Psychedelics as Transformative Therapeutics
Abstract
Over the past decade, psychedelic compounds have emerged as potentially transformative therapeutics for a variety of intractable neuropsychiatric conditions. However, historically most of the basic science has utilized these compounds as probes to interrogate various endogenous neurotransmitter systems—mainly the serotonin 5-HT2A receptor. With the renewed interest in utilizing these compounds as therapeutics and the explosion in clinical trials, psychedelics have been purported to treat many neuropsychiatric disorders, including depression, cluster headaches, migraines, anxiety, and obsessive-compulsive disorder. It is therefore imperative to understand the biology and pharmacology behind their therapeutic mechanisms as well as expose any potential pitfalls in their widespread use as treatments. This review covers the latest advances in understanding the biological mechanisms, the newest efforts in drug discovery, and potential pitfalls when it comes to utilizing this class of compounds as emerging therapeutics.
Hallucinogenic drugs have been reportedly used by indigenous peoples for millennia for spiritual purposes, shamanism, and healing (1–3). In the 1950s and 1960s, psychiatry utilized hallucinogenic drugs as tools to interrogate various neurotransmitter systems and their roles in neuropsychiatric diseases (4–6). Thus, for example, early studies on psychedelic drugs postulated that drugs such as N,N-dimethyltryptamine (DMT) (7) and lysergic acid diethylamide (LSD) (6, 8) might induce a model psychosis in humans and laboratory animals. More recently, psilocybin—the active ingredient of “magic mushrooms” (Psilocybe spp.)—has been shown in several phase 2 clinical trials to robustly and rapidly alleviate depressive symptoms (9–13). Initial clinical studies with LSD have shown a similar rapid action for symptoms of anxiety (14) in terminal cancer patients. In this review, we summarize our current understanding of psychedelic drug actions and how such insights might inform further research and the potential clinical utility of psychedelic drugs.
What Is a Psychedelic Drug?
The term psychedelic was coined by Osmond in 1957 (15) to refer to drugs that are “mind manifesting.” Prior to this, psychoactive drugs such as hallucinogens and dissociative agents (among others) were considered to be psychotomimetic (16), a term that is in common use today. Osmond defined psychotomimetic compounds as follows:
Psychotomimetic agents are substances that produce changes in thought, perception, mood and, sometimes, in posture, occurring alone or in concert, without causing either major disturbances of the autonomic nervous system or addictive craving, and although, with overdosage, disorientation, memory disturbance, stupor, and even narcosis may occur, these reactions are not characteristic (15, p. 418).
Of the various so-called psychotomimetic drugs, Osmond included such compounds as the classic psychedelics (e.g., LSD, psilocybin) as well as the hallucinogens ibogaine (from the iboga plant), mescaline (from the cactus Lophophora williamsii), DMT (from several plant species, and used in hallucinogenic snuff), and atropine (from the mushroom Amanita muscaria) (15). To this list are added synthetic compounds such as the dissociative anesthetic agent ketamine (17), the hallucinogenic kappa opioid receptor agonist salvinorin A (from the plant Salvia divinorum) (2), and others (Figure 1).
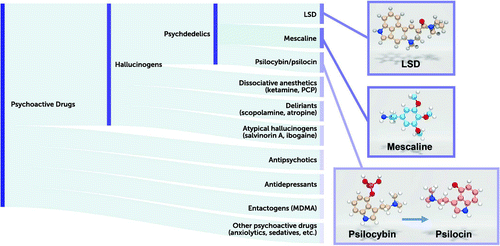
FIGURE 1. Diagram showing the hierarchy of psychoactive molecules and their classificationsa
aThe list is arranged from the most inclusive (top) to the least inclusive (bottom). For example, mescaline, LSD, and psilocybin/psilocin are psychedelics, hallucinogens, and psychoactive compounds, whereas the dissociative anesthetics are not considered psychedelics, but rather hallucinogens and psychoactive compounds, and the entactogen MDMA is not considered a hallucinogen or psychedelic, but a psychoactive compound. Also shown are the chemical structures for the classic psychedelics LSD, mescaline (typically found in the peyote cactus), and psilocybin (found in “magic mushrooms”); the latter is converted to the active compound psilocin after ingestion (indicated by the arrow).
Our current classification of these psychoactive drugs would include hallucinogens as a distinct class (Figure 1), with many different types of hallucinogens based primarily on their pharmacology. Thus, psychedelic drugs are defined as drugs that have an LSD-like action in humans and are 5-HT2A agonists (3, 18). This definition is similar to that offered by the U.S. Food and Drug Administration: “serotonergic 5-HT2A agonists that alter perception, cognition, and mood (i.e., psychedelic effects) and that are currently controlled in Schedule I of the Controlled Substances Act” (19). The European regulatory authorities have provided a similar definition for classic psychedelic drugs as 5-HT2A agonists, exemplified by drugs such as LSD, mescaline, and psilocybin (20). By contrast, drugs such as ketamine and phencyclidine—which can also induce hallucinations along with dissociative states—are classified as dissociative anesthetic agents and are NMDA receptor antagonists. Muscarinic antagonists, such as scopolamine and atropine—which induce delirium and hallucinations in humans—are classified as deliriants. Finally, atypical hallucinogens that have kappa opioid receptor agonist activity (e.g., salvinorin A [2]) constitute another class. MDMA (3,4-methylenedioxymethamphetamine; “Ecstasy”) is not considered a psychedelic drug (as it does not induce an LSD-like effect in humans) and has been classified separately as an entactogen (21).
Psychedelic Drugs Mediate Their Actions via Serotonin 5-HT2A Receptors
There is now considerable evidence that a subclass of serotonin receptors, the 5-HT2A subtype, is essential for the hallucinogenic actions of psychedelics. Initial evidence came from animal studies that indicated that 5-HT2A antagonists block the actions of psychedelics (22) and that their in vivo effects are directly correlated with their affinities for 5-HT2A receptors (23). Subsequent studies in which the 5-HT2A receptor was genetically deleted showed that the effects of psychedelic drugs were blocked (24, 25). The most definitive evidence comes from human studies in which the psychedelic actions of both psilocybin (26) and LSD (27) were blocked by pretreatment with the 5-HT2A-preferring antagonist ketanserin.
Serotonin 5-HT2A receptors are expressed mainly in layer 4 and 5 cortical pyramidal neurons, with sparse expression in parvalbumin-expressing interneurons (28, 29) (Figure 2A). In pyramidal neurons, 5-HT2A receptors are concentrated in apical dendrites (28, 29), complexed with various scaffolding proteins, including PSD-95 and others (30). These interactions with scaffolding proteins are essential for the acute effects of psychedelic drugs in vivo (30, 31). Intriguingly, many atypical antipsychotic drugs are potent 5-HT2A antagonists (32), and these same scaffolding proteins are also essential for the actions of clozapine-like atypical antipsychotic drugs in vivo (30, 31).
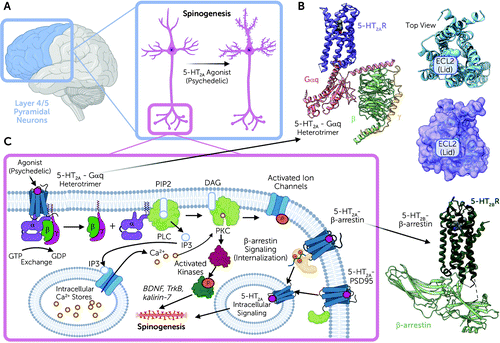
FIGURE 2. Overall schematic showing the protein structure of and the location, downstream signaling, and scaffolding protein for the 5-HT2A receptora
aPanel A highlights the cortex and the layer 4/5 pyramidal neurons, which are where the 5-HT2A receptor is primarily located. Panel B presents cartoon (pictured as the alpha-helical and beta-sheet ribbons) and surface representations (pictured as the space-filling model) of the 5-HT2A/B receptors coupled with various signaling transducers obtained by cryoelectron microscopy. The 5-HT2A-Gq heterotrimer complex (top left: 5-HT2A receptor pictured in cornflower blue, Gαq in salmon, Gβ in green, and Gγ in yellow) is bound with 25CN-NBOH (shown as gray spheres in orthosteric site) (rendered from the PDB accession code: 6WHA). Highlighted in the top right and center panels (shown in both cartoon [top right] and surface [center] representations) is the ECL2 lid that forms over the top of the orthosteric site. This lid forms over the top of LSD and occludes its exit, which is responsible for the long-lasting effects. Finally, the bottom right panel shows the 5-HT2B receptor coupled to β-arrestin bound with LSD (shown as gray spheres) in cartoon representation (PDB accession: 7SRS). The 5-HT2B receptor is shown in dark green, and β-arrestin is shown in a lighter green. Panel C illustrates downstream signal transduction cascade of the canonical Gαq pathway and potential therapeutic mechanism. Also indicated are the interactions with β-arrestin, the scaffolding protein PSD-95, and 5-HT2A-mediated intracellular signaling.
In terms of the potential therapeutic actions of psychedelic drugs, the data are mixed regarding the necessity of 5-HT2A receptor activation. One study in mice showed that ketanserin does not block the potential therapeutic actions of psilocybin (33), while another showed complete blockade with ketanserin (34). The latter study also showed lack of antidepressant-like actions in mice in which 5-HT2A receptors are blocked. Such studies using ketanserin are problematic, however, as this agent also interacts with alpha-1 adrenergic receptors (35) and with vesicular monoamine transporters sensitive to reserpine (36). In future studies, the use of more selective 5-HT2A antagonists—for example, pimavanserin, which is approved for treating Parkinson’s psychosis (37, 38) and is the most selective approved 5-HT2A antagonist (37)—could be used to definitively address this question.
We now have molecular-level details regarding how psychedelic drugs interact with and activate 5-HT2A receptors (39) (Figure 2B). Studies on a related serotonin receptor (5-HT2B) have clarified how LSD can stabilize distinct signaling complexes (40, 41). A key finding of these studies was the discovery that once LSD binds to the 5-HT2A receptor, a lid is formed over the binding pocket, which “traps” LSD for several hours (39, 40) (Figure 2B). These findings imply that at least part of the reason for the long duration of action of drugs like LSD is the trapping of the receptor via conformational changes that occur after drug binding. These studies also showed that this prolonged action of LSD is due in part to a specific residue within the binding pocket, which is found in humans but not in mice or rats (39). This residue (Ser242) also is essential for the high-affinity interactions of LSD, psilocybin, and perhaps other such drugs at the human and nonhuman primate 5-HT2A receptors.
After psychedelic drug binding, 5-HT2A receptors then activate a complex web of signaling processes mediated by interactions of the 5-HT2A receptor with various transducer molecules, including both G proteins and arrestins (42) (Figure 2C). The main G protein activated is Gαq, which leads to the activation of phospholipase C (43, 44) and mobilization of intracellular calcium, leading ultimately to enhanced neurotransmission at cortical pyramidal neurons (45). In addition to G protein activation, 5-HT2A receptors also induce arrestin interactions (39, 41, 46). Interactions with both G proteins and arrestins appear to be essential for the full expression of psychedelic drug–induced behaviors in mice (47, 48). Finally, there is evidence that psychedelic drugs may also induce changes in brain gene transcription (49, 50), although it is unclear whether these effects are central to their putative therapeutic actions.
Psychedelic drugs also rapidly induce enduring changes in spine formation and dendritic arborization in layer 5 cortical pyramidal neurons (51–53). These findings are especially intriguing given the observations that 5-HT2A receptors are enriched in layer 5 cortical neuronal synapses and dendritic arborizations at the microscopic (54) and ultrastructural levels (55). The molecular details regarding the mechanisms by which 5-HT2A receptor activation induces enhanced plasticity are unclear, although pathways involving BDNF (56), TrkB (52), and kalirin-7 signaling (51) (Figure 2B) have been implicated. These findings take on added significance given the long-standing findings that antidepressant drugs enhance spine formation (57–59). Additionally, since antidepressant drug–induced spine formation appears to be central to the therapeutic actions of antidepressants (60, 61), these same pathways could be involved in the antidepressant drug–like actions of psychedelics.
In a recent study by the Olson lab (62) examining the role of psychedelics in inducing spine formation, the authors provide data consistent with the hypothesis that intracellular 5-HT2A receptors are essential for the plasticity-inducing actions of psychedelics (62). These findings are intriguing, as previous studies have shown that 5-HT2A receptors in the brain are found in the dendroplasmic reticulum, where they interact with MAP1A (55). Many anatomical studies have demonstrated a close association between intracellular 5-HT2A receptors and various transducers and effectors, including arrestins (63), RSK2 (64), and many others (65). Further research on the role of intracellular 5-HT2A receptors in mediating the actions of psychedelics is warranted.
Problematic Off-Target Actions of Psychedelics
In addition to their actions at 5-HT2A receptors, most psychedelic drugs have complex polypharmacological interactions with many other receptors in the brain (25, 66, 67). Thus, for instance, LSD is a high-affinity agonist for nearly every serotonin, dopamine, and noradrenergic receptor (67). In fact, LSD has been found to be a high-potency dopamine receptor agonist (8, 68), with significant activity at both D1 and D2 family receptors (67). DMT has a similarly robust agonist profile at several 5-HT receptors (25), and it has been reported that its interactions with sigma-1 receptors may be involved in at least some of its actions in vivo (69), although not its psychedelic effects (25). Finally, psilocin (the active metabolite of psilocybin) has also been found to be a high-affinity agonist for most 5-HT receptors, including 5-HT2C and 5-HT2B (70). In fact, many psychedelic drugs, as well as MDMA, activate 5-HT2C receptors (71–76). Given that many 5-HT2C agonists are anorectic (77), the appetite-suppressant actions of some psychedelics could be related to this effect. What effects, if any, these off-target actions of psychedelic drugs have for their therapeutic actions is unknown.
Most problematic has been the activation of 5-HT2B receptors by nearly all psychedelic drugs and the entactogen MDMA (72, 78). For many years it has been known that drugs with potent 5-HT2B agonist activity induce valvular heart disease in humans (79)—for instance, fenfluramine, which was withdrawn from the market due to drug-induced valvular heart disease in as many as 30% of individuals (80). This was subsequently demonstrated to be due to activation of the 5-HT2B receptor by norfenfluramine, the major metabolite of fenfluramine (81–83). Subsequently, chronic treatment with several ergot derivatives used in treating Parkinson’s disease (84, 85) as well as MDMA (86) were associated with clinically significant valvular heart disease. Finally, ergot derivatives used for treating migraine headache have also been associated with valvular heart disease (87). For drugs like ergotamine, this is due to the main metabolite, methylergonovine, which is a potent 5-HT2B agonist (81, 88, 89).
No studies have yet directly addressed the concern that chronic administration of psychedelic drugs—as might occur with “microdosing”—might induce clinically significant valvular heart disease. Such studies would likely require large numbers of subjects, assessed in a prospective fashion, as was done for the drug lorcaserin (90, 91). Until such time as definitive studies are performed, we must caution against the long-term use of psychedelic drugs and MDMA.
Psychedelic and Psychedelic-Inspired Medications
Since the 1950s and 1960s, there has been evidence that psychedelic drugs might be useful for treating a variety of neuropsychiatric diseases (6, 92), although this area of study was not without controversy (93, 94). Over the decades since, there have been scattered and anecdotal reports of beneficial actions of psychedelics in obsessive-compulsive disorder (70, 95), migraine (96), cluster headaches (97, 98), and other conditions (3).
More recently, phase 2 placebo-controlled trials have reported significant actions of both single and two doses of psilocybin to rapidly reduce symptoms of depression and anxiety (9, 10, 12, 13). A similar phase 2 placebo-controlled trial showed similar significant effects of LSD on depression and anxiety (14). All these trials employed psychotherapy as part of the treatment regimen, and it is unknown to what extent therapist interventions might be key to the potential therapeutic actions of psychedelics (for recent perspectives, see references 3, 18).
Many approved antidepressant medications—including virtually all the tricyclic drugs (99) as well as newer medications such as mirtazapine (100) and brexpiprazole as adjunctive therapy (101)—are potent 5-HT2A antagonists. Related to this, it has long been known that chronic treatment with many antidepressant medications induces a downregulation of 5-HT2A receptors (102–105). Intriguingly, both atypical antipsychotic drugs and psychedelics also can induce rapid downregulation of 5-HT2A receptors (106–108). Collectively, these findings suggest that nonpsychedelic 5-HT2A-active medications can function as therapeutic drugs for a variety of neuropsychiatric conditions (Gumpper and Roth, in press).
Given this background, recent studies have addressed the hypothesis as to whether it is possible to identify 5-HT2A agonists that are not psychedelic and are potentially therapeutic (3). To date, three groups have identified new drug-like molecules that—in mice—are devoid of psychedelic drug–like actions and have antidepressant-like actions (109–111). In all these instances, the new 5-HT2A agonists displayed minimal activity at typical mouse models of psychedelic drug actions (e.g., 110). Simultaneously, these drug-like molecules displayed robust antidepressant drug–like actions in a variety of rodent models (109–111). Although none of these molecules has advanced to clinical trials, these results support the hypothesis, and it will be informative to determine their effects in humans with depression and related disorders.
Conclusions
With the continued widespread use of psychedelic compounds in the clinic, pinpointing the gaps in our current knowledge is paramount. Numerous areas remain to be explored, including 1) furthering our understanding of the downstream signaling mechanisms and how they differ between hallucinogenic and nonhallucinogenic psychedelic compounds; 2) clarifying differences in extracellular and intracellular signaling cascades; 3) thoroughly understanding the molecular interactions between the receptor and psychedelic compounds and how they contribute to downstream signaling events; 4) elucidating the polypharmacology of these compounds to look for potentially harmful off-target side effects as well as other therapeutic mechanisms; 5) identifying the therapeutic mechanisms that are induced by these compounds and whether these are dependent on 5-HT2A activation; and, finally, 6) answering the question of whether nonhallucinogenic 5-HT2A agonists can retain, or exceed, the potential therapeutic properties of the classic psychedelics.
Given the last point, several new compounds have been discovered that may offer some therapeutic potential (109–111) as well as several compounds that have been known for some time (97, 98, 112, 113). While many of them have proven to be successful in rodent models, it will be of great interest to see whether these molecules will be as effective as the emerging psychedelic therapeutics in treating depression and other related disorders in clinical trials.
1. : A prehistoric mural in Spain depicting neurotropic Psilocybe mushrooms. Econ Bot 2011; 65:121–128 Crossref, Google Scholar
2. : A potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 2002; 99:11934–11939Crossref, Medline, Google Scholar
3. : The promises and perils of psychedelic pharmacology for psychiatry. Nat Rev Drug Discov 2022; 21:463–473Crossref, Medline, Google Scholar
4. : Drug-induced psychoses and schizophrenic reactions: a critical comparison. Ann N Y Acad Sci 1962; 96:80–92Crossref, Medline, Google Scholar
5. : Drugs which antagonize 5-hydroxytryptamine. Br J Pharmacol Chemother 1954; 9:240–248Crossref, Medline, Google Scholar
6. : A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci U S A 1954; 40:228–231Crossref, Medline, Google Scholar
7. : The psychedelic model of schizophrenia: the case of N,N-dimethyltryptamine. Am J Psychiatry 1976; 133:203–208Link, Google Scholar
8. : Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology 2005; 180:427–435Crossref, Medline, Google Scholar
9. : Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 2016; 30:1181–1197Crossref, Medline, Google Scholar
10. : Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016; 30:1165–1180Crossref, Medline, Google Scholar
11. : Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 2021; 78:481–489Crossref, Medline, Google Scholar
12. : Trial of psilocybin versus escitalopram for depression. N Engl J Med 2021; 384:1402–1411Crossref, Medline, Google Scholar
13. : Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med 2022; 387:1637–1648Crossref, Medline, Google Scholar
14. : Lysergic acid diethylamide-assisted therapy in patients with anxiety with and without a life-threatening illness: a randomized, double-blind, placebo-controlled phase II study. Biol Psychiatry 2023; 93:215–223Crossref, Medline, Google Scholar
15. : A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci 1957; 66:418–434Crossref, Medline, Google Scholar
16. : Book review: “Neuropharmacology: Transactions of the Second Conference”. Am J Psychiatry 1959; 116:88 Link, Google Scholar
17. : Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther 1965; 6:279–291Crossref, Medline, Google Scholar
18. : The neural basis of psychedelic action. Nat Neurosci 2022; 25:1407–1419Crossref, Medline, Google Scholar
19. : Considerations in assessing the abuse potential of psychedelics during drug development. Neuropharmacology 2023; 224:109352Crossref, Medline, Google Scholar
20. : The therapeutic potential of psychedelics: the European regulatory perspective. Lancet 2023; 401:714–716Crossref, Medline, Google Scholar
21. : Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens: identification of a new therapeutic class: entactogens. J Psychoactive Drugs 1986; 18:305–313Crossref, Medline, Google Scholar
22. : Antagonism of the effects of the hallucinogen DOM, and the purported 5-HT agonist quipazine, by 5-HT2 antagonists. Eur J Pharmacol 1983; 91:189–196Crossref, Medline, Google Scholar
23. : Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 1984; 35:2505–2511Crossref, Medline, Google Scholar
24. : Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007; 53:439–452Crossref, Medline, Google Scholar
25. : Predicting new molecular targets for known drugs. Nature 2009; 462:175–181Crossref, Medline, Google Scholar
26. : Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998; 9:3897–3902Crossref, Medline, Google Scholar
27. : Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife 2018; 7:e35082Crossref, Medline, Google Scholar
28. : Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 1997; 27:79–82Crossref, Medline, Google Scholar
29. : 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A 1998; 95:735–740Crossref, Medline, Google Scholar
30. : PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci 2009; 29:7124–7136Crossref, Medline, Google Scholar
31. : Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psychiatry 2011; 1:e33Crossref, Medline, Google Scholar
32. : Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2, and serotonin2 pKi values. J Pharmacol Exp Ther 1989; 251:238–246Medline, Google Scholar
33. : Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A 2021;118:e2022489118Crossref, Medline, Google Scholar
34. : 5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS Chem Neurosci 2023; 14:351–358Crossref, Medline, Google Scholar
35. : [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites: binding properties, brain distribution, and functional role. Mol Pharmacol 1982; 21:301–314Medline, Google Scholar
36. : Characterization of two [3H]-ketanserin recognition sites in rat striatum. J Neurochem 1987; 49:1833–1838Crossref, Medline, Google Scholar
37. : Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother 2008; 9:3251–3259Crossref, Medline, Google Scholar
38. : Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014; 383:533–540Crossref, Medline, Google Scholar
39. : Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell 2020;182:1574–1588.e19Crossref, Medline, Google Scholar
40. : Crystal structure of an LSD-bound human serotonin receptor. Cell 2017; 168:377–389.e12Crossref, Medline, Google Scholar
41. : Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 2022; 110:3154–3167.e7Crossref, Medline, Google Scholar
42. : SnapShot: psychedelics and serotonin receptor signaling. Cell 2023; 186:232–232.e1Crossref, Medline, Google Scholar
43. : Selective 5HT-2 antagonists inhibit serotonin-stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology 1984; 23:993–996Crossref, Medline, Google Scholar
44. : Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology 1984; 23:1223–1225Crossref, Medline, Google Scholar
45. : Psychedelic compounds directly excite 5-HT2A layer 5 pyramidal neurons in the prefrontal cortex through a 5-HT2A Gq-mediated activation mechanism. bioRxiv, November 15, 2022 Google Scholar
46. : The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT(2A) receptor induces agonist-independent internalization. Mol Pharmacol 2003; 63:961–972Crossref, Medline, Google Scholar
47. : Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology 2007; 52:1671–1677Crossref, Medline, Google Scholar
48. : LSD-stimulated behaviors in mice require beta-arrestin 2 but not beta-arrestin 1. Sci Rep 2021; 11:17690Crossref, Medline, Google Scholar
49. : A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 2002; 26:634–642Crossref, Medline, Google Scholar
50. : Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res 2003; 111:182–188Crossref, Medline, Google Scholar
51. : Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A 2009; 106:19575–19580Crossref, Medline, Google Scholar
52. : Psychedelics promote structural and functional neural plasticity. Cell Rep 2018; 23:3170–3182Crossref, Medline, Google Scholar
53. : Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021; 109:2535–2544.e4Crossref, Medline, Google Scholar
54. : Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 2003; 116:107–117Crossref, Medline, Google Scholar
55. : Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience 2002; 113:23–35Crossref, Medline, Google Scholar
56. : 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 1997; 17:2785–2795Crossref, Medline, Google Scholar
57. : Changes in dendritic spine morphology in response to increased availability of monoamines in rat medial prefrontal cortex. Synapse 1991; 9:235–237Crossref, Medline, Google Scholar
58. : Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse 2001; 42:151–163Crossref, Medline, Google Scholar
59. : Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012; 62:35–41Crossref, Medline, Google Scholar
60. : Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex. eNeuro 2016; 3:ENEURO.0133-15.2016Crossref, Google Scholar
61. : Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019; 364:eaat8078Crossref, Medline, Google Scholar
62. : Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023; 379:700–706Crossref, Medline, Google Scholar
63. : Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J Neurochem 1999; 72:2206–2214Crossref, Medline, Google Scholar
64. : p90 Ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A 2006; 103:4717–4722Crossref, Medline, Google Scholar
65. : Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology 2008; 55:961–968Crossref, Medline, Google Scholar
66. : Psychedelics and the human receptorome. PLoS One 2010; 5:e9019Crossref, Medline, Google Scholar
67. : PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 2015; 22:362–369Crossref, Medline, Google Scholar
68. : LSD as an agonist at mesolimbic dopamine receptors. Psychopharmacologia 1975; 45:221–224Crossref, Medline, Google Scholar
69. : The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009; 323:934–937Crossref, Medline, Google Scholar
70. : SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg Med Chem Lett 2005; 15:4555–4559Crossref, Medline, Google Scholar
71. : (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5HT1C receptor agonist. J Pharmacol Exp Ther 1991; 258:891–896Medline, Google Scholar
72. : Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett 1994; 177:111–115Crossref, Medline, Google Scholar
73. : 1-(2,5-dimethoxy-4-(trifluoromethyl)phenyl)-2-aminopropane: a potent serotonin 5-HT2A/2C agonist. J Med Chem 1994; 37:4346–4351Crossref, Medline, Google Scholar
74. : Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse 2000; 35:144–150Crossref, Medline, Google Scholar
75. : Agonist-directed signaling of serotonin 5-HT2C receptors: differences between serotonin and lysergic acid diethylamide (LSD). Neuropsychopharmacology 1999;21:77S-81SMedline, Google Scholar
76. : Molecular insights into the regulation of constitutive activity by RNA editing of 5HT2C serotonin receptors. Cell Rep 2022; 40:111211Crossref, Medline, Google Scholar
77. : Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest 2013; 123:4986–4991Crossref, Medline, Google Scholar
78. : 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 2003; 63:1223–1229Crossref, Medline, Google Scholar
79. : Drugs and valvular heart disease. N Engl J Med 2007; 356:6–9Crossref, Medline, Google Scholar
80. : Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997; 337:581–588Crossref, Medline, Google Scholar
81. : Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000; 102:2836–2841Crossref, Medline, Google Scholar
82. : Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 2000; 57:75–81Medline, Google Scholar
83. : Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol Pharmacol 2005; 68:20–33Crossref, Medline, Google Scholar
84. : Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 2007; 356:29–38Crossref, Medline, Google Scholar
85. : Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 2007; 356:39–46Crossref, Medline, Google Scholar
86. : Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol 2007; 100:1442–1445Crossref, Medline, Google Scholar
87. : Mitral and aortic valve disease associated with ergotamine therapy for migraine: report of two cases and review of literature. Arch Pathol Lab Med 1990; 114:62–64Medline, Google Scholar
88. : 4IB4: crystal structure of the chimeric protein of 5-HT2B-BRIL in complex with ergotamine. Science 2013; 340:615–619 Crossref, Medline, Google Scholar
89. : Structural determinants of 5-HT2B receptor activation and biased agonism. Nat Struct Mol Biol 2018; 25:787–796Crossref, Medline, Google Scholar
90. : Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009; 17:494–503Crossref, Medline, Google Scholar
91. : A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011; 96:3067–3077Crossref, Medline, Google Scholar
92. : Use of d-lysergic acid diethylamide in the treatment of alcoholism. Q J Stud Alcohol 1959; 20:577–590Crossref, Medline, Google Scholar
93. : A clinical study of LSD treatment in alcoholism. Am J Psychiatry 1969; 126:59–69Link, Google Scholar
94. : Harmful aspects of the LSD experience. J Nerv Ment Dis 1967; 145:464–474Crossref, Medline, Google Scholar
95. : Hallucinogens and obsessive-compulsive disorder. Am J Psychiatry 1999; 156:1123Link, Google Scholar
96. : Lysergic acid diethylamide (LSD-25). 38. Comparison with action of methysergide and psilocybin on test subjects. J Asthma Res 1965; 3:81–96Crossref, Medline, Google Scholar
97. : Response of cluster headache to psilocybin and LSD. Neurology 2006; 66:1920–1922Crossref, Medline, Google Scholar
98. : Indoleamine hallucinogens in cluster headache: results of the Clusterbusters Medication Use Survey. J Psychoactive Drugs 2015; 47:372–381Crossref, Medline, Google Scholar
99. : Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2C receptor. Psychopharmacology 1996; 126:234–240Crossref, Medline, Google Scholar
100. : Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of alpha2-adrenergic and serotonin2C receptors: a comparison with citalopram. Eur J Neurosci 2000; 12:1079–1095Crossref, Medline, Google Scholar
101. : A positron emission tomography occupancy study of brexpiprazole at dopamine D(2) and D(3) and serotonin 5-HT(1A) and 5-HT(2A) receptors, and serotonin reuptake transporters in subjects with schizophrenia. Neuropsychopharmacology 2020; 45:786–792Crossref, Medline, Google Scholar
102. : Differential effects of electroconvulsive shock and antidepressant drugs on serotonin-2 receptors in rat brain. Eur J Pharmacol 1981; 69:515–518Crossref, Medline, Google Scholar
103. : Regulation of serotonin2 (5-HT2) receptors labeled with [3H]spiroperidol by chronic treatment with the antidepressant amitriptyline. J Pharmacol Exp Ther 1980; 215:582–587Medline, Google Scholar
104. : Two distinct central serotonin receptors with different physiological functions. Science 1981; 212:827–829Crossref, Medline, Google Scholar
105. : Different synaptic location of mianserin and imipramine binding sites. Science 1982; 215:1112–1115Crossref, Medline, Google Scholar
106. : Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci 1988; 42:2439–2445Crossref, Medline, Google Scholar
107. : Differential effect of subchronic treatment with various neuroleptic agents on serotonin2 receptors in rat cerebral cortex. J Neurochem 1986; 46:191–197Crossref, Medline, Google Scholar
108. : Antagonist functional selectivity: 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J Pharmacol Exp Ther 2011; 339:99–105Crossref, Medline, Google Scholar
109. : A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021; 589:474–479Crossref, Medline, Google Scholar
110. : Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022; 375:403–411Crossref, Medline, Google Scholar
111. : Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 2022; 610:582–591Crossref, Medline, Google Scholar
112. : Pharmacological mechanism of the non-hallucinogenic 5-HT(2A) agonist ariadne and analogs. ACS Chem Neurosci 2023; 14:119–135Crossref, Medline, Google Scholar
113. : The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series. Cephalalgia 2010; 30:1140–1144Crossref, Medline, Google Scholar



