Patients Admitted to Emergency Services for Drunkenness: Moderate Alcohol Users or Harmful Drinkers?
Abstract
OBJECTIVE: Most of the patients admitted to hospital emergency services are drunk. Some of them may need specific treatment after acute intoxication remits. At present, treatment for alcoholism is offered to less than 5% of these patients. The authors evaluated the biological markers carbohydrate-deficient transferrin (CDT) and γ-glutamyltransferase (GGT) in patients admitted for acute alcohol intoxication (per DSM-IV criteria) supported by blood alcohol assay. These tests distinguished between otherwise moderate alcohol users who were acutely intoxicated and harmful drinkers or alcohol-dependent patients. METHOD: The authors conducted an exhaustive survey 24 hours a day during 2 nonconsecutive months. The study involved 166 patients (124 men and 42 women) who were admitted for acute alcohol intoxication as a principal or additional diagnosis. Their blood was analyzed for alcohol, GGT, and CDT levels. The CAGE questionnaire was administered, and social and demographic data were collected. RESULTS: About 80% of the population studied displayed elevated GGT or CDT levels (65.7% had CDT levels >60 mg/liter; 41.6% had GGT levels >65 IU/liter). Less than 10% of the patients with acute alcohol intoxication revealed results in the normal range for both markers and a negative finding on the CAGE questionnaire. CONCLUSIONS: Patients admitted to emergency services with high blood alcohol levels should not be assumed to be moderate drinkers. Any drunkenness should be interpreted as a sign of likely harmful alcohol consumption or alcohol dependency requiring clinical and biological tests, including GGT and CDT assays. Specific treatment for alcoholism should be systematically offered to these patients.
The prevalence of positive blood alcohol test results or alcohol-related difficulties in patients admitted to hospital emergency services ranges widely (1–3): 10% among elderly patients (4), 50% among nonfatal automobile accident victims (5), and up to 62% for patients who have attempted suicide (6). In one California study (7), the proportion of patients admitted to emergency services who were reported as displaying positive blood alcohol levels (>1 g/liter) was 10%. In France, a study carried out at the Centre Général de Montbéliard emergency services found that 17.4% of admitted patients had positive blood alcohol test results, most of them (14.5%) ≥0.8 g/liter (8).
However, to our knowledge, no information is available on whether the patients recorded as intoxicated at admission were moderate alcohol users who occasionally got drunk, harmful drinkers (per ICD-10 criteria), alcohol abusers (per DSM-IV criteria), or alcohol-dependent patients displaying subacute or chronic alcohol intoxication. In the Californian study (7) of 91 emergency services patients with blood alcohol levels >1 g/liter, Cherpitel et al. showed that only 9% of the patients were identified by the emergency services medical staff as patients with drinking problems. Only one patient was referred for specific treatment for alcoholism. In the French study (8), after a short stay in the emergency services of the Centre Général de Montbéliard, 50% of the patients with blood alcohol levels ≥0.8 g/liter were sent home, 28% were hospitalized, and 22% were held in police custody. Specific treatment for alcoholism was offered to 2% of these patients.
Thus, in the majority of cases, no treatment for alcohol problems was offered to emergency services patients displaying acute alcohol intoxication. There are two possible explanations for this: either harmful drinkers make up only a tiny minority of such patients (2%–10%) (7, 8), or their identification is difficult for the practitioner (because of lack of knowledge and skill).
To further investigate patients being admitted to emergency services with acute alcohol intoxication (per DSM-IV 303.00 criteria) as determined by blood test results and the biological consequences of alcohol consumption, we set out to determine the proportion 1) of moderate drinkers and 2) of alcohol abusers (per DSM-IV 305.00 criteria) or alcohol-dependent patients (per DSM-IV 303.90 criteria) among them, using the biological markers of blood alcohol, GGT (9), and CDT (10) levels and the standard CAGE questionnaire (11–13).
Method
All patients aged 16–70 years who were admitted to the 24-hour-a-day emergency services area during 2 nonconsecutive months who met the criteria for acute alcohol intoxication as a principal or additional diagnosis (per DSM-IV criteria) with blood alcohol levels >0.8 g/liter were included. In France an intoxicated person ends up in emergency services only if there is evidence of need for medical or psychiatric treatment (e.g., trauma from a fall, psychotic behavior, attempted suicide, or danger to the public). Most emergencies are addressed by general practitioners, ambulance personnel, or police officers.
Subjects were excluded from the study for any of the following: refusal to give consent, serious medical condition (e.g., alcohol-induced coma, multiple trauma, or delirium tremens but not minor trauma), attempted suicide, health disorders unrelated to alcohol, or medication that modified GGT levels. A standard CBC count and blood alcohol, GGT, and CDT levels were measured within 15 minutes of admission and installation in an emergency services ward.
Examination of the patient included recording anamnestic data (antecedents and history of illness) and a clinical examination. We then waited for the patient to recover to a condition of clear-headedness compatible with an interview (disappearance of signs of acute alcohol intoxication per DSM-IV criteria). After we fully explained the study to the patients, those who gave written informed consent to participate were included. Those who refused to give informed consent were excluded. During the study, the CAGE questionnaire was used, and social and demographic information was elicited. All the patients subsequently received the results of their blood tests, in particular the GGT and CDT levels, and a specialized consultation was offered to them.
Blood alcohol was measured by means of an automated alcohol dehydrogenase enzyme method (Beckman CX 7, Paris). GGT levels were assayed by means of an enzyme method at 37 ˚C by using a Bayer Diagnostic CHEM 1 automated analyzer (Leverrusen, Germany). Normal GGT values are <65 IU/liter (14, 15). The sensitivity of the GGT measures ranged from 40% to 88%, and the specificity ranged from 50% to 90% (16–18).
Normal values of serum CDT are <60 mg/liter. The serum was stored at –20°C until it was assayed. CDT was measured by means of immunonephelometric assay on the basis of microisocratic anion-exchange chromatography, as described by Schellenberg et al. (19) of France. The CDT level has proved a good marker of harmful drinking; it has a sensitivity of about 82% and a specificity of about 97% (20, 21). Yersin et al. (10) have shown that the CDT level is a particularly sensitive marker in subjects under age 40, which age usually coincides with the clinical stage of alcohol abuse and is therefore in line with our study design.
Various studies have demonstrated the lack of any correlation between GGT and CDT levels, and the independence of these two markers (22–24). Combining them therefore increases sensitivity (86% for determining alcohol abusers and 96% for determining alcohol-dependent patients) (25).
We used the CAGE questionnaire as a standard interview for screening alcoholism. Multiple studies have shown that the CAGE questionnaire has the advantage of being fast, simple, and inexpensive, and it performs well (11, 12). A survey was conducted to validate the French version of the CAGE questionnaire. The sensitivity of the test was 83%, the specificity was 96%, and the percentage of patients correctly classified was 95.5%, resulting in a prevalence rate of 9% alcoholics, revealing that the French version of the CAGE is effective with the use of two or more positive answers as evidence for alcoholism (26).
The significance threshold was set at p<0.05. According to the distribution of the variables, data were expressed as means and standard deviations or as means, medians, and ranges. Comparisons were accomplished by means of the chi-square statistic and nonparametric tests (Mann-Whitney U and Spearman’s rank correlations).
Results
We enrolled all 178 patients who arrived at emergency services at Clermont-Ferrand University Hospital during July 1997 and March 1998 with acute alcohol intoxication. Twelve patients (6.7%) met the initial exclusion criteria; three of these refused to give consent. Thus, 166 eligible patients (93.3%) were included in the study. Most of them (N=95, 57.2%) had been admitted to emergency services between 4:00 p.m. and midnight, compared with the numbers admitted from midnight to 8:00 a.m. (N=36, 21.7%) and from 8:00 a.m. to 4:00 p.m. (N=35, 21.1%). There were 130 patients (78.3%) admitted to the medical section and 36 (21.7%) admitted to the surgical section of emergency services. Their mean age was 41.7 years (SD=11.5); 124 (74.7%) were male, and 42 (25.3%) were female. About one-half (N=87, 52.4%) lived alone, and only 44 (26.5%) were employed.
Their mean blood alcohol level was 2.41 g/liter (median=2.36, range=0.86–4.61). The highest blood alcohol levels were found in the patients admitted between 4:00 p.m. and midnight (mean=2.52 g/liter), compared with those admitted from midnight to 8:00 a.m. (mean=1.83 g/liter) and 8:00 a.m. to 4:00 p.m. (mean=2.44 g/liter). Their mean GGT level was 154 IU/liter (median=56, range=7–2,463), and their mean CDT level was 100.8 mg/liter (median=79.6, range=31.4–355.2). No significant relation was found between positive GGT (>65 IU/liter) and positive CDT (>60 mg/liter) levels (χ2=0.05, df=1, p=0.82) (rs=0.12, N=166, t=1.56, df=164, p=0.12).
The mean CDT level was significantly higher in men (107.9 mg/liter, median=87.7, range=31.4–355.2) than in women (79.8 mg/liter, median=70.5, range=35.3–269.0) (Mann-Whitney U test: z approximation=–2.42, p=0.02). The mean blood alcohol level was also significantly higher in men (2.49 g/liter, median=2.50, range=0.86–4.61) than in women (2.15 g/liter, median=2.06, range=0.98–3.78) (Mann-Whitney U test: z approximation=–2.39, p=0.02). A significant relation was found between CDT and blood alcohol levels in men (rs=0.24, N=124, t=2.68, df=122, p=0.01) but not in women (rs=–0.001, N=42, t=–0.01, df=40, p=1.00). A significant relation was found between GGT and blood alcohol levels in the entire group (rs=0.38, N=166, t=5.25, df=164, p<0.0001).
The CDT level was >60 mg/liter in 109 (65.7%) of the patients, and the GGT level was >65 IU/liter in 69 (41.6%) of the patients. Thus, 132 (79.5%) of the patients had positive GGT levels, positive CDT levels, or both (Figure 1). The CAGE questionnaire gave positive results (yes to two or more of four questions) for 137 (82.5%) of the patients (Figure 1). Hence, 151 (91.0%) of the patients admitted to emergency services with acute alcohol intoxication had at least positive GGT or positive CDT levels or a positive CAGE questionnaire result (Figure 1). In other words, only 15 (9.0%) of the patients had no positive results on the measures used (GGT or CDT levels or CAGE questionnaire).
Discussion
To our knowledge, no studies have been carried out in patients admitted to emergency services for drunkenness to determine how many of them are moderate drinkers with simple acute alcohol intoxication and how many are alcohol abusers or are alcohol dependent, whose drunkenness is a sign of subacute or chronic alcohol intoxication. Our results show that the proportion of alcohol abusers or alcohol-dependent patients among those admitted to emergency services for acute alcohol intoxication was about 80% (positive GGT or CDT levels) and possibly 90% (positive CAGE questionnaire results or positive GGT or CDT levels).
Although the CAGE questionnaire is effective, simple, and quick, biological markers make it easier to initiate a discussion with patients who often deny having drinking problems or are unwilling to address them. The GGT level does not increase in acute alcohol abuse but only after consumption of about 80–200 g of alcohol every day for several weeks (23). CDT levels increase at lower levels of alcohol consumption. Although, to our knowledge, no study of a large group of patients has ever defined the quantity, duration, or frequency of alcohol consumption required to raise CDT levels, it appears that positive values of serum CDT are attained only after a period of alcohol consumption of about 60 g for at least 3 weeks (27–29). The GGT-CDT marker combination is particularly interesting because the alcohol-induced elevation of these parameters occurs by means of different mechanisms, which is why no correlation has been found between them, either in this study or in others (24, 27). Combining the GGT level with the CDT level is thus more sensitive (25) and therefore a good indicator of alcoholism.
Hence, it is wrong to diagnose—or consider—most patients admitted to emergency services with high blood alcohol levels as moderate drinkers seen with acute alcohol intoxication. Therapeutic strategies for moderate drinkers who occasionally get drunk are fundamentally different from those that apply to patients with alcohol abuse or alcohol dependence. In particular, for subjects who consume alcohol at harmful levels, it is vital to intervene early to forestall alcohol dependency, with its increasingly high mortality (30–33). The efficiency of brief interventions for heavy drinkers is well documented (34, 35).
The detection and early treatment of patients with alcohol problems should be a major health care objective today. However, although severe alcohol dependency is easy to diagnose, signs of alcohol abuse can be elusive. Admission to emergency services for drunkenness should be considered an indicator of likely pathological alcohol use. Indeed, patients admitted to emergency services for other reasons often prove to have alcohol problems (e.g., ill-defined episodes of malaise, falls at home, or accidents at work).
It thus seems desirable to use the CAGE questionnaire systematically (24, 36) (this could be done by physicians during clinical questioning on alcohol consumption patterns) for patients admitted to emergency services. All patients with acute alcohol intoxication or with positive CAGE questionnaire results should be systematically tested for elevated GGT and CDT levels as part of a full diagnostic examination (37).
This diagnostic approach might help correct the mistaken medical assumption that most patients with drunkenness are probably merely acutely intoxicated with alcohol. Any case of drunkenness in emergency services should be considered a clinical finding suggestive of alcohol abuse or alcohol dependency, requiring appropriate specialized examination and care, including medical psychological and social therapy.
Received Oct. 26, 1999; revisions received May 2 and July 26, 2000; accepted Aug. 10, 2000. From Medical Psychology Center B, the Biochemistry Department, the Department of Biostatistics and Medical Information Technology, and the Department of Emergency Medicine, University Medical School, Clermont-Ferrand. Address reprint requests to Dr. Schwan, Centre Médico-Psychologique-B, rue Montalembert, F-63003 Clermont-Ferrand Cedex 1, France; [email protected] (e-mail). The authors thank Drs. A. Feral, D. Boussiron, and A. LeBlanc for their work and Drs. M. Plane and E. Nigon for organizational assistance.
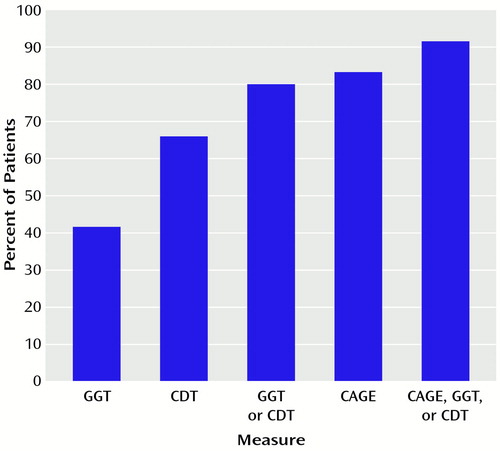
Figure 1. Frequency of Biological Markers of Alcoholisma or a CAGE Questionnaire Positive Findingb Among 166 Patients Admitted to Emergency Services With Acute Alcohol Intoxication
aCarbohydrate-deficient transferrin (CDT) level >60 mg/liter; γ-glutamyltransferase (GGT) level >65 IU/liter.
bYes to two or more of four questions.
1. Sandra C, Lapham SC, Skipper BJ, Brown P, Chadbunchachai W, Suriyawongpaisal P, Paisarnsilp S: Prevalence of alcohol problems among emergency room patients in Thailand. Addiction 1998; 93:1231–1239Google Scholar
2. Macdonald S, Wells S, Giesbrecht N, Cherpitel CJ: Demographic and substance use factors related to violent and accidental injuries: results from an emergency room study. Drug Alcohol Depend 1999; 55:53–61Crossref, Medline, Google Scholar
3. Borges G, Medina-Mora ME, Cherpitel C, Casanova L: Alcohol beverage consumption in the patients of the emergency services of the city of Pachuca, Hidalgo. Salud Publica Mex 1999; 41:3–11Crossref, Medline, Google Scholar
4. Callahan CM, Tierney WM: Health services use and mortality in older primary care patients with alcoholism. J Am Geriatr Soc 1995; 43:1378–1383Google Scholar
5. Gentilello LM, Duggan P, Drummond D, Tonnesen A, Degener EE, Fischer RP, Reed L: Major injury as a unique opportunity to initiate treatment in the alcoholic. Am Surg 1988; 156:558–561Crossref, Google Scholar
6. Soukas J, Lonnqvist J: Suicide attempts in which alcohol is involved: a special group in general hospital emergency rooms. Acta Psychiatr Scand 1995; 91:36–40Crossref, Medline, Google Scholar
7. Cherpitel CJ, Soghikian K, Hurley LB: Alcohol-related health services use and identification of patients in the emergency department. Ann Emerg Med 1996; 28:418–423Crossref, Medline, Google Scholar
8. Allemand H, Villaume M, Deudon P, Monnet E: Etude épidémiologique de l’alcoolisation chez 3079 sujets admis consécutivement dans un service d’accueil-urgence. Alcoologie 1990; 1:6–10Google Scholar
9. Penn R, Worthington LJ, Clarke CA, Whithfield AGW: Gamma-glutamyltranspeptidase and alcohol intake (letter). Lancet 1981; 1:849Google Scholar
10. Yersin B, Nicolet JF, Decrey H, Burnier M, van Melle G, Pécoud A: Screening for excessive alcohol drinking: comparative value of carbohydrate-deficient transferrin, gamma-glutamyl transferase, and mean corpuscular volume. Arch Intern Med 1995; 155:1907–1911Google Scholar
11. Nilssen O, Ries RK, Rivara FP, Gurney JG, Jurkovich GJ: The CAGE questionnaire and the short Michigan Alcohol Screening Test in trauma patients: comparison of their correlations with biological alcohol markers. J Trauma 1994; 36:784–788Crossref, Medline, Google Scholar
12. Liskow B, Campbell J, Nickel EJ, Powell BJ: Validity of the CAGE questionnaire in screening for alcohol dependence in a walk-in clinic. J Stud Alcohol 1995; 56:277–281Crossref, Medline, Google Scholar
13. Beresford H, Blow FC, Hill E, Singer K, Lucy MR: Comparison of CAGE questionnaire and computer-assisted laboratory profiles in screening for covert alcoholism. Lancet 1990; 336:336–348Crossref, Google Scholar
14. Wellman M: Gamma-glutamyltransferase, in Réferences en biologie clinique. Edited by Siest G, Henny J, Schiele F. Paris, Collection Option Bio Elsevier, 1990, pp 275–293Google Scholar
15. Schiele F, Guilmin AM, Detienne H, Siest G: Gamma-glutamyltransferase activity in plasma: statistical distributions, individual variations, and reference intervals. Clin Chem 1977; 23:1023–1028Google Scholar
16. Schellenberg F, Bénard J, Goff AM, Bourdin C, Weill J: Evaluation of carbohydrate-deficient transferrin compared with Tf index and other markers of alcohol abuse. Alcohol Clin Exp Res 1989; 13:605–610Crossref, Medline, Google Scholar
17. Allen JP, Litten RZ, Anton R: Measures of alcohol consumption in perspective, in Measuring Alcohol Consumption. Edited by Litten RZ, Allen JP. Totowa, NJ, Humana Press 1992, pp 99–134Google Scholar
18. Mihas AA, Tavassoli M: Laboratory markers or ethanol intake and abuse: a critical appraisal. Am J Sci 1992; 303:415–428Google Scholar
19. Schellenberg F, Martin M, Cages E, Bernard JX, Weill J: Nephelometric determination of carbohydrate-deficient transferrin. Clin Chem 1996; 42:551–557Medline, Google Scholar
20. Stibler H: Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin Chem 1991; 37:2029–2037Google Scholar
21. Tarentino G, Morlando N, Morelli L, Schipani M, Liquori C: Use of serum carbohydrate-deficient transferrin and other biological markers of excessive drinking for the diagnosis of alcohol abuse with or without liver diseases. Alcologia 1993; 5:211–220Google Scholar
22. Saini RS, Pettinati HM, Semwanga AE, O’Brien P: Carbohydrate-deficient transferrin: an investigation biochemical marker of heavy alcohol consumption. Psychopharmacol Bull 1997; 33:171–175Medline, Google Scholar
23. Sillanaukee P, Löf K, Härlin A, Matensson O, Brandt R, Seppä K: Comparison of different methods for detecting carbohydrate-deficient transferrin. Alcohol Clin Exp Res 1994; 18:1150–1155Google Scholar
24. Reynaud M, Hourcade F, Planche F, Albuisson E, Meunier MN, Planche R: Usefulness of carbohydrate-deficient transferrin in alcoholic patients with normal gamma-glutamyltranspeptidase. Alcohol Clin Exp Res 1998; 22:1–4Crossref, Medline, Google Scholar
25. Reynaud M, Schellenberg F, Loiseaux-Meunier MN, Schwan R, Maradeix B, Planche F, Gillet C: Objective diagnosis of alcohol abuse: compared values of carbohydrate-deficient transferrin (CDT), gamma-glutamyl transferase (GGT), and mean corpuscular volume. Alcohol Clin Exp Res 2000; 24:1414–1419Google Scholar
26. Rueff B, Cernac J, Darne B: Dépistage de malades alcoologiques par l’auto-questionnaire systématique DETA. La Presse Médicale 1989; 18:1654–1656Google Scholar
27. Sillanaukee P: Laboratory markers of alcohol abuse. Alcohol Alcohol 1996; 31:613–616Crossref, Medline, Google Scholar
28. Kapur A, Wild G, Milford-Ward A, Triger DR: Carbohydrate deficient transferrin: a marker for alcohol abuse. Br Med J 1989; 299:427–431Crossref, Medline, Google Scholar
29. Salmela KJ, Laitinen K, Nyström M, Salaspuro M: Carbohydrate-deficient transferrin during three weeks heavy alcohol consumption. Alcohol Clin Exp Res 1994; 18:228–230Crossref, Medline, Google Scholar
30. O’Connor PG, Schottenfield RS: Patients with alcohol problems. N Engl J Med 1998; 338:592–602Crossref, Medline, Google Scholar
31. Camargo CA, Hennekens CH, Gaziano J, Glynn RJ, Manson JE, Stampfer MJ: Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med 1997; 157:79–85Crossref, Medline, Google Scholar
32. Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Doll R: Alcohol consumption and mortality among middle-aged and elderly US adults. N Engl J Med 1997; 337:1705–1714Google Scholar
33. Fuchs CS, Stampfer MJ, Coldditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B, Speizer FE, Willett WC: Alcohol consumption and mortality among women. N Engl J Med 1995; 332:1245–1250Google Scholar
34. Wilk AL, Jensen NM, Havighurst TC: Meta-analysis of randomized controlled trials addressing brief intervention in heavy alcohol drinkers. J Gen Intern Med 1997; 12:274–283Crossref, Medline, Google Scholar
35. Bien TH, Miller WR, Tonigan JS: Brief interventions for alcohol problems: a review. Addiction 1993; 88:315–335Crossref, Medline, Google Scholar
36. Parish DC: Another indication for screening and early intervention: problem drinking. JAMA 1997; 277:1079–1080Google Scholar
37. Sillanaukee P, Aalto M, Seppa K: Carbohydrate-deficient transferrin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary health care. Alcohol Clin Exp Res 1998; 22:892–896Crossref, Medline, Google Scholar



