Traumatic Brain Injury and Schizophrenia in Members of Schizophrenia and Bipolar Disorder Pedigrees
Abstract
OBJECTIVE: Schizophrenia following a traumatic brain injury could be a phenocopy of genetic schizophrenia or the consequence of a gene-environment interaction. Alternatively, traumatic brain injury and schizophrenia could be spuriously associated if those who are predisposed to develop schizophrenia have greater amounts of trauma for other reasons. The authors investigated the relationship between traumatic brain injury and psychiatric diagnoses in a large group of subjects from families with at least two biologically related first-degree relatives with schizophrenia, schizoaffective disorder, or bipolar disorder. METHOD: The Diagnostic Interview for Genetic Studies was used to determine history of traumatic brain injury and diagnosis for 1,275 members of multiplex bipolar disorder pedigrees and 565 members of multiplex schizophrenia pedigrees. RESULTS: Rates of traumatic brain injury were significantly higher for those with a diagnosis of schizophrenia, bipolar disorder, and depression than for those with no mental illness. However, multivariate analysis of within-pedigree data showed that mental illness was related to traumatic brain injury only in the schizophrenia pedigrees. Independent of diagnoses, family members of those with schizophrenia were more likely to have had traumatic brain injury than were members of the bipolar disorder pedigrees. The members of the schizophrenia pedigrees also failed to show the gender difference for traumatic brain injury (more common in men than in women) that was expected and was present in the bipolar disorder pedigrees. Subjects with a schizophrenia diagnosis who were members of the bipolar disorder pedigrees (and thus had less genetic vulnerability to schizophrenia) were less likely to have had traumatic brain injury (4.5%) than were subjects with schizophrenia who were members of the schizophrenia pedigrees (and who had greater genetic vulnerability to schizophrenia) (19.6%). CONCLUSIONS: Members of the schizophrenia pedigrees, even those without a schizophrenia diagnosis, had greater exposure to traumatic brain injury compared to members of the bipolar disorder pedigrees. Within the schizophrenia pedigrees, traumatic brain injury was associated with a greater risk of schizophrenia, consistent with synergistic effects between genetic vulnerability for schizophrenia and traumatic brain injury. Posttraumatic-brain-injury schizophrenia in multiplex schizophrenia pedigrees does not appear to be a phenocopy of the genetic disorder.
Traumatic brain injuries and later serious psychopathology have long been associated. Kraepelin (1), in 1919, stated that head injuries in childhood might either cause or release predisposition to schizophrenia, implicating a causative role for traumatic brain injury for psychiatric illness. Traumatic brain injury is a reasonable exposure to examine with respect to later mental illness. Recent estimates of the annual incidence of head injury range from 100 in 100,000 to 444 in 100,000 (2, 3). Although only 16% of those with head injuries are admitted to the hospital, 80% receive medical attention (4), and their injuries are documented. The period of greatest risk is from the mid-teens through the mid-20s, before the age at risk for onset of most major psychiatric disorders, and men have a several-fold higher risk for traumatic brain injury than women (2, 3), perhaps related to greater impulsivity and/or other psychomotor behavior.
Broad ranges of neuropsychiatric dysfunction, ranging from subtle to devastating, are related to traumatic brain injury, including cognitive, psychotic, mood, anxious, and aggressive disorders and Alzheimer’s dementia (5). Depression is the most common psychiatric sequela of traumatic brain injury, with estimates as high as 39% after mild injury and 77% after severe injury (6–10). Posttraumatic-brain-injury depression occurs within 6 months in about half of the cases (7) and is unrelated to loss of consciousness, skull fracture, or accompanying physical and cognitive impairments (6, 9, 11, 12). The symptoms of posttraumatic depression are similar to those of functional mood disorders (13). Mania following traumatic brain injury is uncommon but may accompany right basal temporal and orbitofrontal damage (6, 14).
Increased rates of psychosis also follow traumatic brain injury. This posttraumatic psychosis is relatively indistinguishable from schizophrenia (15, 16), and its onset is often remote from the injury. Reported rates of posttraumatic-brain-injury psychosis range from 0.07% to 9.8%, with increasing prevalence over time (17). Assuming a lifetime prevalence of 0.8% for schizophrenia, Davison and Bagley (17) concluded that traumatic brain injury increased the prevalence of schizophrenia by two- to threefold over 10–20 years. More recent studies support these findings (18, 19), as do data from a 10,000-member national Finnish cohort, in which 7.6% of patients with traumatic brain injury went on to develop chronic psychotic and delusional disorders (20). Retrospective studies also demonstrate elevated rates of traumatic brain injury among schizophrenia patients (16). Although some studies suggest that subsequent psychosis is related to left-sided and temporal lobe injuries (17, 21), other studies find no relationship of injury type or location to subsequent psychosis (22). The relationship of traumatic brain injury and psychotic disorders is enigmatic, since injury can precede the onset of illness by many years and the type and neuroanatomic location of the injury appears to be unrelated to the ensuing diagnosis.
There are several ways to interpret the relationship of traumatic brain injury and schizophrenia, which are not mutually exclusive. Traumatic brain injury may cause a phenocopy of the genetic form of schizophrenia. If this is the case, then patients with schizophrenia in whom the disorder is highly familial might have lower rates of traumatic brain injury than patients in whom the disorder is nonfamilial. Schizophrenia related to traumatic brain injury could also be the result of a gene-environment interaction, with traumatic brain injury lowering the threshold for expressing schizophrenia in those with genetic vulnerability to the disorder. If so, then those with genetic loading and traumatic brain injury would have higher rates of schizophrenia than those with a similar genetic risk but no traumatic brain injury. The association could also be spurious. Early illness features of schizophrenia such as agitation or psychosis might increase exposure to traumatic brain injury. If that is true, then the head injury does not cause the schizophrenia, even though it is associated with schizophrenia, and rates of traumatic brain injury would be similar among schizophrenia patients who have different etiologies for their illness.
These associations are difficult to examine prior to the identification of disease genes. However, we were able to model the effects of genes and traumatic brain injury by using membership in multiplex schizophrenia pedigrees as a proxy for the greater genetic loading for schizophrenia and membership in bipolar disorder pedigrees as a proxy for the presence of relatively less genetic loading for schizophrenia. These well-characterized subjects were recruited for genetic linkage studies and had received structured diagnostic interviews that included assessments of traumatic brain injury and lifetime symptoms. Each subject was categorized by sex, psychiatric diagnosis (phenotype), history of traumatic brain injury, and whether he or she was a member of the schizophrenia or bipolar disorder pedigree study groups.
Method
The subjects for this study participated in the National Institute of Mental Health (NIMH) Genetics Initiative for Schizophrenia and Bipolar Disorders, a cooperative project to ascertain multiplex schizophrenia and bipolar disorder families involving NIMH and several university sites (Harvard, Washington, and Columbia Universities for schizophrenia, and John Hopkins, Washington, and Indiana Universities for bipolar illness). All sites used common diagnostic procedures so that joint analyses of the two pedigree sets could be undertaken. Subjects came from families with at least two biologically related first-degree relatives diagnosed with the core illness. For schizophrenia sites, enrolled pedigrees contained at least one relative pair in which one member was diagnosed with schizophrenia and the other was diagnosed with schizophrenia or schizoaffective depression. For the bipolar disorder pedigrees, core diagnoses were bipolar illness in one relative and bipolar or schizoaffective disorder (bipolar type) in another biological first-degree relative (23). Subjects were interviewed with the Diagnostic Interview for Genetic Studies (24), and their clinical data were entered into the centralized database established by the NIMH (SRA Technologies, Falls Church, Va.) as of April 1996. The Diagnostic Interview for Genetic Studies directly asks about the subject’s education and occupational functioning. Occupational functioning is expressed numerically, reflecting the level of managerial or professional responsibility, with lower numbers reflecting positions with higher levels of responsibility (according to a modification of the Hollingshead Four-Factor Index of Social Position [25]).
The Diagnostic Interview for Genetic Studies question on head injury (24) showed good reliability in the interviews conducted for an intersite test-retest diagnostic reliability study (kappa=0.83, χ2=45.63, df=1, p<0.0001 [unpublished data]). For each subject affirming traumatic brain injury in the SRA database we obtained copies of the interview notes and reviewed the written information and the interview narrative. From the interview notes we rated the information on head injury, further noting the number of head injuries, the date(s) of injury, the subject’s age, the nature of injury, and the duration of any loss of consciousness. Severity of head injury was also categorized as follows: no loss of consciousness, mild traumatic brain injury (loss of consciousness of “probably” to 5 minutes), moderate traumatic brain injury (loss of consciousness for 6–15 minutes), and severe traumatic brain injury (loss of consciousness for >15 minutes).
The subjects in the two pedigree groups were described demographically, and the two groups’ rates of head injury and gender differences for head injury were compared. The presence of psychiatric diagnoses among those with head injury was determined, and the exposure variable (head injury) was also examined by using more stringent criteria to define its presence (i.e., degree of loss of consciousness and duration). We used univariate analyses to examine the association of traumatic brain injury with psychiatric diagnoses in both the combined pedigree groups and within each pedigree group. Multiple logistic regression analyses were used to examine relationships between traumatic brain injury and particular psychiatric diagnoses, controlling for age, gender, and substance abuse.
Results
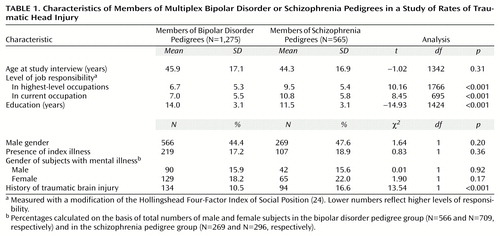
The bipolar disorder pedigree group included 1,275 subjects (566 male and 709 female subjects), and the schizophrenia pedigree group included 565 subjects (269 male and 296 female subjects). As seen in Table 1, the bipolar and schizophrenia pedigree study groups did not differ in mean age at interview, gender, or percentage of individuals with the core illnesses. Compared with the schizophrenia pedigree subjects, the bipolar disorder pedigree subjects had significantly more years of education and higher levels of responsibility in their current occupation and in the highest-level occupation they had ever had.
More schizophrenia than bipolar disorder pedigree subjects reported traumatic brain injury (odds ratio=1.70, 95% confidence interval [CI]=1.28–2.26). However, the distribution of the levels of severity of head injury among those reporting traumatic brain injury did not differ between the study groups. Among schizophrenia and bipolar disorder pedigree members who had traumatic brain injury reported, no loss of consciousness was reported, respectively, by 24.5% (N=23) and 22.4% (N=30), loss of consciousness <5 minutes by 9.6% (N=9) and 13.4% (N=18), loss of consciousness between 6–15 minutes by 3.2% (N=3) and 3.7% (N=5), loss of consciousness >15 minutes by 4.3% (N=4) and 8.2% (N=11), and unconsciousness for an unknown duration by 23.4% (N=22) and 18.7% (N=25); 35.1% (N=33) and 33.6% (N=45), respectively, did not know if they lost consciousness with their brain injury (χ2=2.80, df=5, p=0.73).
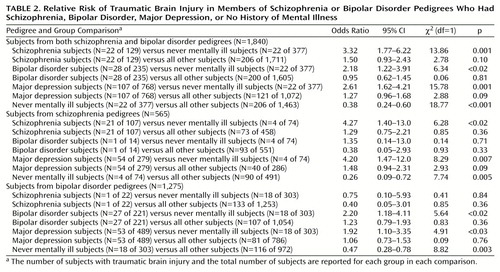
For the combined schizophrenia and bipolar disorder pedigrees, univariate analyses showed that traumatic brain injury was associated with schizophrenia, bipolar disorder, and major depression diagnoses, compared with no history of mental illness (Table 2). However, there was no higher exposure to traumatic brain injury for subjects with any of the three disorders in comparison to all other subjects in their respective pedigrees (Table 2). The odds ratio for traumatic brain injury was elevated for substance abusers only in comparison to the never mentally ill subjects in the schizophrenia pedigrees (odds ratio=4.73, 95% CI=1.61–13.9, χ2=8.19, df=1, p=0.004) and bipolar disorder pedigrees (odds ratio=2.09, 95% CI=1.03–4.24, χ2=3.59, df=1, p<0.06). Compared to all other pedigree members, the substance abusers were not significantly more likely to have traumatic brain injury in either the schizophrenia pedigrees (odds ratio=1.55, 95% CI=0.97–2.48, χ2=3.33, df=1, p<0.07) or the bipolar disorder pedigrees (odds ratio=1.14, 95% CI=0.66–1.99, χ2=0.22, df=1, p=0.64). A similar effect was seen for alcohol abuse, with elevated rates of traumatic brain injury for alcohol abusers in comparison to the never mentally ill subjects in the schizophrenia pedigrees (odds ratio=3.80, 95% CI=1.20–12.0, χ2=4.65, df=1, p<0.04) and the bipolar disorder pedigrees (odds ratio=2.64, 95% CI=1.39–4.99, χ2=8.44, df=1, p=0.004), but not in comparison to all other family members in those respective pedigrees (odds ratio=1.11, 95% CI=0.60–2.03, χ2=0.11, df=1, p=0.75, and odds ratio=1.52, 95% CI=0.95–2.42, χ2=3.07, df=1, p=0.08).
Multiple logistic regression analyses were used to examine relationships between traumatic brain injury and psychiatric diagnoses, controlling for age and gender. In the combined pedigrees, the only significant effects explaining traumatic brain injury were schizophrenia diagnosis (odds ratio=1.96, 95% CI=1.19–3.20,Wald χ2=7.09, df=1, p=0.008) and sex (fewer female subjects: odds ratio=0.46, 95% CI=0.33–0.62, Wald χ2=24.33, df=1, p<0.0001). When substance abuse was also included in the regression equation, the association of traumatic brain injury and gender was unchanged, the association between traumatic brain injury and schizophrenia was strengthened (odds ratio=2.06, 95% CI=1.25–3.38, Wald χ2=8.01, df=1, p=0.005), and no significant relationship was found between traumatic brain injury and substance abuse (odds ratio=1.33, 95% CI=0.89–1.98, Wald χ2=1.91, df=1, p=0.17). Within the bipolar disorder pedigree group, only sex was related to traumatic brain injury, with a threefold lower risk for female than for male subjects (odds ratio=0.34, 95% CI=0.22–0.53, Wald χ2=22.73, df=1, p<0.0001). Including substance abuse as an independent variable did not change the gender differences for traumatic brain injury. Within the schizophrenia pedigrees, only schizophrenia diagnosis was related to traumatic brain injury (odds ratio=2.06, 95% CI=1.04–4.06, Wald χ2=4.32, df=1, p=0.04), with the higher exposure to traumatic brain injury among those with a schizophrenia diagnosis also being unchanged by the inclusion of alcohol and substance abuse as independent variables.
The use of the alternative thresholds to define traumatic brain injury (i.e., no loss of consciousness, and mild, moderate, and severe traumatic brain injury) affected the proportions of patients and nonpatients considered to have had traumatic brain injury but did not change any of the associations between traumatic brain injury and illness. Those who had never been mentally ill were significantly less likely to have had traumatic brain injury compared to the other subjects in all analyses. The never mentally ill subjects from the schizophrenia pedigrees were even less likely to have had traumatic brain injury than those from the bipolar disorder pedigrees. A significant gender effect was present in the bipolar disorder pedigree group, with 14.8% of male subjects (N=84) but only 6.5% of female subjects (N=46) having had traumatic brain injury (χ2=24.04, df=1, p<0.0001, odds ratio=2.51, 95% CI=1.72–3.67). There was only a marginal gender difference in the schizophrenia pedigree group (19.3% of male subjects [N=52] and 13.5% of female subjects [N=40] having had traumatic brain injury) (χ2=3.56, df=1, p<0.07, odds ratio=1.53, 95% CI=0.98–2.41).
In multiple logistic regression analyses examining the association of traumatic brain injury and pedigree type, controlling for age, sex, schizophrenia or bipolar disorder diagnosis, traumatic brain injury was still significantly higher in the schizophrenia pedigree group relative to the bipolar disorder pedigree group (odds ratio=1.92, 95% CI=1.35–2.70, Wald χ2=14.82, df=1, p=0.001). There was still a significant association of traumatic brain injury with male gender (odds ratio=2.17, 95% CI=1.66–3.03, Wald χ2=23.06, df=1, p=0.001), but the association of traumatic brain injury with schizophrenia diagnosis was weakened (odds ratio=1.36, 95% CI=0.80–2.30, Wald χ2=1.54, df=1, p=0.22). Bipolar disorder remained unrelated to traumatic brain injury (odds ratio=1.10, 95% CI=0.67–1.82, Wald χ2=0.22, df=1, p=0.64).
Next we examined if traumatic brain injury among relatives was correlated to their degree of relationship to the proband. A correlation might be expected if a history of traumatic brain injury among relatives was related to genetic vulnerability and if the probands had the highest level of genetic vulnerability among their relatives. The association between traumatic brain injury and degree of relationship to the proband approached statistical significance in the schizophrenia pedigree (χ2=8.89, df=4, p<0.07), but was not significant in the bipolar pedigree group (χ2=5.57, df=4, p=0.23).
Both pedigree types contained subjects diagnosed with schizophrenia. If traumatic brain injury causes an illness phenocopy, then a stronger association of traumatic brain injury and illness would be expected in those with lower genetic risk. This effect was not observed. Only 4.5% of subjects with schizophrenia from the bipolar disorder pedigrees (one of 22 subjects) had traumatic brain injury, compared to 19.6% of subjects with schizophrenia from the schizophrenia pedigrees (21 of 107 subjects). Thus fewer subjects with nonfamilial schizophrenia had traumatic brain injury exposure than those with familial schizophrenia (odds ratio=0.19, 95% CI=0.02–1.56, χ2=2.93, df=1, p<0.09). Likewise, for subjects with bipolar disorder, 7.1% of those from schizophrenia pedigrees (lower genetic load) had traumatic brain injury (one of 14 subjects), compared to 12.2% of those from bipolar disorder pedigrees (27 of 221 subjects) (odds ratio=0.55, 95% CI=0.07–4.39, χ2=0.32, df=1, p=0.57). Subjects with major depression from the schizophrenia pedigrees had greater exposure to traumatic brain injury (19.4%, N=54 of 279 subjects), than did subjects with major depression from the bipolar disorder pedigrees, who had a greater genetic loading for affective illness (10.8%, N=53 of 489 subjects) (odds ratio=1.97, 95% CI=1.31–2.99, χ2=10.74, df=1, p=0.001).
Discussion
This study found a threefold greater rate of reported traumatic brain injury for subjects with schizophrenia and twofold excess for those with bipolar and unipolar depression only when the subjects were compared to their never-mentally-ill family members. This association of illness and traumatic brain injury is similar to that found in other prospective and retrospective studies of individuals with head injuries and psychiatric patients. However, we had the advantage of comparing rates of traumatic brain injury for those with psychiatric diagnoses with the rates for all of their family members, controlling for age and sex. In these multivariate analyses only subjects with schizophrenia retained a significant twofold elevation in rate of traumatic brain injury, compared to the other members of the schizophrenia pedigrees.
Traumatic brain injury was related to schizophrenia even though the subjects selected for the study were at high genetic risk for schizophrenia. If traumatic brain injury related to schizophrenia were a phenocopy of genetic illness, as has been proposed, then greater exposure to traumatic brain injury might be expected for those with lesser genetic vulnerability. However, traumatic brain injury and schizophrenia were associated only in the subjects from the multiplex schizophrenia pedigrees. Subjects with schizophrenia from the bipolar disorder pedigrees actually were less likely to have had traumatic brain injury than the other members of the bipolar disorder pedigrees. Membership in the multiplex schizophrenia pedigrees independently increased the risk for both schizophrenia and traumatic brain injury, and traumatic brain injury exposure was associated with a further elevated risk of schizophrenia. These findings are consistent with a model in which family factors increases exposure to traumatic brain injury and traumatic brain injury further increases the risk of illness in those with genetic vulnerability, as expected from proposed synergistic effects of genes and traumatic brain injury exposure on the risk of schizophrenia. Indeed, a protective role of avoiding traumatic brain injury in schizophrenia families is suggested by the finding that schizophrenia pedigree members who had never been mentally ill were four times less likely than other family members to have had traumatic brain injury, while never-mentally-ill members of the bipolar disorder pedigrees were only half as likely as other family members to have had traumatic brain injury.
We also found that subjects from the schizophrenia pedigrees had significantly greater traumatic brain injury exposure than subjects from the bipolar disorder pedigrees, independent of psychiatric diagnoses. Perhaps increased exposure to traumatic brain injury is a consequence of an inherited diathesis of attentional abnormality in schizophrenia families. If so, this may contribute to the lack of a gender difference in head injury in the schizophrenia pedigrees, as vulnerability to injury is increased for men and women by impaired attention and vigilance (26). This would also be in keeping with models in which certain genotypes are associated with increased exposure to environmental factors (27). Schizophrenia genes may increase exposure to head trauma, with head trauma further increasing the risk for schizophrenia.
A series of studies have demonstrated that rates of schizophrenia and neurological abnormalities are higher in the family members of subjects with schizophrenia and that these higher rates appear to be independent of each other. Schizophrenia probands and relatives show greater sensory cortex dysfunction than normal comparison subjects, substance abusers, and subjects with bipolar disorder; in addition, subjects with schizophrenia show cerebellar dysfunction (28). Patients with familial schizophrenia also have greater focal neurological signs than do patients without familial illness (29), and family members without schizophrenia (from schizophrenia families) have been reported to have greater or similar amounts of focal neurological signs than the patients themselves (30, 31).
We found bipolar illness and traumatic brain injury to be unassociated within the bipolar disorder pedigrees, but only pedigree of origin was associated with the risk for bipolar illness in controlled analyses. Although subjects with major depression from the schizophrenia pedigrees were twice as likely to have traumatic brain injury as those from the affective disorder pedigrees, this excess was similar to the overall surplus of traumatic brain injury in the schizophrenia families. It is noteworthy that the rates of depression in the schizophrenia families were as great as in the bipolar families. This finding may support reports in the literature that family members of those with schizophrenia have higher vulnerability to depression (32, 33). A review of family, twin, and adoption data concluded that affective illness and schizophrenia were associated in at least half of all published studies (34). In addition, Maier et al. (35), in a well-controlled family study comparing relatives of probands with different disorders, found higher risk of major depression for relatives of probands with bipolar disorder, unipolar depression, and schizophrenia. Some investigators have interpreted the familial association of depression and schizophrenia as evidence that psychiatric illnesses exist on a continuum from depression through schizoaffective disorders to schizophrenia, with schizophrenia being the more severe illness (36). This notion of a continuum is in contrast to the original Kraepelinian dichotomy between these syndromes. However, an overlap of brain abnormalities in schizophrenia and severe depression has been noted (34, 37, 38), and no particular abnormality has been found to be pathognomonic for either disorder, including higher ventricular brain ratios, which are also found prior to brain injury in those who develop posttraumatic-brain-injury depression (39).
Recent data support a role for gene-environment interaction in accounting for the sequela of traumatic brain injury in several neurological conditions. For example, functional recovery after brain injury has been related to differences in genes for apolipoprotein E, consistent with genetic susceptibility to the effects of brain injury (40). Likewise, epidemiological studies of Alzheimer’s disease have suggested that traumatic brain injury may not be a sufficient cause of the illness, but may significantly reduce the time to disease onset in those at risk, perhaps again because of an interaction between traumatic brain injury and the presence of the apolipoprotein E-4 allele (41).
The data reported here are limited by our inability to identify which family members carry schizophrenia vulnerability gene(s). The true analysis of joint genetic and environmental effects will require the categorization of subjects on the basis of the presence or absence of a genotype and of exposure to an environmental factor. The appropriate methodologies and conditions for examining gene-environment interaction before the identification of such genes are still being clarified. We used the subjects’ pedigree of origin as a proxy for genetic vulnerability to schizophrenia or bipolar disorder. Although our model assumed that subjects with schizophrenia in the bipolar disorder pedigrees had a nongenetic form of schizophrenia, it is possible that these subjects and the subjects with schizophrenia in the schizophrenia pedigrees share genes for severe psychiatric illness. If so, perhaps only the schizophrenic subjects from the multiplex schizophrenia pedigrees had the type of premorbid inattention or poor coordination that resulted in more accidents. Alternatively, family members in the bipolar disorder pedigrees may be protected in some way from traumatic brain injury.
A potential bias for reporting head injuries may relate to a patient’s desire to attribute severe illnesses to external or physical causes. However, subjects with similarly severe major psychiatric disorders in the alternate pedigrees differed in their rates of reported traumatic brain injury. There may also have been differential ascertainment of traumatic brain injury history for subjects from the schizophrenia and bipolar disorder pedigrees. We consider such differences to be minimal, as the same interview was used with both groups and the interrater reliability for the question on traumatic brain injury was confirmed in an intersite test-retest diagnostic reliability study involving separate interviewers from the sites where the schizophrenia and bipolar disorder pedigree members were assessed.
One might hypothesize that the severity of traumatic brain injury would be associated with greater risk for disease. However, research in this area has not generally found outcome after brain injury to be explained by severity of the injury (9, 12). For this study as well, the use of alternate definitions of traumatic brain injury exposure based on the presence or duration of loss of consciousness did not alter the association of traumatic brain injury with the psychiatric conditions. We did not categorize the severity of the psychiatric conditions of individual subjects, so it is possible that the severity of head injury is related to course, treatment response, or functional outcome among those with particular diagnoses, rather than the development of the illness per se.
The present results suggest that subjects from multiplex schizophrenia pedigrees who have schizophrenia and premorbid traumatic brain injury do not necessarily have a phenocopy of the genetic illness and may be reasonably included as candidates for genetic studies of schizophrenia.
 |
 |
Received May 2, 2000; revision received Aug. 8, 2000; accepted Oct. 16, 2000. From the New York State Psychiatric Institute and Columbia University; the Department of Psychiatry, Harvard Medical School, Boston; the Department of Psychiatry, Washington University, St. Louis; the Department of Psychiatry, Indiana University, Indianapolis; and NIMH, Bethesda, Md. Address reprint requests to Dr. Malaspina, New York State Psychiatric Institute, 722 West 168th St., New York, NY 10032; [email protected] (e-mail). Supported by the G. Harold and Leila Y. Mathers Charitable Foundation and NIMH grants MH-50727 and MH-46289.
1. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
2. Jager TE, Weiss HB, Coben JH, Pepe PE: Traumatic brain injuries evaluated in US emergency departments, 1992–1994. Acad Emerg Med 2000; 7:134–140Crossref, Medline, Google Scholar
3. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. JAMA 1999; 282:974–983Crossref, Medline, Google Scholar
4. Kraus JF, McArthur DL: Epidemiologic aspects of brain injury. Neurol Clin 1996; 14:435–450Crossref, Medline, Google Scholar
5. Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, et al (EURODEM Risk Factors Research Group): Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int J Epidemiol 1991; 20(suppl 2):S28–S35Google Scholar
6. Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F: Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disord 1993; 27:233–243Crossref, Medline, Google Scholar
7. Varney NR, Martzke JS, Roberts RJ: Major depression in patients with closed head trauma. Neuropsychology 1987; 1:7–9Crossref, Google Scholar
8. Rutherford WH: Sequelae of concussion caused by minor head injuries. Lancet 1977; 1:1–4Crossref, Medline, Google Scholar
9. Levin HS, Grossman RG: Behavioral sequelae of closed head injury: a quantitative study. Arch Neurol 1978; 35:720–727Crossref, Medline, Google Scholar
10. Silver JM, Caton CLM, Shrout PE, Dominguez B: Traumatic brain injury and schizophrenia, in 1993 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1993, p 250Google Scholar
11. Bornstein RA, Miller HB, van Schoor JT: Neuropsychological deficit and emotional disturbance in head-injured patients. J Neurosurg 1989; 70:509–513Crossref, Medline, Google Scholar
12. Cummings JL: Organic delusions: phenomenology, anatomical correlations, and review. Br J Psychiatry 1985; 146:184–197Crossref, Medline, Google Scholar
13. Federoff UP, Starkstein SE, Parikh RM, Price TR, Robinson RG: Are depressive symptoms nonspecific in patients with acute stroke? Am J Psychiatry 1991; 148:1172–1176Google Scholar
14. Robinson RG, Boston JD, Starkstein SE, Prince TR: Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry 1988; 145:172–178Link, Google Scholar
15. O’Callaghan E, Larkin C, Redmond O, Stack J, Ennis JT, Waddington JL: “Early-onset schizophrenia” after teenage head injury: a case report with magnetic resonance imaging. Br J Psychiatry 1988; 153:394–396Crossref, Medline, Google Scholar
16. Wilcox JA, Nasrallah HA: Childhood head trauma and psychosis. Psychiatry Res 1987; 21:303–306Crossref, Medline, Google Scholar
17. Davison K, Bagley CR: Schizophrenia-like psychoses associated with organic disorders of the central nervous system, in Current Problems in Neuropsychiatry. Edited by Herrington RN. Ashford, Kent, UK, Headley, 1969, pp 113–184Google Scholar
18. Violon A: Post-traumatic psychoses. Acta Neurochir Suppl (Wien) 1988; 44:67–69Medline, Google Scholar
19. Gualtieri T, Cox DR: The delayed neurobehavioural sequelae of traumatic brain injury. Brain Inj 1991; 5:219–232Crossref, Medline, Google Scholar
20. Achte K, Jarho L, Kyykka T, Vesterinen E: Paranoid disorders following war brain damage: preliminary report. Psychopathology 1991; 24:309–315Crossref, Medline, Google Scholar
21. Davison K: Schizophrenia-like psychoses associated with organic cerebral disorders: a review. Psychiatr Dev 1983; 1:1–34Medline, Google Scholar
22. De Mol J, Violon A, Brihaye J Cloninger CR: [Posttraumatic schizophrenic bouts: with regard to six cases of traumatic schizophrenia.] Encephale 8:17–24 (French)Google Scholar
23. Cloninger CR: Turning point in the design of linkage studies of schizophrenia. Am J Med Genet 1994; 54:83–92Crossref, Medline, Google Scholar
24. Nurnberger J, York Cooler C, Kaufmann CA, Malaspina D, Harkavy Friedman J, Depaul JR, Simpson S, Reich T, Gershon ES, Cloninger CR, Blehar M, Tsuang MT, Faraone SV, Pepple JR, Miller M, Wynne D, Maxwell ME, Guroff J, Kirch D: Diagnostic Interview for Genetic Studies. Arch Gen Psychiatry 1994; 51:849–859Crossref, Medline, Google Scholar
25. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
26. Pless IB, Taylor HG, Arsenault L: The relationship between vigilance deficits and traffic injuries involving children. Pediatrics 1995; 95:219–224Medline, Google Scholar
27. Kendler KS, Eaves LJ: Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry 1986; 143:279–289Link, Google Scholar
28. Kinney DK, Yurgelun-Todd DA, Woods BT: Neurologic signs of cerebellar and cortical sensory dysfunction in schizophrenics and their relatives. Schizophr Res 1999; 35:99–104Crossref, Medline, Google Scholar
29. Woods BT, Kinney DK, Yurgelun-Todd DA: Neurological “hard” signs and family history of psychosis in schizophrenia. Biol Psychiatry 1991; 30:806–816Crossref, Medline, Google Scholar
30. Kinney DK, Yurgelun-Todd DA, Woods BT: Hard neurologic signs and psychopathology in relatives of schizophrenic patients. Psychiatry Res 1991; 39:45–53Crossref, Medline, Google Scholar
31. Kinney DK, Woods BT, Yurgelun-Todd D: Neurologic abnormalities in schizophrenic patients and their families, II: neurologic and psychiatric findings in relatives. Arch Gen Psychiatry 1986; 43:665–668Crossref, Medline, Google Scholar
32. Gershon ES, Hamovit JH, Joel H, Guroff JJ, Nurnberger JI: Birth cohort changes in manic and depressive disorders in relatives of bipolar and schizoaffective patients. Arch Gen Psychiatry 1987; 44:314–319Crossref, Medline, Google Scholar
33. Farmer AE, McGuffin P, Gottesman II: Searching for the split in schizophrenia. Psychiatry Res 1984; 13:109–118Crossref, Medline, Google Scholar
34. Taylor MA: Are schizophrenia and affective disorder related? a selective literature review. Am J Psychiatry 1992; 149:22–32Link, Google Scholar
35. Maier W, Lichterman D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF: Continuity and discontinuity of affective disorders and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 1993; 50:871–883Crossref, Medline, Google Scholar
36. Crow TJ: The continuum of psychosis and its implication for the structure of the gene. Br J Psychiatry 1986; 149:419–429Crossref, Medline, Google Scholar
37. Goetz KL, van Kammen DP: Computerized axial tomography scans and subtypes of schizophrenia: a review of the literature. J Nerv Ment Dis 1986; 74:31–41Crossref, Google Scholar
38. Pearlson GD, Garbacz DJ, Tompkins RH, Ahn HS, Gutterman DF, Veroff AE, DePaulo JR: Clinical correlates of lateral ventricular enlargement in bipolar affective disorder. Am J Psychiatry 1984; 141:253–256Link, Google Scholar
39. Fedoroff JP, Starkstein SE, Forrester AW, Geisler FH, Jorge RE, Arndt SV, Robinson RG: Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Link, Google Scholar
40. Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, Babaey S, Yehuda B, Groswasser Z: Apolipoprotein E-ε4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 1999; 52:244–248Crossref, Medline, Google Scholar
41. Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT: Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol 1999; 149:32–40Crossref, Medline, Google Scholar



