Mindfulness-Oriented Recovery Enhancement for Veterans and Military Personnel on Long-Term Opioid Therapy for Chronic Pain: A Randomized Clinical Trial
Abstract
Objective:
This randomized clinical trial evaluated the efficacy of Mindfulness-Oriented Recovery Enhancement (MORE) among past and present U.S. military personnel with prescriptions for long-term opioid therapy for chronic pain.
Methods:
In this clinical trial, 230 past and present military personnel with prescriptions for long-term opioid therapy were randomized in a 1:1 ratio to MORE or supportive psychotherapy (initially delivered in person and then via videoconferencing after the onset of the COVID-19 pandemic). Primary outcomes were chronic pain, measured by the Brief Pain Inventory, and aberrant drug-related behaviors, measured by the Current Opioid Misuse Measure, through 8 months of follow-up. Opioid dose was a key secondary outcome. Other outcomes included psychiatric symptoms, catastrophizing, positive affect, ecological momentary assessments of opioid craving, and opioid attentional bias.
Results:
MORE was superior to supportive psychotherapy through the 8-month follow-up in reducing pain-related functional interference, pain severity, and opioid dose. MORE reduced daily opioid dose by 20.7%, compared with a dose reduction of 3.9% with supportive psychotherapy. Although there was no overall between-group difference in opioid misuse, the in-person MORE intervention outperformed supportive psychotherapy for reducing opioid misuse. MORE reduced anhedonia, pain catastrophizing, craving, and opioid attentional bias and increased positive affect to a greater extent than supportive psychotherapy. MORE also modulated therapeutic processes, including mindful reinterpretation of pain sensations, nonreactivity, savoring, positive attention, and reappraisal.
Conclusions:
Among past and present U.S. military personnel, MORE led to sustained decreases in chronic pain, opioid use, craving, and opioid cue reactivity. MORE facilitated opioid dose reduction while preserving adequate pain control and preventing mood disturbances, suggesting its utility for safe opioid tapering.
Chronic pain and opioid misuse are threats to U.S. military personnel and veterans, who may develop problematic opioid use coincident with long-term opioid therapy for pain conditions incurred during military service. Many military personnel and veterans who suffer from persistent pain conditions are treated with long-term opioid therapy, which increases the risk of transitioning to opioid misuse (1). Chronic pain is prevalent among military personnel, with 30% having a chronic pain diagnosis (2) and 44% reporting chronic pain following deployment (3). Among active-duty soldiers returning from deployment, 35% received an opioid prescription (4). Opioids were prescribed to 33% of veterans in 2012 (5). Although studies from the Veterans Health Administration have documented decreases in the prevalence rates of long-term opioid therapy (6), 25%–29% of veterans receiving such therapy for pain subsequently exhibit aberrant drug-related behaviors, such as unauthorized dose escalation and opioid self-medication of negative affective states, that mark the transition from medically appropriate opioid use to misuse (7). Opioid misuse among patients receiving long-term opioid therapy for chronic pain may arise from the neuroplastic effects of prolonged opioid use on brain reward circuits, which can increase opioid cue reactivity and craving and simultaneously reduce sensitivity to pleasure derived from natural rewards (8). Such reward dysregulation is thought, in turn, to result in higher levels of anhedonia and dysphoria that compel opioid dose escalation as a means of maintaining hedonic equilibrium (9). In part because of the complexities presented by these intersecting pathogenic mechanisms, there is a dearth of efficacious interventions for veterans and military personnel with chronic pain treated with long-term opioid therapy.
To meet this need, we conducted a randomized clinical trial of Mindfulness-Oriented Recovery Enhancement (MORE) in a sample of veterans and military personnel receiving long-term opioid therapy for chronic pain. MORE unites complementary aspects of mindfulness training, cognitive-behavioral therapy, and principles from positive psychology to restructure dysregulated reward processes underpinning the cycle of behavioral escalation that links chronic pain to opioid misuse. In a recent meta-analysis, MORE was shown to produce statistically significant effects on addictive behavior, chronic pain, and psychiatric symptoms among civilians (10). Yet, its efficacy among veterans and military personnel remains unknown. Here we conducted an efficacy test of MORE versus supportive psychotherapy for outcomes including chronic pain, aberrant drug-related behaviors, and opioid use, as well as psychiatric symptoms, pain catastrophizing, reward-related processes, opioid craving, and opioid cue reactivity.
Methods
Study Design, Setting, and Participants
This was a single-blind, parallel, randomized controlled superiority trial. The University of Utah institutional review board approved the protocol. From April 1, 2017, to October 1, 2021, participants were recruited from the Salt Lake City Veterans Affairs Medical Center, the Utah Army National Guard, and the region of the Salt Lake valley, Utah. Participants were recruited based on data extracted from electronic medical records (EMRs) and from physician referrals and advertisements. Following the onset of the COVID-19 pandemic, the trial shifted from an in-person format to a remote format. Eligible participants were U.S. military personnel or veterans age 18 or older with a physician-confirmed, chronic pain–related diagnosis, daily prescription opioid use for ≥3 months, and an average pain rating ≥3 on a 0–10 numerical rating scale. We excluded individuals receiving cancer treatment (because of potential confounding by treatment effects or disease progression), individuals experiencing elevated suicide risk, psychosis, and severe nonopioid substance use disorder (assessed with the Mini-International Neuropsychiatric Interview [11]), and individuals with previous exposure to mindfulness interventions (e.g., mindfulness-based stress reduction). After obtaining informed consent from the participants (which covered EMR data extraction), study staff collected demographic and outcome data, and the in-person cohorts completed a dot-probe task as a measure of opioid cue reactivity. Participants were compensated after each study visit.
Masking and Randomization
A researcher who was not involved in the assessments or analysis used a computerized random number generator to determine treatment allocation to MORE or supportive psychotherapy with a blocked random assignment method (1:1 ratio, with block sizes of 4 to 8). Participants were not allocated by the coordinator until the day of the first treatment session to maintain allocation concealment and prevent bias. Assessments were conducted by staff blinded to group allocation (which remained concealed throughout the study). The allocation list was not accessible to staff involved in study treatments or assessments, and participants were prompted to keep their treatment assignment undisclosed before each assessment.
Interventions
The study interventions were delivered in eight weekly 2-hour group therapy sessions with 6–12 participants. Sessions were delivered in person until the onset of the COVID-19 pandemic, at which point sessions were implemented through a HIPAA-compliant videoconferencing platform. To control for therapist effects, the same two licensed psychologists conducted an equal number of MORE and supportive psychotherapy sessions.
The manualized MORE intervention (12) provided training in mindfulness, reappraisal, and savoring techniques (N=69 in person; N=47 remote). Mindfulness training entailed mindful breathing and body scan meditations to attenuate pain and opioid craving, by reinterpreting these aversive sensations as innocuous sensory signals, and to self-regulate reactivity to opioid-related cues. Reappraisal training entailed reframing stress appraisals to reduce catastrophizing and negative emotional reactivity, while promoting meaning-making in the face of adversity. Savoring training entailed mindfully focusing attention on pleasant events and pleasurable sensations to boost positive emotions and reduce anhedonia. The psychoeducation component of the intervention addressed opioid misuse and chronic pain. Participants were instructed to complete 15-minute audio-guided mindfulness, reappraisal, and savoring practices each day. Also, before taking each daily opioid dose, participants were instructed to practice a 3-minute mindfulness technique designed to promote opioid sparing (i.e., reduced use of opioids) and help patients delay taking as-needed opioid doses by using mindful breathing to reduce pain and increase self-control over opioid craving and cue reactivity. We defined the minimum intervention dose of MORE (and supportive psychotherapy) as four or more treatment sessions, in accordance with treatment completion thresholds established in previous trials of mindfulness interventions (13, 14).
The active control condition in this trial consisted of supportive psychotherapy, in which therapist-led discussions elicited participants’ thoughts and emotions about coping with chronic pain, opioid misuse, and emotional distress (N=75 in person; N=39 remote). Clinicians encouraged expression of emotions via empathic responding and fostered social support between group members. No skill training or education on mindfulness was provided. To match the homework requirements of the MORE intervention, participants in the supportive psychotherapy group were instructed to journal about themes discussed at weekly therapy sessions for 15 minutes each day. We selected this control condition, which was designed emulate a widely available form of process-oriented, client-centered therapy (15), to control for nonspecific therapeutic factors (e.g., therapeutic relationship and social support). Previous trials of MORE and other mindfulness interventions have validated this control condition and found no significant difference in treatment credibility ratings between mindfulness interventions and supportive psychotherapy (16, 17).
Sessions were audio-recorded, and treatment fidelity was scored with validated measures (18). Adherence and competence scores were excellent, indicating that both manualized protocols were implemented as intended with no treatment diffusion.
Outcomes
Primary and secondary outcomes were collected over an 8-month period, at baseline, after treatment, and then every 2 months for the next 6 months. Our prespecified primary chronic pain outcome was measured with the pain interference and pain severity subscales of the Brief Pain Inventory (BPI) (19). The primary opioid misuse outcome was the Current Opioid Misuse Measure (COMM), a self-reported measure of aberrant drug-related behaviors, including using opioids for reasons other than pain, taking more opioids than prescribed, obtaining opioids from sources other than a prescribing physician, exhibiting signs of intoxication and emotional volatility, and so on (20). We chose this continuous measure as our primary measure of opioid misuse severity, unlike the measurement approach used in our previous trial of MORE (14). In that study, all participants entered the trial with a positive score on a binary measure of opioid misuse, and opioid misuse outcomes were assessed with this binary measure. For the present study of patients on long-term opioid therapy where opioid misuse was not an inclusion criterion, we reasoned that changes in a continuous measure of aberrant drug-related behaviors were clinically meaningful. To triangulate self-reports of aberrant drug-related behaviors, we also performed a clinical assessment of opioid misuse with the Addiction Behaviors Checklist (21), a semistructured interview performed by clinical staff (i.e., psychologists, social workers, and nurses) blinded to treatment assignment. In addition, at each assessment point for the in-person cohort, we performed urine screens, and as an exploratory outcome, we extracted available random urine screen data from EMRs at the 12-month follow-up. Urine screens that were positive for illicit drugs (e.g., heroin, cocaine, methamphetamine) or nonprescribed opioid medications were classified as positive.
Changes in morphine equivalent daily opioid dose were computed in accordance with best practice guidelines (22) as assessed with the timeline followback method (23), and when participants missed follow-up assessments, opioid use was obtained from the EMR.
Other secondary outcome measures included scores on the Depression Anxiety Stress Scale–21 (24), the Posttraumatic Stress Disorder Checklist–Military Version (25), the pain catastrophizing subscale of the Coping Strategies Questionnaire (26), the Snaith-Hamilton Anhedonia and Pleasure Scale (27), and the positive affect subscale of the Positive and Negative Affect Schedule (28). Process measures included the attention to positive information subscale of the Attention to Positive and Negative Information Scale (29), the cognitive reappraisal subscale of the Emotion Regulation Questionnaire (30), the Momentary Savoring Scale (31), the Mindful Reappraisal of Pain Scale (32), and the nonreactivity subscale of the Five Facet Mindfulness Questionnaire (33), which was selected because of its previously demonstrated association with the pain-relieving effects of MORE (16). Opioid craving in daily life was assessed on a 0–10 scale by ecological momentary assessments delivered by smartphone at three random times each day during the 8-week study treatments.
Before the trial switched to a remote format at the onset of the COVID-19 pandemic, opioid cue reactivity was assessed in the laboratory with an opioid dot-probe task designed to measure attentional bias toward opioid-related cues (34). Each trial began with the presentation of a fixation cross (for 500 ms) followed by an opioid-related image and a neutral image, which appeared side by side for either 200 or 2,000 ms. Opioid-related cues included images of opioid pills and pill bottles, which had been validated in previous studies (34). Neutral images were matched to the opioid-related images by visual features, including color, figure-ground relationships, and the presence of human faces. Presentation duration and the left-right position of the images were randomized and counterbalanced within each block of 58 trials. After a 50-ms interstimulus interval, a target probe replaced one of the images. Participants indicated the location of the target by pressing a left or right button, and reaction times were recorded.
Statistical Analysis
Based on our pilot data, a sample size of 200 (after 30% attrition) would provide power greater than 0.90 at an alpha of 0.05 to estimate small clinical effects (Cohen’s d=0.20) from baseline-adjusted treatment effects averaged over four postrandomization time points on the BPI, the COMM, and other continuous outcomes (Cohen’s d values in pilot trials of MORE ranged from 0.50 to 0.84) (16). We planned to enroll 260 patients to account for loss to follow-up; because of the COVID-19 pandemic, the actual number of patients enrolled was 230.
To control for random baseline imbalance between study treatment arms, we used a constrained longitudinal data analysis (cLDA) model in SAS, version 9.4, and Mplus, version 8.10. The cLDA model (for additional details, see the online supplement) provides results similar to the classic analysis of covariance (ANCOVA) approach when there is minimal missing data (35, 36). Yet, unlike ANCOVA, cLDA is a full information maximum likelihood (FIML) procedure that retains every observation and is hence more efficient and less prone to missing data biases. The baseline assessment and four postbaseline assessments (at 2, 4, 6, and 8 months of follow-up) are described above. Baseline measurements were taken before randomization to supportive psychotherapy or MORE conditions, so the treatment group means are assumed equal at baseline in the cLDA model but allowed to differ at follow-up assessments. As detailed above, COVID-19-related restrictions, which were instituted midway through the study, necessitated a change in format. Therapy sessions were initially conducted in person but were subsequently implemented remotely to comply with requirements. Because this format change could not be randomized, we treated delivery method as an observational stratification variable. Therefore, population means for the treatment groups were randomized and necessarily equal within the in-person and remote conditions, but the effects of these two conditions were uncontrolled, and their baseline means were unconstrained. Effects were averaged across the in-person and remote conditions to compute the principal estimate of overall treatment impact. Serial dependence was modeled by normally distributed random intercepts, along with normally distributed random error. To control for multiple comparisons, we first conducted three independent structural equation models to perform a multivariate omnibus test of no treatment impact on primary, secondary, and process outcomes. These omnibus tests, which control for multiple comparisons, are likelihood ratio tests of the null hypothesis (no difference between groups) with four degrees of freedom between the unrestricted model and the constrained model. Examination of individual outcomes was pursued if and only if the omnibus test was rejected at an alpha of 0.05. Opioid dose was square root transformed to reduce skew before using cLDA.
If the omnibus multivariate test was significant, we examined the univariate treatment effects on each outcome using the cLDA approach under a mixed-effects model likelihood framework. For each outcome, the null hypothesis posited equal baseline-adjusted mean differences between treatment arms over the four postbaseline time points, while assuming equal baseline means across treatment arms, with a compound symmetry covariance structure. The single degree-of-freedom estimates and tests were implemented using SAS “estimate” coefficients and evaluated against the null at alpha=0.05 (two-sided, with conservative Kenward-Roger degrees of freedom). Time was treated as a categorical factor with four postbaseline levels. The overall estimate of the between-group treatment effect is reported in unstandardized response variable metrics. No additional covariates were considered in these primary analyses.
Ecological momentary assessment of craving was analyzed with a mixed model that included fixed effects of time, treatment, and the treatment-by-time interaction, as well as a random intercept and a first-order autoregressive covariance structure. In this analysis, the treatment-by-time interaction (difference in craving trajectory across the possible 180 assessments per subject) was the primary fixed effect of interest. Similarly specified mixed models were also used to assess treatment effects on opioid attentional bias scores, with the treatment-by-time interaction being the effect of interest. Attentional bias scores were computed separately for reaction times (RTs) following presentation of the 200- and 2,000-ms cues using the canonical approach (RTneutral−RTopioid), with higher values indicating a greater attentional bias toward opioids (34).
We followed an intent-to-treat approach and sought to obtain follow-up data on all participants. Study discontinuation rates did not differ significantly across treatment arms and were similar to rates observed in trials of psychosocial treatments for opioid use disorder (37). No missing data were imputed, because FIML methods are theoretically accurate even when dropout rates are substantial and when predictors of dropout, such as treatment assignment and prior observations, are modeled as observed variables (38). Sensitivity to missingness at random was evaluated in FIML multivariate analyses that introduced a larger set of auxiliary demographic and clinical variables expected to correlate with missingness—an alternative to multiple imputation that often produces more accurate and precise parameter estimates (38).
Results
Participant Characteristics
Of the 331 patients assessed for eligibility, we enrolled 230 (Figure 1). Of this sample, 83% were male and 91% were veterans, with the remainder being active-duty military personnel. Participants had a mean age of 57.5 years (SD=11.7), mean BPI pain severity score of 5.6 (SD=1.5), mean COMM score of 13.4 (SD=7.8), and a mean morphine equivalent daily opioid dose of 105.3 mg (SD=204.6; interquartile range, 12.0–90.0 mg). More than half of the participants (55%) reported having oxycodone or hydrocodone prescriptions. Participants reported having pain for a mean duration of 19.3 years (SD=12.6), and pain was most commonly reported to be in the lower back (81%). Major depressive disorder was prevalent in the sample (60%), but a substantial proportion of participants also met clinical criteria for opioid use disorder (34%) or posttraumatic stress disorder (PTSD) (19%), based on the Mini-International Neuropsychiatric Interview at baseline, with no between-group differences. Participants in the MORE and supportive psychotherapy conditions attended a mean of 5.5 and 5.7 sessions, respectively. Data for one or more outcome variables from the 8-month follow-up were available for 81% of participants. Demographic and baseline clinical characteristics were similar between the two groups (Table 1).
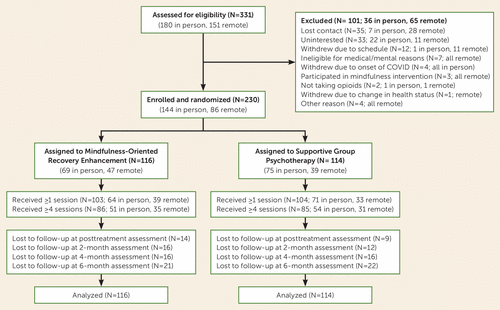
FIGURE 1. Flow diagram for a randomized clinical trial of Mindfulness-Oriented Recovery Enhancement among veterans and military personnel receiving long-term opioid therapy for chronic pain.
| Measure | Mindfulness-Oriented Recovery Enhancement (N=116) | Supportive Psychotherapy (N=114) | ||
|---|---|---|---|---|
| | Mean | SD | Mean | SD |
| Age (years) | 56.4 | 12.9 | 58.6 | 10.4 |
| | N | % | N | % |
| Sex | | | | |
| Male | 97 | 83.6 | 94 | 82.5 |
| Female | 17 | 14.6 | 19 | 16.6 |
| Transgender | 2 | 1.7 | 1 | 0.8 |
| Sexual orientation | | | | |
| Heterosexual | 111 | 95.7 | 103 | 90.4 |
| Gay or lesbian | 1 | 0.9 | 3 | 2.6 |
| Bisexual | 3 | 2.6 | 3 | 2.6 |
| Other | 0 | 0.0 | 1 | 0.9 |
| Missing | 1 | 0.9 | 4 | 3.5 |
| Race | | | | |
| White | 102 | 87.9 | 95 | 83.3 |
| Black or African American | 6 | 5.2 | 5 | 4.4 |
| Hispanic or Latino | 3 | 2.5 | 4 | 3.5 |
| Native American or Alaskan native | 2 | 1.7 | 4 | 3.5 |
| Other or missing | 2 | 1.7 | 6 | 5.3 |
| Highest level of education | | | | |
| Did not complete high school | 2 | 1.7 | 0 | 0.0 |
| Completed high school | 54 | 46.6 | 38 | 33.3 |
| College degree or greater | 59 | 50.9 | 63 | 55.3 |
| Missing | 1 | 0.8 | 4 | 3.5 |
| Estimated household income | | | | |
| <$25,000 | 24 | 20.7 | 28 | 24.6 |
| $25,000–$49,999 | 39 | 33.6 | 27 | 23.7 |
| $50,000–$99,999 | 32 | 27.6 | 43 | 37.7 |
| ≥$100,000 | 10 | 8.6 | 12 | 10.5 |
| Missing | 1 | 0.8 | 4 | 3.5 |
| Pain condition or locationb | | | | |
| Back pain | 90 | 77.6 | 96 | 84.2 |
| Osteoarthritis pain | 63 | 56.9 | 53 | 46.5 |
| Cervical pain | 17 | 14.7 | 19 | 16.7 |
| Neuropathic pain | 14 | 12.1 | 8 | 7.0 |
| Fibromyalgia | 11 | 9.5 | 9 | 7.9 |
| Migraine or tension headache | 8 | 6.9 | 7 | 6.1 |
| Extremity pain | 6 | 5.2 | 8 | 7.0 |
| Other | 3 | 2.3 | 7 | 6.1 |
| Opioid prescriptionb | | | | |
| Oxycodone | 37 | 31.9 | 39 | 34.2 |
| Hydrocodone | 20 | 17.2 | 28 | 24.5 |
| Tramadol | 42 | 36.2 | 34 | 29.8 |
| Morphine | 18 | 15.5 | 20 | 17.5 |
| Buprenorphine | 11 | 9.5 | 11 | 9.6 |
| Methadone | 5 | 4.3 | 7 | 6.1 |
| Other | 3 | 2.6 | 4 | 3.5 |
| | Mean | SD | Mean | SD |
| Pain and opioid use | | | | |
| Pain severity (0–10) (BPI score) | 5.6 | 1.6 | 5.5 | 1.5 |
| Pain duration (years) | 17.9 | 12.3 | 20.8 | 12.8 |
| Duration of opioid use (years) | 9.8 | 7.4 | 11.3 | 8.7 |
| Morphine equivalent daily dose (mg)c | 104.3 | 186.9 | 106.3 | 222.0 |
| Opioid misuse (COMM score) | 14.2 | 8.4 | 12.7 | 7.2 |
| Opioid craving (0–10) (rating scale) | 4.5 | 3.1 | 3.6 | 3.2 |
| | N | % | N | % |
| Psychiatric diagnoses | | | | |
| Opioid use disorder | 39 | 33.6 | 40 | 35.1 |
| Alcohol or nonopioid substance use disorder | 12 | 10.3 | 17 | 14.9 |
| Major depressive disorder | 71 | 61.7 | 68 | 59.6 |
| Generalized anxiety disorder | 24 | 20.6 | 11 | 9.7 |
| Posttraumatic stress disorder | 24 | 20.6 | 20 | 17.4 |
| Military status | | | | |
| Veteran | 106 | 91.4 | 103 | 90.4 |
| Active duty | 10 | 8.6 | 11 | 9.6 |
TABLE 1. Demographic and clinical characteristics of veterans and military personnel receiving long-term opioid therapy for chronic pain (N=230) randomized to Mindfulness-Oriented Recovery Enhancement (MORE) or supportive group psychotherapy conditionsa
Outcomes
Treatment effects of MORE and supportive psychotherapy on primary outcomes, secondary outcomes, and process variables are presented in Table 2. For pain and opioid use outcomes through the 8-month follow-up, the omnibus multivariate likelihood ratio test was significant (χ2=13.09, df=4, p=0.011), indicating the superiority of MORE over supportive psychotherapy. Compared with supportive psychotherapy, MORE produced significantly greater reductions in pain interference (B=0.47, 95% CI=0.10–0.84, p=0.011) and pain severity (B=0.27, 95% CI=0.00–0.54, p=0.048). Although aberrant drug-related behaviors decreased significantly in both groups over time (B=0.50, 95% CI=0.08–0.92, p=0.019) with no significant between-group differences (p=0.43), MORE reduced aberrant drug-related behaviors to a significantly greater extent than supportive psychotherapy in the in-person cohorts (B=0.48, 95% CI=0.01–0.96, p=0.047). No between-group differences were observed in the clinical interview of opioid misuse or in urine drug screen measures, except at an exploratory 12-month follow-up point extracted from EMRs; at this time point, among the patients who completed random urine drug screens (N=38), a smaller percentage of those in the MORE condition (13.3%, N=2) had a positive urine screen compared with those in the supportive psychotherapy condition (43.5%, N=13) (likelihood ratio, χ2=4.13, df=1, p=0.042). With regard to opioid use, MORE reduced daily opioid dose (square root transformed values) to a significantly greater extent than supportive psychotherapy (B=0.65, 95% CI=0.07–1.23, p=0.029); there was a 20.7% reduction in the mean opioid dose (18.88 mg, SD=8.40 mg) in the MORE condition compared with a 3.9% reduction (3.19 mg, SD=4.38 mg) in the supportive psychotherapy condition. Sensitivity analyses controlling for auxiliary variables associated with missingness also found that MORE outperformed supportive psychotherapy in reducing pain interference (p=0.010), pain severity (p=0.025), and opioid dose (p=0.025).
| | Baseline | 2 Months | 4 Months | 6 Months | 8 Months | Between-Group Treatment Effect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Mean | SE | N | Mean | SE | N | Mean | SE | N | Mean | SE | N | Mean | SE | N | Effect | SE | 95% CI | p |
| Pain interference (BPI score) | |||||||||||||||||||
| MORE | 6.04 | 0.30 | 116 | 5.10 | 0.21 | 92 | 5.37 | 0.22 | 84 | 5.44 | 0.22 | 81 | 5.33 | 0.22 | 79 | −0.47 | 0.19 | −0.84, −0.10 | 0.011 |
| SG | 5.93 | 0.32 | 114 | 5.71 | 0.22 | 87 | 5.76 | 0.22 | 80 | 5.71 | 0.23 | 80 | 5.93 | 0.23 | 73 | | | | |
| Pain severity (BPI score) | |||||||||||||||||||
| MORE | 5.58 | 0.20 | 116 | 5.14 | 0.16 | 92 | 5.37 | 0.16 | 84 | 5.43 | 0.16 | 81 | 5.31 | 0.16 | 79 | −0.27 | 0.14 | −0.54, −0.00 | 0.048 |
| SG | 5.59 | 0.22 | 114 | 5.48 | 0.16 | 87 | 5.58 | 0.16 | 80 | 5.53 | 0.16 | 80 | 5.76 | 0.17 | 73 | | | | |
| Aberrant drug-related behaviors (COMM score) | |||||||||||||||||||
| MORE | 10.46 | 1.04 | 116 | 10.84 | 0.63 | 92 | 9.61 | 0.65 | 84 | 9.48 | 0.65 | 81 | 8.89 | 0.66 | 79 | −0.46 | 0.59 | −1.62, 0.69 | 0.430 |
| SG | 12.23 | 0.97 | 114 | 10.42 | 0.66 | 87 | 10.52 | 0.66 | 80 | 9.84 | 0.67 | 80 | 9.87 | 0.69 | 73 | | | | |
| Opioid dose (square root transformed)b | |||||||||||||||||||
| MORE | 7.74 | 0.90 | 116 | 7.25 | 0.49 | 102 | 6.93 | 0.50 | 100 | 6.91 | 0.50 | 84 | 6.84 | 0.50 | 95 | −0.65 | 0.30 | −1.23, −0.07 | 0.029 |
| SG | 8.00 | 0.94 | 114 | 7.75 | 0.50 | 105 | 7.85 | 0.50 | 93 | 7.64 | 0.50 | 98 | 7.29 | 0.51 | 92 | | | | |
| Emotional distress (DASS score) | |||||||||||||||||||
| MORE | 20.60 | 1.88 | 115 | 17.81 | 1.13 | 85 | 17.38 | 1.15 | 81 | 15.79 | 1.15 | 78 | 16.77 | 1.16 | 76 | −1.56 | 0.95 | −3.42, 0.31 | 0.102 |
| SG | 17.52 | 1.93 | 108 | 18.89 | 1.18 | 80 | 19.13 | 1.17 | 80 | 17.41 | 1.21 | 72 | 17.88 | 1.46 | 70 | | | | |
| Posttraumatic stress symptoms (PCL score) | |||||||||||||||||||
| MORE | 45.51 | 2.23 | 114 | 42.52 | 1.31 | 85 | 40.87 | 1.65 | 81 | 40.70 | 1.69 | 78 | 40.81 | 1.69 | 76 | −1.43 | 1.06 | −3.51, 0.64 | 0.175 |
| SG | 41.80 | 2.22 | 108 | 42.52 | 1.35 | 80 | 43.38 | 1.36 | 79 | 41.54 | 1.38 | 72 | 41.57 | 1.38 | 70 | | | | |
| Positive affect (PANAS score) | |||||||||||||||||||
| MORE | 28.54 | 1.07 | 115 | 33.15 | 0.74 | 85 | 32.11 | 0.75 | 81 | 31.95 | 0.76 | 77 | 32.11 | 0.76 | 76 | 1.50 | 0.69 | 0.15, 2.85 | 0.029 |
| SG | 34.22 | 1.22 | 108 | 36.81 | 0.78 | 79 | 36.37 | 0.77 | 80 | 34.47 | 0.80 | 71 | 36.19 | 0.80 | 69 | | | | |
| Anhedonia (SHAPS score) | |||||||||||||||||||
| MORE | 25.78 | 0.96 | 115 | 23.19 | 0.65 | 85 | 23.63 | 0.66 | 81 | 25.53 | 0.66 | 78 | 23.81 | 0.67 | 76 | −2.27 | 0.59 | −3.42, −1.11 | 0.001 |
| SG | 26.87 | 0.95 | 108 | 24.85 | 0.68 | 79 | 25.30 | 0.68 | 80 | 26.57 | 0.71 | 72 | 26.51 | 0.70 | 71 | | | | |
| Attention to positive information (APNIS score) | |||||||||||||||||||
| MORE | 40.53 | 0.91 | 115 | 43.26 | 0.59 | 85 | 43.06 | 0.60 | 80 | 43.28 | 0.60 | 78 | 43.49 | 0.60 | 76 | 1.72 | 0.53 | 0.69, 2.76 | 0.001 |
| SG | 41.97 | 0.95 | 108 | 41.41 | 0.62 | 80 | 41.99 | 0.61 | 80 | 41.31 | 0.63 | 72 | 41.48 | 0.63 | 70 | | | | |
| Mindful reappraisal of pain sensations (MRPS score) | |||||||||||||||||||
| MORE | 13.87 | 1.44 | 115 | 22.36 | 1.04 | 85 | 21.58 | 1.06 | 81 | 20.57 | 1.06 | 78 | 19.84 | 1.12 | 76 | 6.38 | 0.98 | 4.46, 8.31 | 0.001 |
| SG | 12.43 | 1.38 | 108 | 15.02 | 1.09 | 80 | 14.82 | 1.08 | 80 | 16.00 | 1.12 | 73 | 15.70 | 1.12 | 71 | | | | |
| Nonreactivity to distressing thoughts and emotions (FFMQ score) | |||||||||||||||||||
| MORE | 22.31 | 0.63 | 115 | 22.79 | 0.48 | 85 | 23.45 | 0.49 | 81 | 23.18 | 0.49 | 79 | 23.81 | 0.49 | 76 | 0.95 | 0.44 | 0.09, 1.81 | 0.030 |
| SG | 23.41 | 0.78 | 109 | 22.72 | 0.48 | 81 | 22.47 | 0.50 | 79 | 22.55 | 0.52 | 73 | 21.98 | 0.51 | 71 | | | | |
| Pain catastrophizing (CSQ score) | |||||||||||||||||||
| MORE | 13.75 | 1.18 | 115 | 10.32 | 0.75 | 85 | 9.16 | 0.76 | 81 | 9.40 | 0.76 | 78 | 8.91 | 0.77 | 76 | −2.54 | 0.69 | −3.98, −1.19 | 0.001 |
| SG | 12.43 | 1.22 | 108 | 11.98 | 0.78 | 79 | 11.96 | 0.78 | 80 | 11.70 | 0.80 | 73 | 12.31 | 0.80 | 71 | | | | |
TABLE 2. Treatment effects of Mindfulness-Oriented Recovery Enhancement (MORE) versus supportive group (SG) psychotherapy on primary outcomes, secondary outcomes, and process variablesa
For secondary outcomes through the 8-month follow-up, the omnibus multivariate likelihood ratio test was highly significant (χ2=32.51, df=5, p<0.00001). No significant between-group differences were observed for emotional distress (p=0.102) or PTSD symptoms (p=0.175); participants in both treatment conditions improved over time. However, MORE reduced anhedonia (B=2.27, 95% CI=1.11–3.42, p<0.001) and pain catastrophizing (B=2.54, 95% CI=1.19–3.98, p<0.001) and improved positive affect (B=1.50, 95% CI=0.15–2.85, p=0.029) to a significantly greater extent than supportive psychotherapy.
For the process variables, the omnibus multivariate likelihood ratio test was highly significant (χ2=32.79, df=5, p<0.00001). MORE produced greater increases than supportive psychotherapy for all process variables, including mindful reinterpretation of pain sensations (p<0.001), nonreactivity to distressing thoughts and emotions (p=0.030), cognitive reappraisal (p=0.011), savoring (p=0.036), and attention to positive information (p=0.001).
Regarding ecological momentary assessment of opioid craving, craving ratings decreased over the course of treatment by 0.67 points more in the MORE condition than in the supportive psychotherapy condition (95% CI=0.001–0.007, p=0.019). Finally, participants assigned to MORE exhibited significantly greater decreases in the 200-ms opioid attentional bias measure than participants in supportive psychotherapy (B=29.89 ms; 95% CI=3.77–56.01; p=0.025). No significant group differences were observed with the 2,000-ms attentional bias measure.
Discussion
In a sample of past and present U.S. military personnel on long-term opioid therapy, treatment with MORE was associated with significantly greater reductions in chronic pain symptoms and opioid use than supportive psychotherapy. The pain-relieving effects of MORE were coupled with reductions in pain catastrophizing and an increased capacity to mindfully reinterpret pain as an innocuous sensory signal that does not necessarily signify harm. Although aberrant drug-related behaviors decreased substantially over time for both treatment groups, significantly greater reductions in aberrant drug-related behaviors were observed with the in-person MORE intervention when compared with supportive psychotherapy. In a similar manner, both MORE and supportive psychotherapy were associated with reduced emotional distress and PTSD symptoms; however, MORE showed clear superiority for improving reward-related processes, including positive affect, anhedonia, savoring, and attention to positive information. Finally, MORE was associated with reduced opioid craving measured in daily life and decreased opioid cue reactivity measured in the laboratory with a dot-probe task.
These findings converge with our previous research with civilians that demonstrated the efficacy of MORE for reducing pain and opioid use (14). Here, MORE facilitated opioid dose reduction while preserving adequate pain control and preventing disturbances in mood, suggesting the utility of MORE as an adjunctive therapy for safe opioid tapering among veterans and military personnel. MORE produced the largest opioid dose reductions among patients with lower back pain or arthritis. The observed dose reductions are especially remarkable given that participants were not given specific tapering instructions as part of the study. Future trials might combine MORE with a patient-centered opioid-tapering approach to produce even greater reductions in opioid dosing.
The effects of MORE on opioid misuse in this study were less robust than those reported in our previous trial, where we found that MORE reduced opioid misuse by 45%, more than doubling the effect of supportive psychotherapy (14). Delivering MORE via teletherapy may have attenuated its effect size. Alternatively, supportive psychotherapy may have been more effective during the COVID-19 pandemic, when social isolation drove despair that fueled opioid misuse and an unprecedented number of overdose deaths. Or military populations may be more difficult to treat than civilians as a result of chronic exposure to extreme stress. Nonetheless, urine screen data from EMRs suggested that MORE may produce long-term reductions in illicit drug use and nonprescribed opioid use; additional studies with longer-term follow-ups are needed to replicate this effect.
Notably, MORE decreased attentional bias for opioid-related cues presented for 200 ms but not for cues presented for 2,000 ms, suggesting that the effects of MORE were most evident during the initial automatic attentional orienting to the cue and not during the stage of attentional disengagement (39). Thus, instead of an effortful shifting of attention away from drug cues (e.g., avoidance), MORE might decrease drug cue reactivity in a bottom-up fashion by dampening the incentive salience of the drug. Congruent with this result, previous studies indicated that MORE reduces electrocortical indices of opioid cue reactivity (40). According to our restructuring reward hypothesis (9), MORE restructures bottom-up reward processing from the valuation of drug-related reward back to a valuation of natural reward; this shift occurs by strengthening cognitive control over reward processing through techniques that devalue the drug (e.g., mindfulness of craving and reappraisal of negative consequences of drug use) and increase the competing value of nondrug natural rewards (e.g., savoring). In that regard, previous studies have shown that MORE increases EEG markers of cognitive control (41) and natural reward responsiveness (9, 40). Concomitantly, in the present study, MORE significantly boosted positive affect, savoring, and attention to positive information while reducing anhedonia, suggesting that MORE improves responsiveness to natural rewards and providing additional support for the restructuring reward hypothesis.
The primary limitation of the trial was the rate of loss to follow-up, which was exacerbated by the COVID-19 pandemic. Nonetheless, our trial retention rate was superior to that of other psychotherapy trials of opioid users (mean discontinuation rate of 42%) (37) and trials of medications for opioid use disorder of shorter durations (e.g., 24 weeks) (42). Because dropout rates did not differ between treatment arms, missing data were unlikely to bias outcome analyses toward one of the groups. Sensitivity analyses controlling for auxiliary covariates associated with missingness showed the superiority of MORE over supportive psychotherapy. The study was also limited because it was not possible to blind participants to treatment assignment; nonetheless, participants were informed that the study was designed to compare two active treatments, and the experimental conditions were not identified. Because the sample was predominantly White and male, the findings may not generalize to non-White racial groups. Finally, because we had originally intended to test the in-person MORE intervention and switched to remote delivery only after the onset of the COVID-19 pandemic, we did not have adequate statistical power to test remote versus in-person formats as a treatment moderator. Future fully powered noninferiority trials should assess whether delivering MORE remotely produces results comparable to the in-person format.
In summary, MORE demonstrated efficacy in reducing chronic pain and opioid use among veterans and military personnel being treated with long-term opioid therapy. Implementation and dissemination research should assess how to best deliver MORE to individuals across the Veterans Health Administration and the Department of Defense.
1. : The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162:276–286Crossref, Medline, Google Scholar
2. : A burden and prevalence analysis of chronic pain by distinct case definitions among active duty US military service members, 2018. Pain Physician 2020; 23:E429–E440Crossref, Medline, Google Scholar
3. : Chronic pain and opioid use in US soldiers after combat deployment. JAMA Intern Med 2014; 174:1400–1401Crossref, Medline, Google Scholar
4. : Predictors of postdeployment prescription opioid receipt and long-term prescription opioid utilization among Army active duty soldiers. Mil Med 2019; 184:e101–e109Crossref, Medline, Google Scholar
5. : Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004–2012. J Gen Intern Med 2015; 30:597–604Crossref, Medline, Google Scholar
6. : Opioid discontinuation among patients receiving high-dose long-term opioid therapy in the Veterans Health Administration. J Gen Intern Med 2020; 35:903–909Crossref, Medline, Google Scholar
7. : Prescription opioid misuse and its correlates among veterans and military in the United States: a systematic literature review. Drug Alcohol Depend 2020; 216:108311Crossref, Medline, Google Scholar
8. : Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry 2020; 87:44–53Crossref, Medline, Google Scholar
9. : Mindful positive emotion regulation as a treatment for addiction: from hedonic pleasure to self-transcendent meaning. Curr Opin Behav Sci 2021; 39:168–177Crossref, Medline, Google Scholar
10. : Mindfulness-Oriented Recovery Enhancement for addictive behavior, psychiatric distress, and chronic pain: a multilevel meta-analysis of randomized controlled trials. Mindfulness (NY) 2022; 13:2396–2412Crossref, Medline, Google Scholar
11. : Mini International Neuropsychiatric Interview–Version 7.0.0 (DSM-5). Tampa, FL, Harm Research Press, 2016Google Scholar
12. : Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain. Washington, DC, NASW Press, 2013Google Scholar
13. : Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet 2015; 386:63–73Crossref, Medline, Google Scholar
14. : Mindfulness-oriented recovery enhancement vs supportive group therapy for co-occurring opioid misuse and chronic pain in primary care: a randomized clinical trial. JAMA Intern Med 2022; 182:407–417Crossref, Medline, Google Scholar
15. : Client-Centered Therapy: Its Current Practice, Implications, and Theory. Boston, Houghton Mifflin, 1951Google Scholar
16. : Mindfulness-Oriented Recovery Enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol 2014; 82:448–459Crossref, Medline, Google Scholar
17. : Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol 2011; 106:1678–1688Crossref, Medline, Google Scholar
18. : The Mindfulness-Oriented Recovery Enhancement Fidelity Measure (MORE-FM): development and validation of a new tool to assess therapist adherence and competence. J Evid Based Soc Work (2019) 2021; 18:308–322Crossref, Medline, Google Scholar
19. : Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994; 23:129–138Medline, Google Scholar
20. : Development and validation of the Current Opioid Misuse Measure. Pain 2007; 130:144–156Crossref, Medline, Google Scholar
21. : The Addiction Behaviors Checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage 2006; 32:342–351Crossref, Medline, Google Scholar
22. : CDC guideline for prescribing opioids for chronic pain: United States, 2016. JAMA 2016; 315:1624–1645Crossref, Medline, Google Scholar
23. : The Timeline Followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol 2000; 68:134–144Crossref, Medline, Google Scholar
24. : The Depression, Anxiety and Stress Scale (DASS-21) as a screener for depression in substance use disorder inpatients: a pilot study. Eur Addict Res 2017; 23:260–268Crossref, Medline, Google Scholar
25. : Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996; 34:669–673Crossref, Medline, Google Scholar
26. : The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983; 17:33–44Crossref, Medline, Google Scholar
27. : A scale for the assessment of hedonic tone: the Snaith–Hamilton Pleasure Scale. Br J Psychiatry 1995; 167:99–103Crossref, Medline, Google Scholar
28. : Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063–1070Crossref, Medline, Google Scholar
29. : Cognitive tendencies of focusing on positive and negative information. J Res Pers 2006; 40:891–910Crossref, Google Scholar
30. : Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 2003; 85:348–362Crossref, Medline, Google Scholar
31. : Does savoring increase happiness? A daily diary study. J Posit Psychol 2012; 7:176–187Crossref, Google Scholar
32. : The Mindful Reappraisal of Pain Scale (MRPS): validation of a new measure of psychological mechanisms of mindfulness-based analgesia. Mindfulness (NY) 2023; 14:192–204Crossref, Medline, Google Scholar
33. : Using self-report assessment methods to explore facets of mindfulness. Assessment 2006; 13:27–45Crossref, Medline, Google Scholar
34. : Attentional bias in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2018; 191:270–278Crossref, Medline, Google Scholar
35. : Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med 2009; 28:2509–2530Crossref, Medline, Google Scholar
36. : To condition or not condition? Analysing “change” in longitudinal randomised controlled trials. BMJ Open 2016; 6:e013096Crossref, Medline, Google Scholar
37. : Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction 2020; 115:201–217Crossref, Medline, Google Scholar
38. : Missing data analysis: making it work in the real world. Annu Rev Psychol 2009; 60:549–576Crossref, Medline, Google Scholar
39. : Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 2008; 97:1–20Crossref, Medline, Google Scholar
40. : Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: neural and affective evidence of target engagement. Sci Adv 2019; 5:eaax1569Crossref, Medline, Google Scholar
41. : Mindfulness-induced endogenous theta stimulation occasions self-transcendence and inhibits addictive behavior. Sci Adv 2022; 8:eabo4455Crossref, Medline, Google Scholar
42. : Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2019; 393:778–790Crossref, Medline, Google Scholar



