completed the study. One subject dropped out in the first week of sustained-release bupropion treatment owing to a rash thought to be secondary to the study medication. Two patients complained of orthostatic dizziness, and one of them required a decrease in the sustained-release bupropion dose. One patient complained of an initial feeling of agitation that resolved without intervention.
Of the subjects who completed the study, five were Caucasian, three were African American, and seven were male. The mean age of the eight subjects who completed the study was 48.05 years (SD=9.29), and their mean duration of illness was 29.68 years (SD=10.95). Their mean baseline score on the Fagerstrom Test for Nicotine Dependence was 6.13 (SD=2.10), which represents a high level of dependency.
Subjects’ mean end expired breath carbon monoxide level decreased over the treatment phase (Figure 1). The mean carbon monoxide level was 39.44 ppm (SD=21.30) at baseline (the average of the two measures during the stabilization period) and 18.38 (SD=11.96) at week 14, for a mean change of 21.06 (SD=22.25) (t=–2.68, df=7, p≤0.05). The decrease in carbon monoxide level between baseline and treatment week 2 was not significant (p=0.07).
Between baseline and week 14, there were no significant changes (all p>0.20) in the BPRS positive symptom score (mean=10.63, SD=4.53, versus mean=10.63, SD=3.81), the BPRS anxiety item (mean=2.63, SD=1.19, versus mean=1.88, SD=0.83), or the BPRS depression item (mean=1.63, SD=1.19, versus mean=1.75, SD=1.16). There were no significant changes from baseline to week 14 on any of the cognitive measures (all p>0.05) or on the measure of P50 suppression (p=0.80). Mean scores on these measures at baseline and week 14 were as follows: Rey Auditory Verbal Learning Test, mean=37.63 (SD=6.21) versus 35.63 (SD=9.65); Brief Visuospatial Memory Test, mean=18.38 (SD=7.11) versus 20.25 (SD=9.24); Continuous Performance Task total correct, mean=35.75 (SD=8.14) versus 31.25 (SD=9.38); and P50 S2/S1 ratio, mean=0.56 (SD=0.34) versus 0.57 (SD=0.34). Treatment with sustained-release bupropion was associated with an 18% decrease in the mean SANS total score from 29.38 (SD=5.53) at baseline to 24.13 (SD=7.61) at week 14, but the difference was not significant (t=–1.78, df=7, p=0.12). There was a significant reduction in the mean SANS alogia factor score over the course of the study from 3.00 (SD=2.98) to 1.13 (SD=1.25), for a mean change of 1.88 (SD=1.89) (t=2.18, df=7, p≤0.05).
Four of eight patients chose to continue treatment with sustained-release bupropion for an additional 12 weeks after the conclusion of the open-label study. One of those patients subsequently stopped smoking completely. Two of the remaining three had further decreases in their expired breath carbon monoxide levels, and the third patient maintained the decrease he had achieved at study completion.
Conclusions
Although none of the subjects quit smoking during the study, adjunctive use of sustained-release bupropion in combination with supportive group therapy seemed to help patients with schizophrenia decrease their cigarette consumption, as measured by the change in their expired breath carbon monoxide levels. The decrease in cigarette consumption began before the initiation of sustained-release bupropion and before the designated quit day and then stabilized after treatment week 4. The early reduction in cigarette consumption may have been due to the group therapy itself and/or to the subjects’ heightened attention to smoking cessation.
Similarly positive findings have been seen with group therapy and adjunctive use of the nicotine patch (15) and in studies of smoking behavior before and after treatment with clozapine (16). It is difficult to compare these findings with those of the current study, since the primary outcome measures and treatment settings were different.
Patients generally tolerated sustained-release bupropion well. There was no associated worsening of positive symptoms or anxiety. There was no evidence of cognitive worsening or of change in suppression of the P50 event-related potential, which might have been expected with loss of nicotine stimulation. These findings are difficult to interpret, as no patients stopped smoking completely during the study, and it is not known how much nicotine stimulation is required to enhance these cognitive processes. The finding of a potential benefit of sustained-release bupropion in improving negative symptoms is of clinical interest but would need to be evaluated with a more rigorous study design.
Although the current study is limited by the small sample size, the open-label design, and the lack of strict inclusion criteria regarding cigarette consumption, it provides preliminary support for the use of a combination of group therapy and adjunctive sustained-release bupropion for the treatment of nicotine addiction in schizophrenia. Further double-blind placebo-controlled studies of this combination of interventions are warranted.
Received June 13, 2000; revision received Sept. 29, 2000; accepted Oct. 9, 2000. From the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland at Baltimore. Address reprint requests to Dr. Weiner, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228; [email protected] (e-mail). Supported in part by NIMH grant MH-40279.
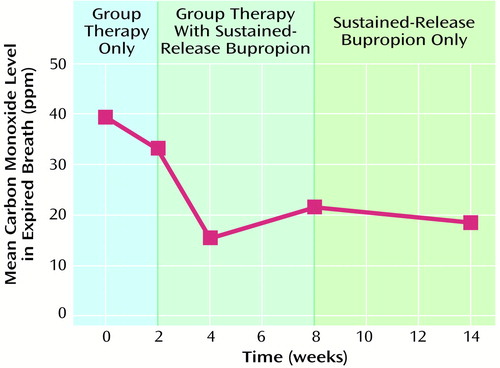
Figure 1. Expired Breath Carbon Monoxide Levels in a 14-Week Open-Label Trial of Supportive Group Psychotherapy and Adjunctive Sustained-Release Bupropion for Nicotine Addiction in Patients With Schizophrenia (N=8)
1. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
2. Addington J, el-Guebaly N, Campbell W, Hodgins DC, Addington D: Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998; 155:974–976Link, Google Scholar
3. Benowitz NL: Drug therapy: pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 1988; 319:1318–1330Google Scholar
4. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB: A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340:685–691Crossref, Medline, Google Scholar
5. Dalack GW, Meador-Woodruff JH: Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res 1996; 22:133–141Crossref, Medline, Google Scholar
6. White HK, Levin ED: Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999; 143:158–165Crossref, Medline, Google Scholar
7. Henningfield JE, Stitzer ML, Griffiths RR: Expired air carbon monoxide accumulation and elimination as a function of number of cigarettes smoked. Addict Behav 1980; 5:265–272Crossref, Medline, Google Scholar
8. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
9. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO: The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Google Scholar
10. Rey A: L’Examen Clinique en Psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
11. Benedict RHB, Schretlen D, Groninger L, Dobraski M, Sphritz B: Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychol Assess 1996; 8:145–153Crossref, Google Scholar
12. Gordon M, Mettelman B: The assessment of attention: I. Standardization and reliability of a behavior-based measure. J Clin Psychol 1988; 44:682–690Crossref, Medline, Google Scholar
13. Klove H: Clinical neuropsychology. Medical Clinics of North America 1963; 47:1647–1658Google Scholar
14. Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M: Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Arch Gen Psychiatry 1996; 53:1114–1121Google Scholar
15. Hartman N, Leong GB, Glynn SM, Wilkins JN, Jarvik ME: Transdermal nicotine and smoking behavior in psychiatric patients. Am J Psychiatry 1991; 148:374–375Link, Google Scholar
16. McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J: Clozapine decreases smoking in patients with chronic schizophrenia. Biol Psychiatry 1995; 37:550–552Crossref, Medline, Google Scholar



