Maternal Psychological Resilience During Pregnancy and Newborn Telomere Length: A Prospective Study
Abstract
Objective:
In the context of the importance of elucidating the determinants of the initial, newborn setting of telomere length (TL), it is increasingly evident that maternal stress and stress-related processes during pregnancy play a major role. Although psychological resilience may function as a buffer, research in this area has not yet examined its potential role vis-à-vis that of stress. The authors examined the relationship between maternal psychological resilience during pregnancy and newborn TL.
Methods:
In a sample of 656 mother-child dyads from the Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction cohort, multiple serial assessments were conducted over the course of pregnancy to quantify maternal stress, negative and positive emotional responses to pregnancy events, positive affect, and perceived social support. Principal component analysis identified two latent factors: stress and positivity. A measure of resilience was computed by regressing the positivity factor on the stress factor, in order to quantify positivity after accounting for stress. TL was measured using quantitative polymerase chain reaction in leukocytes extracted from cord blood shortly after birth. Linear regression was used to predict newborn TL from maternal resilience during pregnancy, adjusting for other potential determinants.
Results:
Maternal stress significantly predicted shorter newborn TL (β=−0.079), and positivity significantly predicted longer TL (β=0.135). Maternal resilience (positivity accounting for stress) was significantly and positively associated with newborn TL (β=0.114, 95% CI=0.035, 0.189), with each standard deviation increase in resilience predicting 12% longer newborn TL.
Conclusions:
The results indicate that maternal psychological resilience may exert a salubrious effect on offspring telomere biology and highlight the importance of enhancing maternal mental health and well-being during pregnancy.
The critical importance of telomere biology for health, disease risk, and longevity is well established. The telomere system, consisting of telomeres, repeated double strands of DNA that serve to protect chromosomal ends during replication, and telomerase, the enzyme capable of elongating telomeres, is one of the key regulatory systems of cellular aging and senescence (1). Shortened telomere length (TL) is not only a biomarker of but appears to play a causal role in a wide range of physical and psychological disorders and mortality risk (2).
The initial, newborn setting of the telomere biology system has important implications for the trajectory of cellular aging and long-term health and susceptibility to common age-related disorders (3). We and others have reported that this initial setting of the system exhibits developmental plasticity and that the effects of suboptimal developmental conditions appear to be mediated in part by the programming actions of maternal-placental-fetal biological processes (4–6).
Among the suboptimal developmental conditions that have been associated with newborn TL, maternal stress and stress-related biological processes feature prominently. Maternal psychological stress during pregnancy (and even prior to becoming pregnant) has been linked to offspring TL at the time of birth, during childhood, and in adulthood (7–12). To date, the focus has been almost exclusively on negative exposures and risk factors (summarized in reference 13), despite the growing recognition of the independent impact of positive emotions (over and beyond that of the mere absence of negative emotions) on biological substrates that underlie health and disease risk. Thus, although positive emotions and psychological resilience have been shown to exert a protective effect on health and disease risk, and in the specific context of pregnancy, maternal positive affect (14) and social support (15) have been positively associated with obstetric and infant outcomes, fetal programming research on telomere biology has not yet addressed the important question of the potential salubrious effects of maternal positivity and resilience. To our knowledge, this is the first study of the potential programming effects of maternal resilience and positive psychological state in pregnancy on the initial (newborn) setting of offspring TL.
Theories of psychological resilience emphasize the importance of positive traits, state, or affect that can buffer the impact of stress. Induction of positive emotions or positivity can lead individuals to recover more quickly from the negative physiological sequelae of stress (16), reduce the allostatic wear and tear of repeated or prolonged exposure to stress, turn on positive restorative mechanisms, and perhaps even prevent or attenuate telomere shortening (17). Positive states of mind have furthermore been theorized to be a driving force in our development of key resilience resources, including rewarding social relationships (16), which have been shown to provide psychological and neurobiological resilience to stress (18).
A relatively small number of studies have examined the effects of positive affect on adult TL. Positivity and optimism have been associated with TL in some (19–21) but not other (22, 23) studies. Of note, these effects on TL were evident even after adjustment for depression (19), posttraumatic stress disorder symptoms, and traumatic life events (20), suggesting that positivity and resilience represent constructs that are not merely the absence of negative psychological states. Social support has also been associated with TL in adults in multiple studies, with greater levels of perceived social support predicting longer telomeres (24–27), including during pregnancy (28), although one study has found the opposite association (29). However, as stated above, the question of the role of positive psychological states in telomere biology, also understudied in adults as compared with negative states, has yet to be addressed in the context of fetal programming of the telomere system.
This is also the first study in the Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO) cohort to examine potential programming effects of maternal psychology on telomere biology. Previous studies in this cohort have linked maternal psychological state during pregnancy to placental functioning (30), birth outcomes (31), and child outcomes (32, 33). Each of these outcomes has been linked in other studies (but not in this cohort) to newborn TL, supporting the scientific premise underlying our hypotheses. Especially relevant is the finding in this cohort that maternal positive affect during pregnancy was inversely associated with risk of preterm birth (31). Our study builds on this foundation to explore the ways in which a mother’s positive emotional state may influence the development of her offspring’s telomere system during intrauterine development.
Our goal in this study was to examine a hypothesized positive relationship between maternal psychological resilience during pregnancy and newborn TL. We conceptualized resilience as the extent to which an individual is able to maintain positivity and satisfying social relationships in the face of stress. Our study is a secondary analysis conducted using data from a large prospective mother-child cohort in which serial measures of maternal psychological state were collected across pregnancy and DNA was isolated from cord blood to assess newborn TL.
Methods
Participants and Procedure
The study population consisted of pregnant women enrolled between 2006 and 2010 in the PREDO cohort at 10 hospitals in Finland and their live-born singleton children (for the study protocol, see reference 34). Of the 4,777 mother-child dyads recruited to the study, TL data were available for 688 newborns. Participants were enrolled at antenatal clinics early in gestation (12 weeks to 13 weeks 6 days) and followed up extensively through pregnancy and beyond. The PREDO cohort was enriched for women with at least one risk factor for preeclampsia (clinical sample, N=602). The cohort also included women recruited from the community irrespective of obstetric risk status (community sample, N=54). Data from both samples were combined and analyzed together, as the two groups did not differ significantly in newborn TL, stress, positivity, or resilience factor scores. The study was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District and by participating hospitals, and written informed consent was obtained from all participants (34).
Sociodemographic, health, and lifestyle data were collected at baseline. As there was no variation in race in this sample (this was an all-white sample representative of the Finnish population), we did not adjust for race. Psychological questionnaires were administered throughout pregnancy, and newborn cord blood samples were obtained at birth. Questionnaires relating to resilience (positivity, social support, and stress) are described in detail below, and the timeline of their administration is presented in Table 1.
| Week of Pregnancy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Pregnancy | Mid Pregnancy | Late Pregnancy | ||||||||||||
| Measure | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | 32 | 34 | 36 | 38 |
| PANAS | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| STAI, positive state | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| VAS for social support | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Pregnancy Experience Scale | ||||||||||||||
| Frequency of uplifts | x | x | x | x | ||||||||||
| Intensity of uplifts | x | x | x | x | ||||||||||
| Frequency of hassles | x | x | x | x | ||||||||||
| Intensity of hassles | x | x | x | x | ||||||||||
| Perceived Stress Scale | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| VAS for stress | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
TABLE 1. Timeline of psychological and psychosocial measures collected from the PREDO cohorta
The final sample for the present analysis included 656 mother-child pairs for whom psychosocial measurements during pregnancy and newborn cord blood TL were available.
Psychological Measures
We quantified positive affectivity using three scales: affect from the Positive and Negative Affect Schedule (PANAS) (35), positive state from the State-Trait Anxiety Inventory (STAI) (36), and positive mood reactivity to pregnancy-related events (“uplifts”) from the Pregnancy Experience Scale (PES) (37). Social support satisfaction was determined using a visual analogue scale (VAS) for social support. Similarly, we quantified negative affectivity using three scales: perceived stress from the Perceived Stress Scale (PSS) (38), biweekly perceived stress from a visual analogue scale for stress, and negative mood reactivity to pregnancy-related events (“hassles”) from the PES. The entire course of pregnancy was well represented, with the affect and social support measures being repeated up to 14 times, and the hassles and uplift measures up to four times, across early, mid, and late gestation.
For each assessment, a within-subject mean was calculated for each participant who completed at least 50% of the questionnaire in question. A summed score across pregnancy was then computed as a product of the within-subject mean and number of assessments completed by the participant. The average completion rate across all waves for all the questionnaires used in the principal component analysis was approximately 90%. In both the principal component analysis and linear regression analysis, incomplete cases were deleted listwise.
Principal component analysis was used to isolate two latent factors, positivity and stress, as described below, in the Statistical Analysis section. The questionnaires used to yield the factors are detailed below.
A positivity factor was created from positive emotion/affect-related items from the following questionnaires and a social support scale:
Positive affect from the positive scale of the PANAS: Participants rated a list of 10 positive emotions (e.g., “interested,” “excited”) on a 5-point Likert scale according to how strongly they felt the emotion in the moment (state affect). Responses were summed to create positive affect scores.
Positive state sum scores from the STAI: The STAI comprises 10 positive items (e.g., “I feel pleasant,” “I feel secure”), rated on a 4-point scale and summed. The state version of the instrument, which asks about feelings in the moment, was administered at each visit.
Pregnancy-related uplifts from the PES: The PES consists of 41 pregnancy-related items (e.g., “how much the baby is moving,” “discussions with spouse about pregnancy/childbirth issues”), which respondents rate on two 4-point Likert scales. One scale asks them to what degree the item was uplifting, the other to what degree it was experienced as a hassle (described below). Responses to the question “How much has this made you feel happy, positive, or uplifted?” were used to determine frequency and intensity of uplifts. Frequency of uplifts was determined by totaling the number of items on the positive scale that were rated above 0 (“not at all”). Intensity of uplifts was calculated by summing the responses scored from 1–3 and dividing the result by the frequency.
Social support satisfaction over the past 2 weeks, from the visual analogue scale for social support: Participants were asked to rate how much support they felt they had received from loved ones over the past 2 weeks by marking the level along a 65-mm horizontal scale from “no support at all” to “a great degree of support.” The responses were scored by measuring the distance in millimeters from the starting point to the line drawn.
A stress factor was computed from the following scales:
1. Perceived stress in the past month from the PSS: The PSS-4 includes four stress items rated on a 5-point scale from “never” to “very often.” Two items were reverse scored, then all responses were summed.
2. Pregnancy-related hassles from the PES, using the responses to the question “How much has this made you feel unhappy, negative, or upset?” scored on a 4-point Likert scale. Frequency and intensity of hassles were computed as described above.
3. Perceived stress over the past 2 weeks, from the visual analogue scale for stress: Participants were asked to rate their overall stress level over the past 2 weeks by marking how much stress they felt along a 65-mm horizontal scale from “no stress at all” to “very high levels of stress.” The distance in millimeters from the starting point to this line was measured and served as a score. The same scale was administered four times, with each repetition focusing on a different aspect of stress: work or studying, close interpersonal relationships, taking care of children/household duties, and pregnancy-related stress. The scores on the subscales were combined to create a total stress score.
To control for the potential effect of personality on the experience of positive emotions and stress, in a subsequent analysis we further adjusted the resilience regression for trait neuroticism, measured at 12 weeks’ gestation using the Finnish version of the NEO Personality Inventory (NEO-PI) (39).
Obstetric Risk Conditions and Birth Outcomes
Obstetric risk conditions, including chronic hypertension, gestational hypertension, preeclampsia, and gestational and type 1 diabetes, were obtained from medical records and the Finnish Medical Birth Register (FMBR). For women in the high obstetric risk group, diagnoses were confirmed by an expert jury (34). We created dummy variables to indicate presence of chronic/gestational hypertension, preeclampsia, and diabetic disorders.
Body mass index (BMI), maternal age, parity, and smoking status were extracted from the FMBR. Birth outcomes were also obtained from the FMBR, including child sex, birth weight, and gestational age at birth (34).
Telomere Length
TL was analyzed in leukocytes from cord blood samples collected at birth. DNA was isolated from whole blood. Leukocyte TL is the most commonly used measure of TL in human epidemiological studies, and it has been postulated that TL dynamics in leukocytes mirror those of the entire hematopoietic stem cell population (40), the original pool of which is formed early in gestation and serves as the progenitor for cells in all blood lineages (41).
Relative TL was measured by quantitative polymerase chain reaction (qPCR), expressed as the ratio of telomere to single-copy gene abundance (T/S ratio), as previously described (42). The telomere qPCR primers were tel1b (5′-CGGTTT[GTTTGG]5GTT-3′), used at a final concentration of 100 nM, and tel2b (5′-GGCTTG[CCTTAC]5CCT-3′), used at a final concentration of 900 nM. The single-copy gene (human beta-globin) qPCR primers were hbg1 (5′-GCTTCTGACACAACTGTGTTCACTAGC-3′), used at a final concentration of 300 nM, and hbg2 (5′-CACCAACTTCATCCACGTTCACC-3′), used at a final concentration of 700 nM. The final reaction mix consisted of the following: 20 mM Tris-hydrochloride, pH 8.4; 50 mM potassium chloride; 200 μM each deoxyribonucleotide triphosphate; 1% dimethyl sulfoxide; 0.4×SYBR green I; 22 ng Escherichia coli DNA; 0.4 units of platinum Taq DNA polymerase (Invitrogen, Carlsbad, Calif.); and approximately 6.6 ng of genomic DNA per 11 µL reaction. A threefold serial dilution of a commercial human genomic DNA containing 26, 8.75, 2.9, 0.97, 0.324, and 0.108 ng of DNA was included in each PCR run as the reference standard. The quantity of targeted templates in each sample was determined relative to the reference DNA sample by the maximum second derivative method in the Roche LC480 program. The reaction was carried out in a Roche LightCycler 480 in 384-well plates, with triplicate wells for each sample. The Dixon Q test was used to exclude outliers from the triplicates. The average of the T and S triplicate wells after outlier removal was used to calculate the T/S ratio for each sample. The same reference DNA was used for all PCR runs.
We applied a telomere (T) thermal profile consisting of denaturing at 96°C for 1 minute followed by 30 cycles of denaturing at 96°C for 1 second and annealing or extension at 54°C for 60 seconds with fluorescence data collection and a single copy gene (S) thermal profile consisting of denaturing at 96°C for 1 minute followed by eight cycles of denaturing at 95°C for 15 seconds, annealing at 58°C for 1 second, and extension at 72°C for 20 seconds, followed by 35 cycles of denaturing at 96°C for 1 second, annealing at 58°C for 1 second, extension at 72°C for 20 seconds, and holding at 83°C for 5 seconds with data collection. The T/S ratio for each sample was measured in duplicate runs, each with triplicate wells. When the duplicate T/S values disagreed by more than 7%, the sample was run in triplicate and the two closest values were used.
Eight control genomic DNA samples were included to calculate a normalizing factor for each run. In each batch, the T/S ratio of each control DNA was divided by the average T/S ratio for the same DNA from 10 runs to generate a normalizing factor that was then used to correct the participant DNA samples to generate the final T/S ratio. Assays were performed with the same lot of reagent throughout the whole experiment. All samples that were included for TL measurements passed quality control criteria of an optical density 260 nm/280 nm ratio between 1.7 and 2.0. The majority of samples had a DNA concentration of 30 ng/dL, with a few exceptions at concentrations of 20 ng/mL (1% of samples). The average interassay coefficient of variation for this study was 2.3%.
DNA was extracted from 344 samples via the NucleoSpin system, 293 via the phenol-chloroform method, and 19 via the automated Gentra method. Although it is possible that DNA extraction method can lead to systematic differences in measured TL (43), it has been shown that the rank order of TL in a population is not affected by the DNA extraction method (43). We therefore created standardized (z) scores of the TL measurements and furthermore adjusted the model for DNA extraction method. The observation that DNA extraction method was a significant predictor of TL in one of our subgroup analyses (specifically, the subgroup with resilience and neuroticism data) highlights the importance of accounting for the effect of this factor.
Statistical Analysis
Analyses were conducted using SPSS 25.0 for Windows (IBM, Armonk, N.Y.).
Pregnancy sum scores.
To obtain a measure of cumulative levels of maternal stress, positivity, and resilience, scores from each questionnaire were averaged across pregnancy. The intercorrelation between the scores at different time points supports using an average (correlations for all instruments between 0.328 and 0.732). The average score of the PES intensity of hassles scale was transformed to achieve a normal distribution (natural log plus one transformation).
Principal component analysis of the positive and negative affectivity measures.
We created two composite variables, positivity and stress, from the various questionnaires described above. The scores of the individual measures that went into each composite were weighted using principal component analysis. Principal component analysis allows the number of covariates to be reduced by transforming intercorrelated variables into a new set of uncorrelated principal components encompassing the variation of the original variables, allowing models to be simplified while retaining maximum variance and predictive value. This strategy was particularly suitable for our data, as multiple questionnaires measured similar constructs, yielding correlated scores. Principal component analysis was deemed preferable to principal axis factoring, as no a priori hypothesis was made regarding the number of underlying factors (44). Incomplete cases were deleted listwise so that only women who had completed more than half of each component questionnaire at least at one time point were included.
A positivity factor was computed using the PANAS and STAI positive subscales, the frequency and intensity of PES uplifts, and the visual analogue scale for social support. Factorability of these scales was supported on several grounds. All scores were significantly correlated (at least r=0.27, p<0.001), and Bartlett’s test of sphericity was significant (χ2=1130.67, df=10, p<0.001), indicating covariance between the scales. Furthermore, the diagonals of the anti-image correlation matrix were all greater than 0.658 (above the accepted cutoff of 0.50), and Kaiser-Meyer-Olkin sampling adequacy was above the established threshold of 0.50, at 0.709 (44). One component had an eigenvalue of 2.71, above the standard Kaiser criterion cutoff of 1, and explained 54.01% of the total variation. A scree plot revealed a sharp drop in predictive value and subsequent leveling off for further components, supporting the recognition of a single factor (44). Because only one component was extracted, a rotated factor matrix could not be produced. We identified this component as the positivity factor.
A stress factor was similarly constructed from PES hassle frequency, hassle intensity, PSS, and visual analogue scale stress scores using principal component analysis. Interrelatedness of the variables was established by correlation (at least r=0.45, p<0.001) and Bartlett’s test of sphericity (χ2=837.49, df=6, p<0.001). Sample adequacy was confirmed by a Kaiser-Meyer-Olkin statistic of 0.76 and anti-image correlation matrix diagonals of above 0.71. A single component was extracted with an eigenvalue of 2.50 and visually confirmed by scree plot, indicating that including further components would not increase the predictive value of the model (44). This component explained 62.51% of the total variance in the data and was considered the stress factor.
Principal component analysis factors were centered with a mean of zero and a standard deviation of one. The positivity factor had a range of −3.34 to 2.68. The stress factor ranged from −2.75 to 3.49. The stress and positivity factors were significantly inversely correlated (r2=−0.514, p<0.001).
Resilience factor.
To create a resilience factor, we regressed the positivity factor against the stress factor. This approach quantifies for each subject the variation in positivity that is not accounted for by the variation in stress. Thus, at a given level of stress, individuals who exhibit higher positivity have more resilience. The resilience factor was also zero-centered with a standard deviation of one, and had a minimum value of −4.18 and a maximum of 2.66.
Regression models.
Linear regression models were developed to predict newborn TL from maternal resilience, positivity, and stress, with adjustment for the effects of other potential determinants of newborn TL. Cases were deleted listwise so that only women with pregnancy sum scores for each of the psychological measures composing the factors and complete covariate data were included.
Repeated-measures analyses of variance revealed no significant within-subject effects for the resilience (F=0.036, df=2, 1088, p=0.850), positivity (F=0.170, df=2, 1128, p=0.681), or stress (F=0.058, df=2, 1090, p=0.810) factors. Given this lack of interindividual variation across time, we performed analyses using the average scores of these factors across pregnancy.
Covariates were specified a priori based on previously published determinants of newborn telomere biology and included child sex, gestational age at birth, birth weight, maternal age at childbirth, maternal prepregnancy BMI, maternal educational attainment (classified as primary, secondary, lower tertiary, or upper tertiary and scored from 1 to 4), obstetric risk conditions (hypertension, preeclampsia, and diabetes), and maternal smoking status during pregnancy. Parity was also included as a covariate because of its expected influence on maternal emotional state during pregnancy.
Results
Data on newborn TL and maternal prenatal resilience were available for 656 mother-child dyads. The sociodemographic and clinical characteristics of the sample are summarized in Table 2. Newborn T/S ratio ranged from 1.58 to 3.35, with a mean of 2.39 (SD=0.24). Scores on the individual questionnaires (before they were collapsed into positivity and stress factors) are presented in Table 3.
| Maternal, Obstetric, and Child Factors | ||
|---|---|---|
| Maternal factors | ||
| Mean | SD | |
| Age at childbirth (years) | 33.24 | 5.48 |
| Prepregnancy BMI | 26.97 | 6.31 |
| N | % | |
| Educational attainment | ||
| Primary | 27 | 4.1 |
| Secondary | 269 | 41.0 |
| Lower tertiary | 152 | 23.1 |
| Upper tertiary | 208 | 31.8 |
| Parity | ||
| 0 | 205 | 31.3 |
| 1 | 293 | 44.7 |
| 2 | 117 | 17.8 |
| 3 | 27 | 4.1 |
| ≥4 | 14 | 2.2 |
| Obstetric factors | ||
| Hypertension | ||
| Chronic hypertension | 101 | 15.4 |
| Gestational hypertension | 57 | 8.7 |
| Preeclampsia | 47 | 7.2 |
| Diabetes | ||
| Type 1 diabetes | 8 | 1.2 |
| Gestational diabetes | 131 | 20.0 |
| Smoking during pregnancy | ||
| No smoking | 626 | 95.4 |
| Any smoking | 30 | 4.6 |
| Child factors | ||
| Sex | ||
| Male | 341 | 52.0 |
| Female | 315 | 48.0 |
| Mean | SD | |
| Gestational age at birth (weeks) | 39.73 | 1.71 |
| Birth weight (grams) | 3,541.20 | 569.44 |
TABLE 2. Sociodemographic and clinical characteristics of participants in a study of maternal psychological resilience during pregnancy and newborn telomere length
| Measure | Mean | SD | Range | Maximum Score |
|---|---|---|---|---|
| PANAS | 29.98 | 7.43 | 38.29 | 50 |
| STAI, positive state | 30.43 | 5.24 | 25.29 | 40 |
| VAS for social support | 42.80 | 11.82 | 58.36 | 65 |
| Pregnancy Experience Scale | ||||
| Frequency of uplifts | 27.89 | 7.38 | 40.50 | 41 |
| Intensity of uplifts | 1.85 | 0.39 | 2.60 | 3 |
| Frequency of hassles | 16.35 | 6.79 | 36.70 | 41 |
| Intensity of hassles | 1.39 | 0.28 | 1.80 | 3 |
| Perceived Stress Scale | 5.33 | 2.55 | 15.76 | 20 |
| VAS for stress | 75.50 | 36.73 | 208.71 | 260 |
| NEO-PI, trait neuroticism | 71.71 | 23.05 | 142.00 | 192 |
TABLE 3. Psychological questionnaire data prior to collapse into factors by principal component analysis, in a study of maternal psychological resilience during pregnancy and newborn telomere lengtha
Consistent with findings from previous studies, maternal stress during pregnancy was significantly and inversely associated with newborn TL (β=−0.079, p=0.044, 95% CI=−0.155, −0.002, R2=0.044, F=2.272, df=13, 642, p=0.006). A one standard deviation change in maternal stress was associated with a 4% difference in average newborn TL. Among the covariates included in the model, sex of the child (TL was longer in girls: β=0.099, p=0.013, 95% CI=0.022, 0.177) and maternal age at childbirth (β=0.095, p=0.024, 95% CI=0.011, 0.179) were also significant predictors of TL at birth.
Maternal positivity during pregnancy was significantly and positively associated with newborn TL (β=0.135, p=0.001, 95% CI=0.059, 0.211, R2=0.055, F=2.786, df=13, 642, p<0.001). Each one standard deviation change in maternal positivity was associated with a 13% difference in average newborn TL.
Lastly (and most importantly), maternal resilience during pregnancy was significantly and positively associated with newborn TL (model R2=0.050, F=2.500, df=13, 642, p=0.002; β (resilience)=0.112, p=0.004, 95% CI=0.035, 0.189) (Figure 1). Each one standard deviation change in maternal resilience was associated with a 12% difference in newborn TL. As in previous models, sex of the child (girls had longer telomeres: β=0.093, p=0.020, 95% CI=0.015, 0.171) and maternal age at childbirth (β=0.099, p=0.019, 95% CI=0.017, 0.182) remained significant predictors of TL. These findings persisted when our model was further adjusted for maternal trait neuroticism (β=0.148, p=0.001, 95% CI=0.060, 0.235, R2=0.080, F=2.979, df=14, 481, p<0.001).
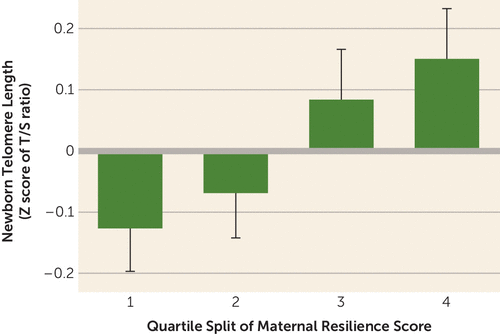
FIGURE 1. Association between maternal resilience factor during pregnancy (in quartiles) and newborn telomere lengtha
a Newborn telomere length is reported as the Z score of the ratio of telomere to single-copy gene abundance (T/S ratio). Resilience was conceptualized as positivity in the face of stress. Factor score is centered at zero. Error bars indicate standard error.
Detailed results of the resilience regression are presented in Table 4. Results of the other regressions (stress, positivity, and resilience with adjustment for neuroticism) are provided in the online supplement.
| Variable | B | SE | 95% CI | β | t | p |
|---|---|---|---|---|---|---|
| Intercept | –1.327 | 1.063 | –3.415, 0.761 | –1.248 | 0.212 | |
| Resilience factor | 0.111 | 0.039 | 0.035, 0.187 | 0.112 | 2.875 | 0.004 |
| DNA extraction method | 0.091 | 0.070 | –0.047, 0.228 | 0.050 | 1.290 | 0.197 |
| Sex of the child | 0.184 | 0.079 | 0.030, 0.339 | 0.093 | 2.341 | 0.020 |
| Gestational age at birth | 0.025 | 0.029 | –0.033, 0.083 | 0.043 | 0.849 | 0.396 |
| Child birth weight | –9.032E–5 | 0.000 | 0.000, 0.000 | –0.052 | –1.022 | 0.307 |
| Maternal age at childbirth | 0.018 | 0.008 | 0.003, 0.033 | 0.099 | 2.360 | 0.019 |
| Parity | –0.014 | 0.043 | –0.097, 0.070 | –0.013 | –0.320 | 0.749 |
| Maternal prepregnancy BMI | –0.012 | 0.007 | –0.025, 0.001 | –0.075 | –1.749 | 0.081 |
| Maternal education level | –0.046 | 0.046 | –0.136, 0.043 | –0.043 | –1.017 | 0.310 |
| Hypertension in pregnancy | 0.151 | 0.093 | –0.032, 0.334 | 0.065 | 1.625 | 0.105 |
| Preeclampsia | 0.217 | 0.156 | –0.090, 0.524 | 0.056 | 1.388 | 0.166 |
| Diabetes in pregnancy | –0.032 | 0.100 | –0.228, 0.164 | –0.013 | –0.319 | 0.750 |
| Smoking in pregnancy | –0.052 | 0.186 | –0.418, 0.314 | –0.011 | –0.278 | 0.781 |
TABLE 4. Linear regression predicting newborn telomere length from maternal resilience during pregnancy adjusted for maternal and child determinants of telomere length
Several subsequent sensitivity analyses were performed on the resilience regression. To examine a potential ceiling or floor in the effect of maternal prenatal resilience on newborn TL, we stratified the regression according to stress factor tertile. We found a stronger effect of resilience among mother-child dyads in the highest tertile of stress (β=0.159, p=0.018) than in the lower tertiles (lowest tertile: β=0.085, p=0.893; middle tertile: β=0.086, p=0.225).
To further investigate the robustness of this relationship, models were run excluding all women with obstetric conditions (hypertensive conditions, preeclampsia, and diabetes). This reduced the sample size from 656 to 366. Although no longer statistically significant, associations between maternal psychological factors and newborn TL length continued to be observed in the expected directions (maternal resilience was positively associated with newborn TL [β=0.072]; maternal positivity was positively associated with newborn TL [β=0.087]; and maternal stress was inversely associated with newborn TL [β=−0.049]).
We found no interaction between child sex and resilience (β=0.139, p=0.263), positivity (β=0.151, p=0.233), or stress (β=−0.076, p=0.535) on child TL.
Discussion
The principal and novel finding of our study is that in a comparably large cohort of mother-child dyads assessed serially over the course of early, mid, and late pregnancy, maternal psychological resilience, conceptualized and operationalized as maternal positive affect and social support satisfaction adjusted for maternal stress during pregnancy, was prospectively associated with newborn TL. To our knowledge, this is the first time that maternal resilience and positivity have been studied in the context of prenatal programming, and this study therefore adds a new perspective to this field of research. The magnitude of the effect of resilience was considerable, with each standard deviation increase in maternal resilience being associated with a 12% difference (increase) in newborn TL. Our findings also replicate previous reports linking maternal stress during pregnancy with offspring TL. We were further able to demonstrate that resilience evinced a stronger association with newborn TL among those women in our sample with the highest levels of stress, which indicates that the benefits of maternal positive emotions may be especially pronounced among the most stressed individuals.
In this study, the impact of maternal resilience on newborn TL was greater than that of maternal stress alone. Given our conceptualization and operationalization of resilience as a multidimensional measure that incorporates positive affect and perceived social support as well as stress, our finding suggests that effects of positivity are not merely the opposite or inverse of those of stress, as well as demonstrating that these positive states can even exert transgenerational effects. Positive emotion, independently of and after accounting for stress, significantly influenced aspects of fetal development that regulate the initial, newborn setting of TL. We also note that although we observed the expected inverse relationship between positivity and stress, this relationship accounted for only about 25% of their shared variance. This suggests that there is considerable between-subject variability in the level of positivity at the same or an equivalent level of stress, thereby supporting the importance of assessing both positive and negative affect and responsivity in the context of development and health.
There are several plausible mechanisms by which maternal resilience could influence newborn TL, broadly by promoting telomere elongation and/or by protecting against telomere attrition. Resilience, positivity, and social support have been shown to have an impact on biological pathways involved in the neuroendocrine stress response (46), which in turn has well-documented effects on telomere biology. Several measures of positive psychological functioning and social support (47) have been associated with reduced or healthier patterns of cortisol output, including among pregnant women (48). Conversely, excessive hypothalamic-pituitary-adrenal (HPA) axis activation and cortisol release have been implicated in telomere shortening (49). Cortisol also has been shown to reduce telomerase activity in vitro (50); thus, lower cortisol levels as a consequence of greater resilience may support the maintenance or even elongation of telomeres by promoting telomerase activity.
Resilience also may exert a protective effect on TL via the immune system. Resilient individuals and those who experience satisfying social support seem to exhibit immune profiles that contrast with those of chronically stressed individuals (who, for their part, are at a higher likelihood of developing unfavorable health outcomes) (46, 51). Positive psychological states and social support have been associated with lower levels of cytokines and inflammatory markers (51, 52) and a reduced risk of infection (53), which, in the context of prenatal development, may result in the embryo/fetus being exposed to a lower inflammatory load and consequently less telomere erosion (54).
Resilience, positivity, and social support are known to diminish basal and stress-related autonomic arousal and lead to a more rapid and complete recovery from stress, and may even directly preserve or promote restorative physiology (16, 55). Improved vagal tone is associated with the experience of positive emotions (56) and social support (51) and in turn may be linked to greater telomerase activity (57). Higher levels of estrogen, which may be protective against negative mood (58), have also been shown to predict longer TL (59). Clearly, additional research is warranted to better identify and characterize the pathways by which maternal positivity and resilience promote fetal development and influence the initial setting of the telomere biology system.
In addition to the above-discussed strength in our conceptualization and operationalization of maternal resilience, other strengths of our study are the comparably large sample size and the breadth and depth of prenatal psychological data, some of which were collected as often as every 2 weeks throughout the course of pregnancy. Some of the limitations of this study include the nonavailability of maternal or paternal TL and paternal age. One of the study findings was an independent and positive association between maternal age and newborn TL. Paternal age is a well-established predictor of newborn TL (60), and this may account for the observed association between maternal age and newborn TL, as older mothers are more likely to have older partners. It is also important that these findings be replicated in a cohort with clinically relevant levels of stress to better understand the robustness of the protective effects of resilience and positivity. Based in part on our finding that the magnitude of the association between maternal resilience and newborn TL was greater among women experiencing higher levels of stress than among those with medium or lower levels of stress, we suggest that the effect size observed in our study is likely a conservative estimate of what might be expected in a population with higher levels of stress.
Another important limitation is the lack of data in this cohort on negative life events, including baseline (prior to occurrence) and subsequent (following occurrence) psychological state and functioning. Such data facilitate examination of the temporal elements of the development of resilience (61). However, the focus of our study was on ascertainment of the transgenerational (mother to child) effects of maternal resilience in pregnancy, and not on how the mothers came to develop psychological resilience. Moreover, other theories of resilience, such as the broaden-and-build hypothesis, specifically emphasize the role of positive emotions in creating resilience, from the immediate biological effects of a positive state of mind to the coping, social, and lifestyle resources that such emotions confer on individuals (62). In this context, we submit that the experience of positive emotions in everyday life after accounting for stress is a reflection of both an individual’s current resilience and of their future capacity to react to adverse circumstances. Given the growing evidence of the direct role that positivity plays in combating stress and building resilience, we believe that there is much insight to be gained from studying resilience even when temporal data on negative life events are not available.
Potential biological mediators of stress and resilience, such as cortisol or proinflammatory cytokines, were also not measured. Future research should seek to identify specific pathways by which maternal resilience may act on the developing fetal telomere biology system.
Our study contributes new insight into the role of maternal prenatal psychological resilience in the initial setting of the offspring telomere system, with potential lifelong consequences for the offspring’s health, disease risk, and aging process. This beneficial effect of resilience underscores the importance of attending to mothers’ mental as well as physical health during pregnancy to optimize the health of both mother and child.
1 : Switching and signaling at the telomere. Cell 2001; 106:661–673Crossref, Medline, Google Scholar
2 : Telomere length and all-cause mortality: a meta-analysis. Ageing Res Rev 2018; 48:11–20Crossref, Medline, Google Scholar
3 : Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013; 12:615–621Crossref, Medline, Google Scholar
4 : The developmental origins of chronic adult disease. Acta Paediatr Supplement 2004; 93:26–33Crossref, Medline, Google Scholar
5 : The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci 2018; 373:20170151Crossref, Medline, Google Scholar
6 : Telomere biology in healthy aging and disease. Pflugers Arch 2010; 459:259–268Crossref, Medline, Google Scholar
7 : Adverse childhood experiences: implications for offspring telomere length and psychopathology. Am J Psychiatry 2020; 177:47–57Link, Google Scholar
8 : Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 2013; 208:134.e1–134.e7Crossref, Google Scholar
9 : Prenatal stress and newborn telomere length. Am J Obstet Gynecol 2016; 215:94.e1–94.e8Crossref, Google Scholar
10 : Association between maternal-perceived psychological stress and fetal telomere length. South Med J 2016; 109:767–772Crossref, Medline, Google Scholar
11 : Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology 2017; 42:2407–2413Crossref, Medline, Google Scholar
12 : Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci USA 2011; 108:E513–E518Crossref, Medline, Google Scholar
13 : Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci Signal 2012; 5:pt12Crossref, Medline, Google Scholar
14 : Maternal positive affect over the course of pregnancy is associated with the length of gestation and reduced risk of preterm delivery. J Psychosom Res 2013; 75:336–340Crossref, Medline, Google Scholar
15 : Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosom Med 2000; 62:715–725Crossref, Medline, Google Scholar
16 : The undoing effect of positive emotions. Motiv Emot 2000; 24:237–258Crossref, Medline, Google Scholar
17 : Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci 2009; 1172:34–53Crossref, Medline, Google Scholar
18 : Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont) 2007; 4:35–40Medline, Google Scholar
19 : Multisystem resiliency moderates the major depression-telomere length association: findings from the Heart and Soul Study. Brain Behav Immun 2013; 33:65–73Crossref, Medline, Google Scholar
20 : Posttraumatic stress disorder symptoms, temperament, and the pathway to cellular senescence. J Trauma Stress 2018; 31:676–686Crossref, Medline, Google Scholar
21 : The relationship between positive psychological characteristics and longer telomeres. Psychol Health 2016; 31:1466–1480Crossref, Medline, Google Scholar
22 : Telomere length and mental well-being in elderly men from the Netherlands and Greece. Behav Genet 2012; 42:278–286Crossref, Medline, Google Scholar
23 : Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun 2009; 23:446–449Crossref, Medline, Google Scholar
24 : Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis. Psychosom Med 2013; 75:171–177Crossref, Medline, Google Scholar
25 : Social support sources matter: increased cellular aging among adults with unsupportive spouses. Biol Psychol 2016; 115:43–49Crossref, Medline, Google Scholar
26 : Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci USA 2014; 111:4519–4524Crossref, Medline, Google Scholar
27 : Perceived social support, loneliness, and later life telomere length following wartime captivity. Health Psychol 2018; 37:1067–1076Crossref, Medline, Google Scholar
28 : Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology 2018; 87:43–52Crossref, Medline, Google Scholar
29 : Social relationships and salivary telomere length among middle-aged and older African American and white adults. J Gerontol B Psychol Sci Soc Sci 2019; 74:1053–1061Crossref, Medline, Google Scholar
30 : Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychol Med 2015; 45:2023–2030Crossref, Medline, Google Scholar
31 : Maternal prenatal positive affect, depressive and anxiety symptoms, and birth outcomes: the PREDO study. PLoS One 2016; 11:e0150058Crossref, Medline, Google Scholar
32 : Maternal depressive symptoms during and after pregnancy and psychiatric problems in children. J Am Acad Child Adolesc Psychiatry 2017; 56:30–39.e7Crossref, Medline, Google Scholar
33 : Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS One 2017; 12:e0177506Crossref, Medline, Google Scholar
34 : Cohort profile: Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO) study. Int J Epidemiol 2017; 46:1380–1381gMedline, Google Scholar
35 : Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063–1070Crossref, Medline, Google Scholar
36 : STAI Manual. Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
37 : Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol 2004; 25:189–201Crossref, Medline, Google Scholar
38 : Perceived stress in a probability sample of the United States, in The Claremont Symposium on Applied Social Psychology: The Social Psychology of Health. Edited by Spacapan S, Oskamp S. Newbury Park, Calif, Sage Publications, 1988, pp 31–67Google Scholar
39 : A Big Five personality inventory in two non-Indo-European languages. Eur J Pers 1995; 9:109–124Crossref, Google Scholar
40 : Synchrony of telomere length among hematopoietic cells. Exp Hematol 2010; 38:854–859Crossref, Medline, Google Scholar
41 : The journey of developing hematopoietic stem cells. Development 2006; 133:3733–3744Crossref, Medline, Google Scholar
42 : Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30:e47Crossref, Medline, Google Scholar
43 : Method specific calibration corrects for DNA extraction method effects on relative telomere length measurements by quantitative PCR. PLoS One 2016; 11:e0164046Crossref, Medline, Google Scholar
44 : Principal component analysis: a review and recent developments. Philos Trans- Royal Soc, Math Phys Eng Sci 2016; 374:20150202Crossref, Medline, Google Scholar
45 : Reliability and validity of the 4-item perceived stress scale among pregnant women: results from the OTIS antidepressants study. Res Nurs Health 2012; 35:363–375Crossref, Medline, Google Scholar
46 : In the search for integrative biomarker of resilience to psychological stress. Neurosci Biobehav Rev 2017; 74(Pt B):310–320Crossref, Medline, Google Scholar
47 : Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 2003; 54:1389–1398Crossref, Medline, Google Scholar
48 : Positive life events predict salivary cortisol in pregnant women. Psychoneuroendocrinology 2012; 37:1336–1340Crossref, Medline, Google Scholar
49 : Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav 2012; 106:40–45Crossref, Medline, Google Scholar
50 : Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 2008; 22:600–605Crossref, Medline, Google Scholar
51 : Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med 2006; 29:377–387Crossref, Medline, Google Scholar
52 : Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol 2008; 167:96–102Crossref, Medline, Google Scholar
53 : Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza a virus. Psychosom Med 2006; 68:809–815Crossref, Medline, Google Scholar
54 : Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008; 44:235–246Crossref, Medline, Google Scholar
55 : Is benefit finding good for your health? Pathways linking positive life changes after stress and physical health outcomes. Curr Dir Psychol Sci 2009; 18:337–341Crossref, Google Scholar
56 : How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci 2013; 24:1123–1132Crossref, Medline, Google Scholar
57 : Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 2006; 31:277–287Crossref, Medline, Google Scholar
58 : Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015; 59:14–24Crossref, Medline, Google Scholar
59 : Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res 2011; 1379:224–231Crossref, Medline, Google Scholar
60 : The paternal age at conception effect on offspring telomere length: mechanistic, comparative, and adaptive perspectives. Philos Trans R Soc Lond B Biol Sci 2018; 373:373Crossref, Google Scholar
61 : The temporal elements of psychological resilience: an integrative framework for the study of individuals, families, and communities. Psychol Inq 2015; 26:139–169Crossref, Google Scholar
62 : The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. Am Psychol 2001; 56:218–226Crossref, Medline, Google Scholar



