Mood Improvement Following Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients With Depression: A Placebo-Controlled Crossover Trial
Abstract
OBJECTIVE: Preliminary studies have indicated that daily left prefrontal repetitive transcranial magnetic stimulation might have antidepressant activity. The authors sought to confirm this finding by using a double-blind crossover design. METHOD: Twelve depressed adults received in random order 2 weeks of active treatment (repetitive transcranial magnetic stimulation, 20 Hz at 80% motor threshold) and 2 weeks of sham treatment. RESULTS: Changes from the relevant phase baseline in scores on the 21-item Hamilton depression scale showed that repetitive transcranial magnetic stimulation significantly improved mood over sham treatment. During the active-treatment phase, Hamilton depression scale scores decreased 5 points, while during sham treatment the scores increased or worsened by 3 points. No adverse effects were noted. CONCLUSIONS: These placebo-controlled results suggest that daily left prefrontal repetitive transcranial magnetic stimulation has antidepressant activity when administered at these parameters. Further controlled studies are indicated to explore optimal stimulation characteristics and location, potential clinical applications, and possible mechanisms of action. (Am J Psychiatry 1997; 154:1752–1756)
Nonpharmacologic methods for alleviating depression have recently garnered renewed attention (1, 2). ECT is the most effective nonpharmacologic treatment of depression (3). Although the mechanisms of antidepressant action of ECT are unknown, recent data indicate that production of a generalized convulsion alone is not sufficient for treating depression; the effect of the ECT seizure on regional brain function is also important in determining therapeutic benefit (4, 5). These data are consistent with evidence from functional neuroimaging studies that have implicated prefrontal, temporal, and limbic structures in depression (for review see reference 6).
Transcranial magnetic stimulation has been developed as a new tool to explore brain-behavior relationships noninvasively for potential therapeutic applications (7). First developed by neurophysiologists and neurologists, transcranial magnetic stimulation involves placing a small but powerful electromagnet on the scalp, which causes cortical neurons just below the skull to depolarize in response to the electrical current generated in them by rapid oscillations in the magnetic field. This technology can map regional brain function (8) and temporarily alleviate the bradykinesia of Parkinson's disease (9). A single magnetic pulse, which typically generates a field of approximately 1.5 tesla 1–2 cm below the coil, will cause a cluster of neurons to discharge. Repetitive pulses (repetitive transcranial magnetic stimulation) can cause neurons to repeatedly discharge (10). At high frequencies, these neurons may be unavailable for communication with other brain regions, in effect creating a temporary “functional deficit” (11). For example, repetitive transcranial magnetic stimulation over temporal cortex blocks speech production (12), stimulation over left dorsolateral prefrontal cortex impairs working memory (13), and repetitive transcranial magnetic stimulation over the occipital cortex causes visual field defects (14).
Several groups have begun investigating whether transcranial magnetic stimulation or repetitive transcranial magnetic stimulation might have antidepressant activity (15–19). Preliminary animal evidence suggests that repetitive transcranial magnetic stimulation is similar to electroconvulsive shock in animal models of depression (20) and that it down-regulates beta receptors (21) and produces changes in brain monoamine concentrations (22). We initially employed repetitive transcranial magnetic stimulation over the left prefrontal cortex in six subjects with treatment-refractory depression in an open study and found modest group effects but robust individual responses in two of six patients (18). This initial study was promising yet inconclusive because of its open design and small study group size. We designed and carried out the following placebo-controlled crossover study to test the hypothesis that daily left prefrontal repetitive transcranial magnetic stimulation has antidepressant effects.
METHOD
We enrolled 12 right-handed outpatients from the Washington, D.C., area (11 women) (mean age=41.8 years, SD=12.4). Subjects were recruited through local advertisements and were not paid. Structured interviews used for screening were the Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (23) and the Structured Clinical Interview for DSM-IV (24). Subjects were also screened with a thorough general and neurological examination, a urine drug screen, an HIV test, and a magnetic resonance imaging (MRI) scan of the head. Patients with abnormalities on any of these measures, as well as patients with pacemakers or a history of seizures or major head trauma, were excluded. All subjects met DSM-IV criteria for current major depressive disorder; 11 subjects had recurrent unipolar depression, and one was diagnosed with bipolar II disorder. The mean score on the 21-item Hamilton Depression Rating Scale (25) at entry was 28.5 (SD=4.2).
After complete description of the study to subjects, written informed consent was obtained through a protocol approved by the National Institute of Mental Health institutional review board. The antidepressant medication regimens of nine subjects were tapered. Three subjects had experienced a partial response to a 10-week, stable-dose antidepressant trial. The regimens (venlafaxine, 25 mg/day; venlafaxine, 200 mg/day; and nefazadone, 200 mg/day) were continued in order to not risk a worsening of depression, which would confound the interpretation of the effect of repetitive transcranial magnetic stimulation or sham treatment.
Magnetic stimulation was performed by using a high-speed magnetic stimulator (Cadwell, Inc., Kennewick, Wash.). On the initial visit, the motor threshold of the right abductor pollicis brevis muscle (the thumb) for the subjects was determined by using the method of limits as previously described (11, 26). For the remainder of the study, the subjects then received stimulation at 80% of this level. Each weekday the subjects received 20 2-second, 20-Hz stimulations over 20 minutes (800 pulses per session, 10 sessions per treatment phase, total of 20 sessions overall per subject). Stimulation occurred over the left dorsolateral prefrontal cortex, which was defined for each person as the site 5 cm anterior and in a parasagittal plane from the point of maximum stimulation of the right abductor pollicis brevis muscle. We used a figure 8-shaped water-cooled coil, with the extensions of the coil perpendicular to a line running from the site to the subject's nose.
Sham stimulation occurred in exactly the same manner as active repetitive transcranial magnetic stimulation, except that the angle of the coil, rather than being tangential to the skull, was at 45 degrees off of the skull. This produces a similar sensation in the scalp but appears not to stimulate the brain, since such angled stimulation when applied over motor cortex is ineffective in producing a motor evoked potential and does not produce changes in [18F]fluorodeoxyglucose positron emission tomography (27).
Patients were selected at random to initially receive either placebo or active daily repetitive transcranial magnetic stimulation. Seven patients with a mean Hamilton depression scale score of 30.0 (SD=4.1) initially received active repetitive transcranial magnetic stimulation; five patients with a mean Hamilton depression scale score of 26.4 (SD=3.7) initially received the sham procedure. Subjects were serially rated (at phase baseline and at the end of each week) with the 21-item Hamilton depression scale by trained investigators who were blind to the treatment phase. Hamilton depression scale scores were analyzed as change from the relevant phase baseline (active, sham) by using repeated measures analysis of variance (ANOVA) with order (sham-active versus active-sham) as a between-subjects term and treatment (sham versus active conditions) and time (weeks 1 and 2) as within-subject factors.
RESULTS
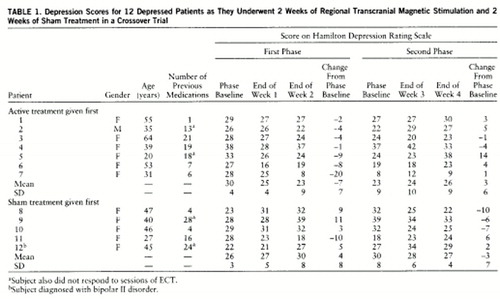
All subjects enrolled completed the study with no unexpected side effects (table 1). Four subjects reported the occurrence of mild headaches, from immediately after to 3 hours after active treatment, that were relieved by acetaminophen. No subjects reported problems with memory or attention. There were no seizures (a possible adverse effect of repetitive transcranial magnetic stimulation).
One subject who initially received sham treatment significantly worsened over the first 10 days of the sham stimulation, stopped going to work, and became actively suicidal. She was deemed capable of providing continued informed consent, and she chose to advance to the next phase (active). She clinically improved over the next 2 weeks and was no longer actively suicidal, although she only returned to her entry level of depression.
A repeated measures ANOVA of changes in Hamilton depression scale scores from relevant phase baseline, with order (sham-active versus active-sham) as a between-subjects term and treatment (sham versus active conditions) and time (weeks 1 and 2) as within-subject factors, found no significant effect for order (F=1.2, df=1,10, p<0.30) or time (F=0.25, df=1,10, p<0.60). During the active phase, mean Hamilton scores decreased by 5.25 points, and during the placebo phase they increased by 3.33 points. This treatment effect was statistically significant (F=6.02, df=1,10, p<0.03).
DISCUSSION
This study confirms the hypothesis that daily left prefrontal repetitive transcranial magnetic stimulation (20 Hz at 80% motor threshold) for 10 days over 2 weeks has an antidepressant effect in a group of mainly unipolar depressed outpatients. We have also demonstrated that repetitive transcranial magnetic stimulation can be safely used in outpatient studies of depression.
Although our findings were statistically significant, there were numerous limitations to this study. It was only designed to test whether repetitive transcranial magnetic stimulation in this population, at these stimulation parameters, and for a limited duration of treatment, would have more robust antidepressant effects than sham treatment. Our study was not a naturalistic “clinical” trial that occurred over 4–6 weeks such as those seen in most antidepressant and ECT studies. However, when several patients from this study openly received additional repetitive transcranial magnetic stimulation at the same parameters after completion of the blind study, further clinical improvement was noted. It also should be noted that, although patients and raters were blind, the physician who administered the repetitive transcranial magnetic stimulation was not, which leaves open the possibility of biasing the results. Future studies may consider the use of a head-holder or other measures to minimize interaction between subjects and clinicians who know the treatment phase of the patient.
The site of stimulation was functionally defined for each person on the basis of the location of motor cortex in that subject. It is likely that we have stimulated slightly different regions of the prefrontal cortex. Future studies might consider the use of MRI-guided stimulation to control for differences in brain morphology and skull size and thickness (28), as well as explorations of other measures. Finally, this study was largely composed of women, and although we are not aware of a differential response by gender, such a phenomenon is possible and thereby limits the ability to generalize from these results.
Despite these limitations, this placebo-controlled study demonstrated an antidepressant effect of left prefrontal active repetitive transcranial magnetic stimulation versus sham treatment. This builds on previous open studies with transcranial magnetic stimulation (15–17) and repetitive transcranial magnetic stimulation (18) in patients with treatment-refractory depression. In a recently published double-blind study that was carried out while this study was ongoing, Pascual-Leone and colleagues (19) demonstrated, with slightly “stronger” stimuli (20 10-second, 10-Hz stimulations at 90% motor threshold or a total of 2000 stimuli per day), that daily left but not right prefrontal repetitive transcranial magnetic stimulation had antidepressant properties in 11 of 17 severely depressed patients.
These treatment studies follow in the path of new information gleaned from functional neuroimaging studies, which have most often implicated the prefrontal and temporal cortex as dysfunctional in depression, with normal function returning after clinical recovery (4, 29). In contrast, in nondepressed healthy volunteers, studies of the acute effect of repetitive transcranial magnetic stimulation on mood have found that left prefrontal repetitive transcranial magnetic stimulation causes subtle increases in self-rated sadness, while right prefrontal stimulation resulted in greater happiness (30–32). This lateralized effect on mood was also seen in a repetitive transcranial magnetic stimulation study in nondepressed patients with obsessive-compulsive disorder, which demonstrated that right prefrontal repetitive transcranial magnetic stimulation decreased compulsive urges and also improved mood (33).
This reversal in hemispheric effects of repetitive transcranial magnetic stimulation on mood in studies of nondepressed comparison subjects and clinically depressed patients is intriguing (34). It has been hypothesized that the prefrontal cortex acts in a regulatory manner on underlying limbic structures, which directly determine mood and may be different in depressed patients and in healthy subjects (6, 35–38). These data suggest the possibility that depression involves not only a relative prefrontal hypofunction but a qualitative change in relative hemispheric modulation of mood.
The mechanisms that underlie the antidepressant effect of repetitive transcranial magnetic stimulation are unknown. It appears that repetitive transcranial magnetic stimulation does not induce “silent” focal seizures, since previous studies in healthy subjects at these parameters showed no change in serum prolactin (31) or in surface EEG activity recorded before and after stimulation (26). We also reason that because of the known rapid drop-off in magnetic field intensity as a function of distance from the coil, we are only directly stimulating neurons immediately beneath the coil at the surface of the cortex (28, 39). Previous studies have demonstrated that repetitive transcranial magnetic stimulation at similar parameters over the prefrontal cortex results in increases in serum thyroid-stimulating hormone, which suggests the possibility of increases in thyrotropin-releasing hormone and an indirect effect of repetitive transcranial magnetic stimulation on hypothalamo-pituitary structures (31). Functional neuroimaging studies and somatosensory evoked responses have demonstrated that prefrontal repetitive transcranial magnetic stimulation has effects at brain regions remote from the site of stimulation, presumably through transsynaptic signaling (18, 27, 40). Of note, a recent SPECT study in healthy adults that used left prefrontal repetitive transcranial magnetic stimulation at parameters identical to those in this study demonstrated that compared to baseline, there was reduced blood flow at the coil site and in the anterior cingulate during stimulation, with increases in brainstem activity (40). Future studies that combine neuroimaging with regional stimulation will likely be very helpful in clarifying neurobiological effects of repetitive transcranial magnetic stimulation (41).
CONCLUSIONS
Daily left prefrontal repetitive transcranial magnetic stimulation over a 2-week period had an antidepressant effect in outpatient depressed patients that was superior to sham stimulation. Further studies appear warranted to examine which of the many variables used in this study are crucial to this antidepressant effect (location, intensity, frequency, train length, intertrain interval, dosing schedule, and length of treatment) and which, if any, can be optimized to induce more robust effects. Until this effect is further replicated and these technical and methodological issues are clarified, it is unclear what role repetitive transcranial magnetic stimulation might have in the clinical management of depression. Regardless of its potential clinical role in the treatment of depression, further repetitive transcranial magnetic stimulation work will likely provide important information about the neuroanatomical structures and pathophysiological mechanisms that are important in mood regulation.
 |
Presented in part at the 149th annual meeting of the American Psychiatric Association, New York, May 4–9, 1996. Received Sept. 13, 1996; revisions received April 29 and June 18, 1997; accepted July 1 7, 1997. From the Biological Psychiatry Branch and the Laboratory of Clinical Science, NIMH; and the Departments of Psychiatry, Radiology, and Neurology, Medical University of South Carolina. A ddress correspondence to Dr. George, Radiology Department, Medi cal University of South Carolina, 171 Ashley Ave., Charleston, SC 29425; [email protected] (e-mail). Address reprint requests to Dr. Post, NIMH, Rm. 3N212, Bldg. 10, 9000 Rockville Pike, Bethesda, MD 20892. Supported in part by the National Alliance for Research in Schizophrenia and Depression and the Ted and Vada Stanley Foundation. The authors thank Drs. Harold Sackeim, Alvaro Pascual-Leone, and Robert Belmaker for their advice regarding the use of repetitive transcranial magnetic stimulation.
1. Levitt AJ, Joffe RT, Kennedy SH: Bright light augmentation in antidepressant nonresponders. J Clin Psychiatry 1991; 52:336–337Medline, Google Scholar
2. Wehr TA: Sleep loss: a preventable cause of mania and other excited states. J Clin Psychiatry 1989; 50:8–16Medline, Google Scholar
3. Kellner CH (ed): Electroconvulsive Therapy. Psychiatr Clin North Am 1991; 15(1)Google Scholar
4. Nobler MS, Sackeim HA, Prohovnik I, Moeller JR, Mukherjee S, Schnur DB, Prudic J, Devanand DP: Regional cerebral blood flow in mood disorders, III: treatment and clinical response. Arch Gen Psychiatry 1994; 51:884–897Crossref, Medline, Google Scholar
5. Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimmons L, Moody BJ, McElhinney MC, Coleman EA, Settembrino JM: Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993; 328:839–846Crossref, Medline, Google Scholar
6. George MS, Ketter TA, Post RM: What functional imaging studies have revealed about the brain basis of mood and emotion, in Advances in Biological Psychiatry. Edited by Panksepp J. Greenwich, Conn, JAI Press, 1996, pp 63–113Google Scholar
7. George MS, Wassermann EM, Post RM: Transcranial magnetic stimulation: a neuropsychiatric tool for the 21st century. J Neuropsychiatry Clin Neurosci 1996; 8:373–382Crossref, Medline, Google Scholar
8. Pascual-Leone A, Grafman J, Cohen LG, Roth BJ, Hallett M: Transcranial magnetic stimulation: a new tool for the study of higher cognitive functions in humans, in Handbook of Neuropsychology, vol 2. Edited by Grafman J, Boller F. Amsterdam, Elsevier, 1997, pp 267–290Google Scholar
9. Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M: Akinesia in Parkinson's disease, I: shortening of simple reaction times with focal, single-pulse transcranial magnetic stimulation. Neurology 1994; 44:884–891Crossref, Medline, Google Scholar
10. Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M: Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994; 117:847–858Crossref, Medline, Google Scholar
11. Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M: Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain 1992; 115:1045–1059Google Scholar
12. Pascual-Leone A, Gates JR, Dhuna A: Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology 1991; 41:697–702Crossref, Medline, Google Scholar
13. Pascual-Leone A, Hallett M: Induction of errors in a delayed response task by repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuroreport 1994; 5:2517–2520Google Scholar
14. Pascual-Leone A, Gomez-Tortosa E, Grafman J, Alway D, Nichelli P, Hallett M: Induction of visual extinction by rapid-rate transcranial magnetic stimulation (rTMS) of parietal lobe. Neurology 1994; 44:494–498Crossref, Medline, Google Scholar
15. Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller HJ: Application of transcranial magnetic stimulation in treatment of drug-resistant major depression—a report of two cases. Hum Psychopharmacol 1993; 8:361–365Crossref, Google Scholar
16. Grisaru N, Yarovslavsky U, Abarbanel J, Lamberg T, Belmaker RH: Transcranial magnetic stimulation in depression and schizophrenia. Eur Neuropsychopharmacol 1994; 4:287–288Crossref, Medline, Google Scholar
17. Kolbinger HM, Hoflich G, Hufnagel A, Moller HJ, Kasper S: Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Hum Psychopharmacol 1995; 10:305–310Crossref, Google Scholar
18. George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853–1856Google Scholar
19. Pascual-Leone A, Rubio B, Pallardo F, Catala MD: Beneficial effect of rapid-rate transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233–237Crossref, Medline, Google Scholar
20. Fleischmann A, Steppel J, Leon A, Belmaker RH: The effect of transcranial magnetic stimulation compared with electroconvulsive shock on rat apomorphine-induced stereotypy. Eur Neuropsychopharmacol 1994; 4:449–450Crossref, Google Scholar
21. Fleischmann A, Sternheim A, Etgen AM, Li C, Grisaru N, Belmaker RH: Transcranial magnetic stimulation downregulates beta-adrenoreceptors in rat cortex. J Neural Transm 1996; 103:1361–1366Google Scholar
22. Belmaker RH, Grisaru N, Ben-Shahar D, Klein E: The effects of TMS on animal modes of depression, beta-adrenergic receptors and brain monoamines. CNS Spectrums: Int J Neuropsychiatric Med 1997; 2:26–30Google Scholar
23. Mannuzza S, Fyer AJ, Klein DF, Endicott J: The Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA): rationale and conceptual development. J Psychiatr Res 1986; 20:317–325Crossref, Medline, Google Scholar
24. First MB, Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). Washington, DC, American Psychiatric Press, 1995Google Scholar
25. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
26. Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, Valls-Sole J, Brasil-Neto JP, Wassermann EM, Cohen LG, Hallett M: Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol 1993; 89:120–130Crossref, Medline, Google Scholar
27. Wassermann EM, Kimbrell TA, George MS, Danielson AL, Herscovitch P, Hallett M, Post RM: Local and distant changes in cerebral glucose metabolism during repetitive transcranial magnetic stimulation over motor cortex (abstract). Neurology 1997; 48:A107Google Scholar
28. Bohning DE, Pecheny AP, Epstein CM, Specs AM, Vincent DJ, Dannels W, George MS: Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. NeuroReport 1997; 8:2535–2538Google Scholar
29. Kimbrell TA, George MS, Danielson A, Dunn RT, Benson BE, Little JT, Herscovitch P, Hallett M, Post RM, Wassermann E: Changes in cerebral metabolism during transcranial magnetic stimulation (abstract). Biol Psychiatry 1997; 41:108SGoogle Scholar
30. Pascual-Leone A, Catala MD, Pascual AP: Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996; 46:499–502Crossref, Medline, Google Scholar
31. George MS, Wassermann EM, Williams W, Steppel J, Pascual-Leone A, Basser P, Hallett M, Post RM: Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation of the prefrontal cortex. J Neuropsychiatry Clin Neurol 1996; 8:172–180Crossref, Medline, Google Scholar
32. Martin JD, George MS, Greenberg BD, Wassermann EM, Schlaepfer TE, Murphy DL, Hallett M, Post RM: Mood effects of prefrontal repetitive high-frequency TMS in healthy volunteers. CNS Spectrums: Int J Neuropsychiatric Med 1997; 2:53–68Google Scholar
33. Greenberg BD, George MS, Martin JD, Benjamin J, Schlaepfer TE, Altemus M, Wassermann EM, Post RM, Murphy DL: Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry 1997; 154:867–869Link, Google Scholar
34. George MS, Speer AM, Wassermann EM, Kimbrell TA, Williams WA, Kellner CH, Risch SC, Stallings LE, Post RM: Repetitive TMS as a probe of mood in health and disease. CNS Spectrums: Int J Neuropsychiatric Med 1997; 2:39–44Google Scholar
35. Wheeler RE, Davidson RJ, Tomarken AJ: Frontal brain asymmetry and emotional reactivity: a biological substrate of affective style. Psychophysiology 1993; 30:82–89Crossref, Medline, Google Scholar
36. Abercrombie HC, Davidson RJ, Ward RT, Larson CL, Traski PA, Perlman SB, Holden JE: Metabolic activity in the amygdala predicts negative affect in depressives but positive affect in nondepressed controls (abstract). Psychophysiology 1995; 32:S35Google Scholar
37. Morgan MA, Romanski L, LeDoux JE: Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 1993; 163:113Crossref, Google Scholar
38. George MS, Ketter TA, Post RM: Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59–72Crossref, Google Scholar
39. Wassermann EM, Wang B, Zeffiro TA, Sadato N, Pascual-Leone A, Toro C, Hallett M: Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET. Neuroimage 1996; 3:1–9Crossref, Medline, Google Scholar
40. Stallings LE, Speer AM, Spicer KM, Cheng KT, George MS: Combining SPECT and repetitive transcranial magnetic stimulation (rTMS)—left prefrontal stimulation decreases relative perfusion locally in a dose dependent manner (abstract). Neuroimage 1997; 5:S521Google Scholar
41. George MS, Wassermann EM, Kimbrell T, Speer AM, Stallings L, Roberts D, Vincent DJ, Beale M, Cheng K, Spicer KM: An overview of initial studies combining conventional functional imaging (PET, SPECT, fMRI) with transcranial magnetic stimulation (TMS) to actively probe brain-behavior relationships (abstract). J Neuropsychiatry Clin Neurosci 1997; 131:6Google Scholar



