Digital Intervention for Cognitive Deficits in Major Depression: A Randomized Controlled Trial to Assess Efficacy and Safety in Adults
Abstract
Objective:
The authors evaluated AKL-T03, an investigational digital intervention delivered through a video game–based interface, designed to target the fronto-parietal network to enhance functional domains for attentional control. AKL-T03 was tested in adult patients with major depressive disorder and a demonstrated cognitive impairment at baseline.
Methods:
Adults ages 25–55 years on a stable antidepressant medication regimen with residual mild to moderate depression and an objective impairment in cognition (as measured using the symbol coding test) were enrolled in a double-blind randomized controlled study. Participants were randomized either to AKL-T03 or to an expectation-matched digital control intervention. Participants were assessed at baseline and after completion of their 6-week at-home intervention. The primary outcome measure was improvement in sustained attention, as measured by the Test of Variables of Attention (TOVA).
Results:
AKL-T03 (N=37) showed a statistically significant medium-effect-size improvement in sustained attention compared with the control intervention on the TOVA primary outcome (N=37) (partial eta-squared=0.11). Additionally, a composite score derived from all cognitive measures demonstrated significant improvement with AKL-T03 over the control intervention. Individual secondary and exploratory endpoints did not demonstrate statistically significant between-group differences. No serious adverse events were reported, and two patients (5.5%) in the AKL-T03 group reported an intervention-related adverse event (headache).
Conclusions:
Treatment with AKL-T03 resulted in significant improvement in sustained attention, as well as in cognitive functioning as a whole, compared with a control intervention. AKL-T03 is a safe digital intervention that is effective in the treatment of cognitive impairment associated with major depression. Further research will be needed to understand the clinical consequences of this treatment-induced change.
People with major depressive disorder report a range of difficulties with concentrating, making decisions, slowed thinking, and forgetfulness (1). These symptoms reflect one of the core symptoms of the disorder in DMS-5, defined as “diminished ability to think or concentrate, or indecisiveness” (2). Numerous studies have also reported poorer neuropsychological performance on tests of memory, attention, and executive function in patients with depression (3). These cognitive challenges can have a serious impact on patients’ daily activities, especially in education and work (4, 5). Many patients with major depression complain of and/or manifest cognitive difficulties even after their symptoms have responded to antidepressant treatment, and cognitive impairment can persist during periods of symptom remission (6, 7). These findings suggest that personalized interventions targeting key cognitive domains may promote recovery from a major depressive episode (8).
The treatment options for providing benefit to patients with major depression with cognitive deficits are limited. Most pharmacologic treatments provide little or no benefit for cognitive impairment in major depression, suggesting that additional mechanisms of action besides those of conventional medications are needed to target the cognitive impairments associated with depression (9–11). As an example, vortioxetine was the first treatment for major depressive disorder for which data on objective cognitive assessment, showing a positive effect on processing speed, was permitted in the label (12). Additionally, the results of studies using psychological approaches such as cognitive-behavioral therapy and problem-solving therapy that target cognition distortions alongside symptoms suggest that these treatments predominantly improve affective symptoms (13, 14). Siegle et al. (15) reported that a metacognitive attention training task aimed at enhancing functioning of prefrontal regions of the brain resulted in improvement in cognitive control and affective symptoms. However, all of these methods of cognitive training present some limitations, most notably difficulty of access. Mobile technologies that provide home-based cognitive treatment may be particularly useful in overcoming this constraint.
The underlying pathophysiology of cognitive impairment in patients with major depression appears to be associated with inefficient functioning of the fronto-parietal cognitive control networks (16), which have a high degree of connectivity across brain regions (17) and can rapidly modify functional connections in response to changing internal and environmental demands (18). Digital interventions that can adapt the difficulty of a cognitive exercise based on the user’s performance level may hold promise as a personalized tool to target the cognitive impairments of major depression.
AKL-T03 is an investigational digital therapeutic designed to activate the fronto-parietal networks of the brain to improve attention and related attentional control processes. In three different feasibility and proof-of-concept trials, AKL-T01—an investigational digital therapeutic similar to AKL-T03, with the same proprietary algorithms but developed with a video game appearance more appropriate for children—was tested in two studies in patients with major depression: a fully remote randomized virtual clinical trial (ClinicalTrials.gov identifiers NCT00540865, NCT02891564) and a randomized clinical trial with at-home treatment (NCT02229188). AKL-T01 significantly improved affective symptoms following a 4-week intervention, similar to an electronic version of traditional problem-solving therapy, compared with a control condition (an app that provided daily health tips). Patients receiving the AKL-T01 intervention had a response rate (with response defined as a reduction of 50% from preintervention scores) of 46%, compared with 45% with problem-solving therapy and 34% with the health tips condition (13). These initial results supported the feasibility of this intervention in a large, representative sample of patients with major depression, although the fully remote nature of this trial presented challenges in adherence to the prescribed protocol. To better assess the intervention, Anguera et al. (19, 20) conducted a clinical trial of AKL-T01, evaluating improvement in cognitive symptoms in older adults (age 60 and older) with depression using additional outcome measures (e.g., performance on cognitive tests). The results replicated those from the feasibility study (similar improvement in depression symptoms with problem-solving therapy and AKL-T01), but only AKL-T01 resulted in improvement in the cognitive assessments measuring sustained attention and delayed working memory (19, 20). Additionally, Gunning et al. (21) demonstrated an increase in resting-state functional connectivity of the fronto-parietal networks following a 4-week intervention with AKL-T01 in patients with major depression, providing preliminary evidence of the mechanism of action of AKL-T01.
The aim of the present study was to evaluate whether AKL-T03, a version of AKL-T01 adapted for an adult population, would improve cognitive performance in adults with depression and demonstrated cognitive impairment when compared with an educational-styled digital control intervention.
Methods
Study Design
The Software Treatment for Actively Reducing the Severity of Cognitive Deficits in Major Depressive Disorder study (STARS-MDD; ClinicalTrials.gov identifier NCT03310281) was a randomized, double-blind, parallel-group, 6-week controlled trial conducted at four sites in the United States from October 16, 2017, to December 14, 2018 (see Figure S1 in the online supplement). The study was conducted in accordance with the International Conference on Harmonization regulations and was approved by each site’s institutional review board (WIRB-Copernicus Group). Written informed consent was obtained from all participants included in the study.
Study Participants
Eligible patients were 25–55 years of age and had a confirmed diagnosis of major depressive disorder according to DSM-5 criteria and confirmed via the Mini International Neuropsychiatric Interview, version 7.0.2. Other key inclusion criteria included confirmation of a score between 14 and 22 on the 17-item Hamilton Depression Rating Scale (HAM-D) (24) during the screening phase (day −28) and at baseline (day 0) and a symbol coding T-score ≤50 on the Brief Assessment of Cognition (25). Participants were required to have been on antidepressant medication for ≥8 weeks prior to screening and baseline, with a stable dosage for ≥4 weeks prior to baseline. Key exclusion criteria included significant comorbid psychiatric diagnoses and active suicide risk or ideation as measured by the Columbia-Suicide Severity Rating Scale. A full list of inclusion and exclusion criteria is provided in the online supplement.
Randomization and Blinding
Participants were randomized in a 1:1 ratio to receive AKL-T03 or a digital control (a letter-word video game; see below). The randomization scheme was provided by Precision for Medicine using validated computer software–generated pseudorandom numbers. Unblinded staff at clinical sites enrolled patients through an electronic data capture system, obtained the randomized intervention, and trained patients on the assigned device. Devices were provided to the unblinded site staff by Akili along with the list that linked the device serial number to the intervention. Akili remained blinded as to which subject received which intervention until after database lock. Investigators completing outcome measure assessments were blinded to intervention allocation.
To minimize bias, participants were informed that the study was evaluating the effect of two different investigational interventions for cognitive impairments associated with major depression. Expectancy analysis performed on the two different interventions (AKL-T03 and control) showed comparable expectation of benefit from both. Participants were discouraged from discussing their randomized intervention with anyone other than an unblinded study coordinator. Investigators and other blinded site staff were restricted from access to source documents and case report forms.
Intervention Training and Adherence
Both the AKL-T03 and control conditions were administered using Apple iPad mini 2 tablets. After randomization, eligible participants were trained in the use of their intervention by unblinded study staff and then instructed to use the intervention for at least 10 minutes or until the coordinators were confident that the participants were following all rules and using the devices properly. Participants assigned to AKL-T03 were instructed to complete five sessions at least 5 days per week for 6 weeks, for a total of approximately 25 minutes of game-play per day. The software automatically locked after the five sessions were completed, to preclude excessive use of the intervention. Participants assigned to the control condition were also instructed to complete 25 minutes of game-play at least 5 days per week for 6 weeks, and this software also automatically locked after 25 minutes. Adherence was monitored remotely by unblinded study coordinators using an electronic dashboard created by Akili. Unblinded study coordinators notified participants and reminded them to engage with their intervention if they had not done so over a 48-hour period. Devices also generated automatic reminders for participants who had not engaged within 24 hours. Data involving overall adherence and adherence to the study regimen and game-play metrics were received and maintained on Akili servers whenever study devices were connected to the Internet.
Digital Interventions
AKL-T03 is an investigational digital therapeutic built using Akili’s proprietary algorithm (Selective Stimulus Management Engine [SSME]) designed to train interference management at an adaptive and personalized degree of difficulty. Interference is instantiated through a video game–based interface displaying two tasks that are to be done in parallel (multitasking). In brief, users are presented a perceptual discrimination targeting task in which they must respond to the designated stimulus targets and ignore the stimulus distractors (similar to a go/no-go task) and a sensory motor navigation task in which they must continuously adjust their location to interact with or avoid positional targets. Performance in each task is assessed during single and multitask conditions. AKL-T03 differs from Akili’s original SSME intervention, AKL-T01, in its appearance and theme, and it was designed to appeal to an adult population while maintaining the same cognitive mechanism of action (13, 19, 20).
The control intervention is an engagement-, expectancy-, and time-on-task-matched software program developed by Akili. The goal of the video game is to connect letters in a grid on the screen horizontally, vertically, or diagonally to form as many words of at least two letters as possible during a 25-minute period. Points awarded in the game are based on increasing difficulty, as measured by number of words formed, length of the words, and difficulty of letters used (22).
Outcome Measures
The a priori primary outcome was change from baseline in cognitive performance following the AKL-T03 intervention compared with the control group, as measured by change in sustained attention using the Test of Variables of Attention (TOVA) (23) reaction time to rare target stimuli (first half), normalized by age and sex. The TOVA is a validated computerized continuous performance test of attention and inhibitory control (24). Change from baseline scores from the first half of the TOVA between day 0 (baseline) and day 42 (study exit) were compared between the two intervention groups. Secondary outcome measures included the symbol coding test from the Brief Assessment of Cognition (25), Trail Making Test parts A and B (Trails A and B) (26), and a letter-number sequencing task (27). A post hoc analysis was performed to evaluate the overall impact on cognition. To do that, a composite score was calculated by creating a simple average of the z-scores from each cognitive test. In exploratory analyses, we assessed changes in mood, subjective cognitive symptoms, and quality of life. After each intervention, participants completed the HAM-D, the Patient Health Questionnaire–9 (PHQ-9), the Cognitive and Physical Functioning Questionnaire (CPFQ), the Work and Social Adjustment Scale (WSAS), and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q). A summary of cognitive and clinical assessments measured in the study is provided in the online supplement.
Statistical Analysis
Sample size estimation determined that 40 participants per intervention would be sufficient to detect an effect size of 0.70 with >90% confidence and an alpha criterion of 0.05 on the primary outcome.
All analyses were conducted following a prespecified statistical analysis plan. The intent-to-treat analysis was defined to include each participant who was randomized to an intervention and began the at-home portion of treatment. Only participants who had both pre- and postintervention assessments (completers) were considered to be in the intent-to-treat population, given the nature of the statistics used (change scores).
Between-group statistical tests were conducted on each metric using an analysis of covariance (ANCOVA) on the difference between posttreatment (day 42) and pretreatment (day 0) scores, with age, sex, and the baseline score of the corresponding metric included as covariates. Inferential tests for the primary metric were conducted, with an alpha of 0.05 (two-sided). Statistical comparisons for secondary endpoints only were adjusted for multiple comparisons using the Hochberg step-up method. Effect sizes (partial eta-squared) were interpreted as small=0.01, medium=0.06, and large=0.14 (28).
Results
Demographic Characteristics
Table 1 summarizes the demographic characteristics and baseline scores for each group. Notably, the two groups differed significantly in HAM-D score at baseline, suggesting that the control group participants may have been more depressed.
| Characteristic or Measure | AKL-T03 Group (N=37) | Control Group (N=37) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | df | p | |
| Female | 23 | 62 | 24 | 65 | 0 | 1 | 1.000 |
| Race | 5.39 | 2 | 0.067 | ||||
| White | 27 | 73 | 19 | 51 | |||
| Other | 10 | 27 | 19 | 51 | |||
| Hispanic | 5 | 14 | 11 | 30 | 1.99 | 1 | 0.158 |
| Mean | SD | Mean | SD | t | df | p | |
| Age (years) | 43.11 | 41.24 | 0.97 | 70.68 | 0.336 | ||
| HAM-D | 17 | 2.45 | 18.6 | 2.37 | 2.84 | 72 | 0.006 |
| TOVA, reaction time during first half (sustained attention) | 101.72 | 16.48 | 93.38 | 27.86 | −1.57 | 72 | 0.121 |
| Symbol coding test | 46.51 | 9.51 | 44.14 | 10.04 | −1.05 | 72 | 0.299 |
| Trail Making Test, part A | 32.89 | 10.48 | 35.68 | 13.45 | 0.93 | 72 | 0.324 |
| Trail Making Test, part B | 87.89 | 50.7 | 88.83 | 38.95 | 0.09 | 72 | 0.930 |
| Letter-number sequencing | 13.95 | 3.24 | 12.97 | 3.66 | −1.21 | 72 | 0.230 |
| Cognitive and Physical Functioning Questionnaire | 23.73 | 6.72 | 26.73 | 5.93 | 2.04 | 72 | 0.045 |
| Perceived Deficits Questionnaire | 33.19 | 16.98 | 39.73 | 19.35 | 1.55 | 72 | 0.127 |
| Work and Social Adjustment Scale | 18.94 | 10.42 | 23.91 | 8.6 | 2.08 | 72 | 0.042 |
| Quality of Life Enjoyment and Satisfaction Questionnaire | 45.38 | 8.33 | 39.32 | 8.73 | −3.06 | 72 | 0.003 |
| Patient Health Questionnaire–9 | 14.22 | 5.55 | 16.43 | 5.21 | 1.77 | 72 | 0.081 |
TABLE 1. Baseline demographic characteristics and scores for the AKL-T03 and control groupsa
Adherence
There was no statistical difference (χ2=3.01, df=1, p=0.083) between the two groups in the proportion of participants who adhered to the recommended treatment schedule (defined as completing ≥50% of the recommended sessions; AKL-T03 group: 70.3%: control group: 89.2%). However, across the total number of sessions played in each group, there was a statistically significant difference (t=3.61, df=71.96, p<0.001) between groups (control group: 105%, SD=38.6; AKL-T03 group: 72.8%, SD=37.7).
Primary Efficacy Endpoint
The a priori primary endpoint for the study was change from baseline in sustained attention as measured by reaction time for performance on the first half of the TOVA (TOVA-H1). Group differences were evaluated with an ANCOVA using age, sex, and baseline TOVA-H1 as covariates. The results showed a significant between-group difference (F=8.550, df=1, 69, p=0.005; partial η2=0.11, medium), with the AKL-T03 group showing more improvement than the control group (Figure 1).
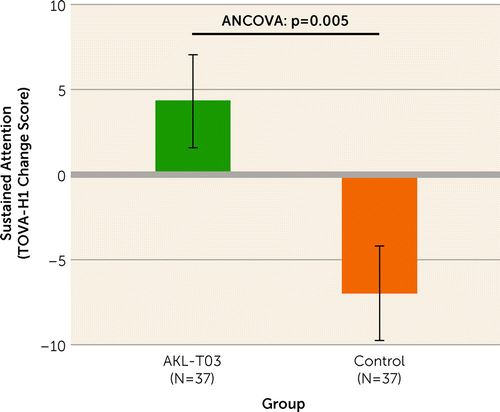
FIGURE 1. Difference in sustained attention scores between patients with major depressive disorder and cognitive impairment who received the AKL-T03 intervention or a control interventiona
aThe graph shows least-square means, with errors bars indicating standard error of the mean. The p value shown is from an analysis of covariance with age, sex, and baseline sustained attention as covariates.
Secondary Efficacy Endpoints
No statistically significant differences were found between the two groups on the four secondary endpoints (change from baseline for the symbol coding test, Trails A, Trails B, and letter-number sequencing). However, the change score values were consistently of higher magnitude in the AKL-T03 group compared with the control group (Table 2).
| Measure | AKL-T03 Group | Control Group | Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| LS Mean | SE | LS Mean | SE | F | df | p | Effect Size (η2) | |
| Symbol coding test | 7.68 | 1.23 | 5.68 | 1.25 | 0.52 | 1, 69 | 0.252 | 0.02 |
| Trail Making Test, part A | −5.75 | 1.18 | −4.08 | 1.21 | 0.99 | 1, 69 | 0.323 | 0.01 |
| Trail Making Test, part B | −18.93 | 4.91 | −7.56 | 5.01 | 2.72 | 1, 69 | 0.104 | 0.04 |
| Letter-number sequencing | 0.66 | 0.38 | 0.26 | 0.39 | 0.58 | 1, 69 | 0.454 | 0.01 |
| Cognitive and Physical Performance Questionnaire | −3.89 | 1.21 | −5.26 | 1.22 | 0.64 | 1, 69 | 0.42 | 0.01 |
| Perceived Deficits Questionnaire | −3.95 | 2.33 | −10.60 | 2.36 | 4.90 | 1, 69 | 0.05 | 0.06 |
| Work and Social Adjustment Scale | −2.94 | 1.51 | −4.07 | 1.57 | 0.27 | 1, 59 | 0.61 | 0.01 |
| Quality of Life Enjoyment and Satisfaction Questionnaire | 5.27 | 1.70 | 7.49 | 1.73 | 0.82 | 1, 69 | 0.37 | 0.01 |
| Patient Health Questionnaire–9 | −4.24 | 1.00 | −4.45 | 1.01 | 0.02 | 1, 69 | 0.88 | 0 |
| HAM-D total | −3.70 | 0.77 | −3.57 | 0.77 | 0.01 | 1, 69 | 0.91 | 0 |
TABLE 2. Statistics for the secondary and exploratory efficacy endpoint, intent-to-treat populationa
Exploratory Efficacy Endpoints
No statistically significant differences were found between the two groups among the four exploratory endpoints (change from baseline on the PHQ-9, CPFQ, WSAS, and Q-LES-Q) (see Table 2).
Depression Symptoms
No statistically significant differences were found in change in depression symptoms between the two intervention groups. Both groups showed significant within-group statistical improvements in depressive symptoms as measured by total score on the HAM-D (AKL-T03 group: one-tailed t test, t=5.30, df=36, p<0.001, least-square mean=−3.70, SE=0.78; control group; one-tailed t test, t=4.63, df=36, p=0.001, least-square mean=−3.57, SE=0.77). For patients in the AKL-T03 group, Pearson’s correlation between sustained attention (TOVA-H1) and depression symptom (HAM-D) change scores showed a medium-sized correlation that fell short of significance (r=−0.30, p=0.073). For patients in the control group, the correlation was smaller (r=0.15, p=0.364).
Post Hoc Analysis
We calculated a composite score as a simple average of all cognitive tests and compared the effect of AKL-T03 to the control condition on the change in this score from baseline. The results showed a significant difference between the interventions (F=4.09, df=1, 67, p=0.047; partial η2=0.06, medium).
Safety
Two (5.5%) of the 37 patients in the AKL-T03 group reported an intervention-related adverse event (headache); none of the 37 in the control group reported any intervention-related adverse events. There were no serious intervention-related adverse events in either group.
Discussion
The results in this randomized controlled trial of an at-home digital intervention for cognitive impairment in patients diagnosed with major depressive disorder indicate that the active intervention, AKL-T03, significantly improved performance on the primary outcome measure of sustained attention compared with the control condition in adults 25–55 years old. Across a range of secondary and exploratory outcome measures targeting a variety of cognitive domains, including working memory, processing speed, task switching (e.g., letter-number sequencing, symbol coding test, Trails A, Trails B), depressive symptoms, and subjective cognitive symptoms (e.g., HAM-D, PHQ, and CPFQ) and quality of life (e.g., WSAS and Q-LES-Q), the benefit of AKL-T03 was not superior to the control intervention. Post hoc analysis indicated a significant difference between interventions on a general composite measure of cognition in favor of AKL-T03, although this result should be interpreted with caution as it may be driven by the improvement in sustained attention. The present findings of improved cognition following a 6-week intervention with AKL-T03 in patients with major depression are consistent with those previously reported in studies of different age groups with a variety of inclusion criteria (20, 21). Additionally, both interventions were well tolerated.
Clinically, patients undergoing depressive episodes as well as those who have recovered from depressive episodes frequently complain of cognitive impairments (1, 4–7). Up to 50% of patients who have otherwise experienced symptomatic mood improvement with antidepressant treatment (like the population included in this clinical trial) still display persistent measurable cognitive impairment. Such impairment is associated with diminished psychosocial functioning (29) and a higher rate of relapse (30), beyond the impact of depressive symptoms (31).
AKL-T03 shows promise for reducing cognitive impairment during a current episode of depression. Further research looking at longitudinal data and durability of effect is needed to confirm the hypothesis that cognitive improvements may have an impact on functional outcomes and potentially on future relapses.
In this study, there were no differences between AKL-T03 and the control intervention on secondary and exploratory measures. There are several factors that might explain these findings. First, the patients in our sample were all on stable antidepressant treatment when they entered the study, presenting mild to moderate depressive symptoms and cognitive impairments. It is possible that the AKL-T03 and control interventions served as a boost to the effects of their medication, as the patients needed to become engaged in a more active, daily routine in order to fulfill the study requirements. Both interventions required continued perseverance (daily commitment for approximately 25 minutes) even when in many cases study participants experienced failure in the intervention tasks (increasing difficulty associated with progress). Potentially, these experiences may have trained coping and reappraisals skills or even increased the sense of self-efficacy and mastery (32). Consequently, patients may have perceived improvement in symptoms independent of the intervention. Second, expectations of efficacy have been shown to moderate intervention effects, and this could be also applied to digital interventions (33). In the study, patients in both arms believed that they received a novel intervention for addressing cognitive impairment in major depression; thus, an expectation of an intervention effect can be assumed for both interventions and may partially explain improvement in both groups. Additionally, in an expectancy survey, a cohort of 40 unrelated depressed patients who were presented with a description and visual display of either AKL-T03 or the control intervention reported no difference in expectation of benefits in objective measures of cognition, perceived cognitive changes, or mood symptoms (three items in each category). Interestingly, interviewees thought that the control intervention may have a more positive impact on their everyday function (work, home). Because AKL-T03 looks and feels more like a video game than the control intervention and is inherently more demanding, patients with major depression may have experienced reduced perceived benefit due to a diminished tendency to persevere on difficult tasks as a part of reduced hedonic response (34). While adherence to the protocol was not statistically different between the two groups, participants in the control group played more sessions. Since AKL-T03 has a time-pressure component that requires more active engagement than the control condition, which is self-paced, the control condition may have been more relaxing and enjoyable. It is possible that any engagement at all is beneficial to patients with major depression and that the control condition provided benefit also.
It is important to highlight some caveats regarding the study results. First, while randomization was performed as indicated, the two participant groups presented with different levels of symptom severity as measured with the HAM-D at baseline, with the control group being more depressed. This mismatch, however, did not appear to have an impact on engagement in the control group. While mean engagement was not statistically significantly different between the groups, it was higher among participants in the control group. Completion of more sessions may have resulted in participants’ experiencing confirmation bias and an increase in perceived improvement. Second, the study engaged clinical research centers for recruitment and applied specific inclusion and exclusion criteria, which may limit the generalizability of our results.
These limitations notwithstanding, the trial is strengthened by its minimization of potential biases and differences in expectation of benefit between the interventions—for example, by engaging blinded raters and discouraging discussion about intervention assignment. Additional issues to be addressed in future studies include the effectiveness of a sham control to reduce perceived subjective benefit, development of a treatment condition inherently more enjoyable for this population, and an examination of the durability of the effect of treatment beyond the 6-week treatment period.
In summary, compared with a control condition, AKL-T03 demonstrated significant improvement on the primary objective measure of cognition in patients with major depression. The intervention was well tolerated and presented minimal adverse events. The digital nature of the intervention could help to increase access for patients who otherwise might not find a solution for their depression-related cognitive difficulties.
1 : The role of cognitive dysfunction in the symptoms and remission from depression. Ann Gen Psychiatry 2015; 14:27Crossref, Medline, Google Scholar
2 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
3 : Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med 2013; 43:2017–2026Crossref, Medline, Google Scholar
4 : Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry 2014; 59:649–654Crossref, Medline, Google Scholar
5 : Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety 2013; 30:515–527 Crossref, Medline, Google Scholar
6 : A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 2008; 106:1–27Crossref, Medline, Google Scholar
7 : The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs 2015; 29:577–589Crossref, Medline, Google Scholar
8 : Defining and measuring functional recovery from depression. CNS Drugs 2010; 24:267–284Crossref, Medline, Google Scholar
9 : Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Clin Psychiatry 2014; 75:864–876Crossref, Medline, Google Scholar
10 : Contemporary methods of improving cognitive dysfunction in clinical depression. Expert Rev Neurother 2019; 19:431–443Crossref, Medline, Google Scholar
11 : The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol 2016; 19:pyw055Crossref, Medline, Google Scholar
12 : A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 2015; 40:2025–2037Crossref, Medline, Google Scholar
13 : The use and effectiveness of mobile apps for depression: results from a fully remote clinical trial. J Med Internet Res 2016; 18:e330Crossref, Medline, Google Scholar
14 : A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013; 58:376–385Crossref, Medline, Google Scholar
15 : Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cogn Ther Res 2007; 31:235–262Crossref, Google Scholar
16 : Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72:603–611Crossref, Medline, Google Scholar
17 : Identifying the brain’s most globally connected regions. NeuroImage 2010; 49:3132–3148Crossref, Medline, Google Scholar
18 : Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013; 16:1348–1355Crossref, Medline, Google Scholar
19 : Conducting a fully mobile and randomised clinical trial for depression: access, engagement, and expense. BMJ Innov 2016; 2:14–21Crossref, Medline, Google Scholar
20 : Improving late life depression and cognitive control through the use of therapeutic video game technology: a proof-of-concept randomized trial. Depress Anxiety 2017; 34:508–517Crossref, Medline, Google Scholar
21 : A digital intervention targeting cognitive control network dysfunction in middle age and older adults with major depression. Transl Psychiatry 2021; 11:269Crossref, Medline, Google Scholar
22 : A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Health 2020; 2:e168–e178Crossref, Medline, Google Scholar
23 : TOVA Continuous Performance Test Manual. Los Alamitos, Calif, Universal Attention Disorders, 1996Google Scholar
24 : TOVA Professional Manual: Test of Variables of Attention Continuous Performance Test, 2016. www.tovatest.com/Google Scholar
25 : Validation of the tablet-administered Brief Assessment of Cognition (BAC App). Schizophr Res 2017; 181:100–106Crossref, Medline, Google Scholar
26 : The Halstead-Reitan Neuropsycholgical Test Battery: Therapy and Clinical Interpretation. Tucson, Ariz, Neuropsychological Press, 1985Google Scholar
27 : Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 1997; 54:159–165Crossref, Medline, Google Scholar
28 : SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS. London, Routledge, 2020Google Scholar
29 : Neurocognitive deficits and disability in major depressive disorder. Psych Res 2006; 145:39–48Crossref, Medline, Google Scholar
30 : Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med 2004; 34:1453–1463Crossref, Medline, Google Scholar
31 : Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNS Neurol Disord Drug Targets 2014; 13:1804–1818Crossref, Medline, Google Scholar
32 : The therapeutic and health benefits of playing video games, in The Oxford Handbook of Cyberpsychology. Edited by Attrill-Smith A, Fullwood C, Kuss DJ, . Oxford, UK, Oxford University Press, 2019Google Scholar
33 : The pervasive problem with placebos in psychology: why active control groups are not sufficient to rule out placebo effects. Perspect Psychol Sci 2013; 8:445–454Crossref, Medline, Google Scholar
34 : Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008; 43:76–87Crossref, Medline, Google Scholar



