Mindful Pharmacogenetics: Drug Dosing for Mental Health
Pharmacogenetics may be said to have paved the way to personalized medicine. Genotype-adjusted dosing recommendations for psychopharmacological treatment are decades old (1). These recommendations provide a practical tool for individualizing drug therapy in everyday clinical practice. Leading the way through the jungle of clinically relevant effects arising from pharmacogenetic polymorphisms, evidence-based guidelines summarize evidence from an ever-growing research and clinical data set and show how to combine genetic information with therapy planning (2).
The article by Jukić et al. in this issue (3) adds to this body of evidence by reporting pharmacogenetic data on escitalopram drug exposure. The authors’ data are also relevant to citalopram dosage adjustments—escitalopram being the S-enantiomer of citalopram, which is the most commonly used antidepressant drug in clinical practice today. However, besides the much-needed evidence on pharmacogenetics and drug exposure in large patient samples, the article also highlights the role of pharmacogenetic diagnostics to preemptively avoid treatment failures. The study findings add fuel to the never-ending discussion on the primacy of therapeutic drug monitoring (TDM) or pharmacogenetics in personalized psychopharmacological treatment (4).
In their analyses, the authors present data from 2,087 patients undergoing TDM while in treatment with escitalopram. Different endpoints were considered to estimate the effect of the CYP2C19 polymorphism in the genotype groups Null/Null, *1/Null, *17/Null, *1/*1, *1/*17, and *17/*17, with *17 being a gain-of-function allele. The first endpoint was escitalopram exposure, which is the typical outcome of TDM in clinical follow-up. Here, the expected exposure (adjusted serum concentration) increase was observed with decreasing CYP2C19 enzyme activity, as predicted by the genotype groups. Since pharmacogenetic dosage recommendations are mostly calculated from data on pharmacokinetic parameters like drug exposure or oral clearance (deduced from dosage-adjusted trough levels or area under the curve), this analysis provides powerful evidence for the quantitative effect of the CYP2C19 genotype on escitalopram dosage adjustment. While poor metabolizers (Null/Null) may need no more than half the normal dosage, ultrarapid metabolizers may need up to one and a half times the normal dosage to achieve adequate serum concentrations of escitalopram (see Table 2 in the Jukić et al. article).
The data from this TDM cohort of patients in escitalopram treatment, the largest available to date, may be used for the next pharmacogenetics dosing guideline on escitalopram, such as the evidence-based Clinical Pharmacogenetics Implementation Consortium guidelines for pharmacogenetics-based dosing of the other SSRIs (for example, see reference 5).
Up to this point, the article could well serve as an argument for “TDM first,” with pharmacogenetics adding an explanation but no new information for clinicians. However, the much more interesting finding concerns the authors’ second endpoint: switches in antidepressant therapy due to therapeutic failure. Here, switches from escitalopram as first-line therapy to another antidepressant within 1 year after TDM were considered as failures, presumably due either to insufficient clinical response or to adverse events. Indeed, switches during first-line antidepressant drug therapy usually indicate nonresponse to therapy or are a consequence of intolerable side effects (6). If side effects or nonresponse could be predicted in the individual patient, measures could be taken to reduce the number of switches. Although in general cohorts, the proportion of nonresponse is about 30% (7), the analysis of this TDM cohort reported switching rates of 10%−15%. However, the rate for poor metabolizers (Null/Null) and ultrarapid metabolizers of CYP2C19 (*17/*17) was almost triple that of patients carrying the wild-type alleles (*1/*1) (30% compared with 12%).
These findings suggest that there may be additional value in genotyping patients, even when followed up with TDM. The fraction of patients who switched from escitalopram to another antidepressant increased on both sides of the genotype spectrum. Even in patients undergoing TDM, there was still a high risk of treatment failure and antidepressant switch in the Null/Null and *17/*17 genotype groups, resulting in a U-shaped risk curve (see Figure 1B in the Jukić et al. article). This indicates that while TDM provided information on the risk of therapy failure associated with low and insufficient serum concentrations, genotype groups were potential predictors of residual higher risk. This was the case not only in the ultrarapid metabolizers but also in the poor metabolizers, who have high to very high drug exposure. As the study authors note, the TDM-adjusted plasma concentrations may have different effects in different patients, as shown by the therapy outcomes in the genotype groups.
The question arises as to why the data suggest that residual genotype-based adjustment was possible even under TDM. One possibility is that information from monitoring led to underadjustment of escitalopram dosages (8). Another, not necessarily incompatible, possibility is related to the constitutive role of drug-metabolizing enzymes in transforming endogenous substrates and transforming drugs locally (9) or in transforming endogenous substrates (10). While not expressed in the adult brain, CYP2C19 presents functionally relevant expression levels during neurodevelopment. As shown in previous work by this group, poor CYP2C19 metabolizer genotypes are associated with a mitigated anxious-depressed phenotype and increased hippocampal volume in neuroimaging studies (11, 12).
As illustrated in Figure 1, the dual role of drug-metabolizing enzymes in the brain may lead to interfering effects ensuing from drug exposure on the one hand and from their constitutive role on the other. The constitutive role of CYP2C19 during neurodevelopment may have implications for individual susceptibility affecting a drug’s benefit-risk profile. This adds new insights to drug therapy during pregnancy, where high citalopram exposure can be expected in the infant, according to findings from amniotic fluid and umbilical cord blood analyses (14). Therapy outcome may depend not only on the individual drug exposure as measured by TDM but also on the patient’s vulnerability. This may include vulnerability to mental disorders, deterioration of disease symptoms, or susceptibility to expression of certain symptom profiles (such as anxiety or disease-associated impulse control failure leading to suicidality in depression). For example, higher suicidality has been reported not only in CYP2C19 ultrarapid metabolizers but also in CYP2D6 ultrarapid metabolizers (12), even when no drug therapy was known.
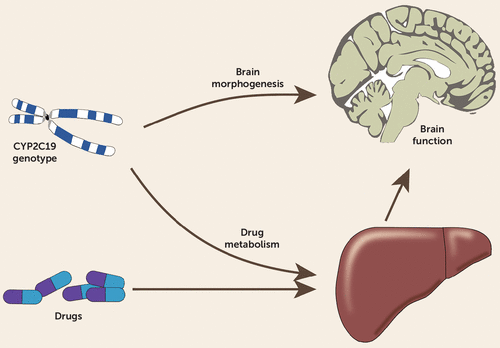
FIGURE 1. Diagram of the Dual Role in Brain Morphogenesis and Drug Metabolism of Drug-Metabolizing Enzymes in the Braina
a Figure adapted from Stingl and Viviani (13), with permission; copyright 2014, Association for the Publication of the Journal of Internal Medicine.
The findings of the Jukić et al. study suggest that, far from being either/or alternatives, TDM and genotyping may usefully complement each other. The plausibility of this conclusion is strengthened by considering the different type of information they convey. Preemptive genotyping provides information on a stable functional status of the enzymes in question that remains unaltered for life. In contrast, TDM captures contingent factors determining variability in drug concentration. In contrast to genotyping, however, TDM can be performed only after the treatment is initiated and is more appropriate as a supplement after initiating treatment if genotype-guided dosing turns out to be unsuccessful. Thus, pharmacogenetic diagnostics may be integrated into a holistic concept of genome medicine, where therapy of mental disorders should consider the dual role of some drug-metabolizing enzymes, whose polymorphism contains information on aspects directly relevant to therapy and dosing as well as on patient vulnerability profiles. This may lead to a deeper understanding of personalized drug therapy and to a mindful application of pharmacogenetics in psychiatry.
1 : CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001; 104:173–192Crossref, Medline, Google Scholar
2 : CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther 2011; 89:464–467Crossref, Medline, Google Scholar
3 : Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. Am J Psychiatry 2018; 175:463–470 Link, Google Scholar
4 : Consensus Guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 2018; 51(1-02):9–62 Medline, Google Scholar
5 : Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther 2015; 98:127–134Crossref, Medline, Google Scholar
6
7 : Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Control Clin Trials 2004; 25:119–142Crossref, Medline, Google Scholar
8 : Therapeutic drug monitoring (TDM) in psychiatry (part I): why studies attempting to correlate drug concentration and antidepressant response don’t work. J Psychiatr Pract 2014; 20:133–137Crossref, Medline, Google Scholar
9 : Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects. Neuropsychopharmacology 2011; 36:692–700Crossref, Medline, Google Scholar
10 : Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry 2013; 18:273–287Crossref, Medline, Google Scholar
11 : Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol Psychiatry 2014; 19:733–741Crossref, Medline, Google Scholar
12 : Elevated CYP2C19 expression is associated with depressive symptoms and hippocampal homeostasis impairment. Mol Psychiatry 2017; 22:1224Crossref, Medline, Google Scholar
13 : Polymorphism in CYP2D6 and CYP2C19, members of the cytochrome P450 mixed-function oxidase system, in the metabolism of psychotropic drugs. J Intern Med 2015; 277:167–177Crossref, Medline, Google Scholar
14 : Pregnancy exposure to citalopram: therapeutic drug monitoring in maternal blood, amniotic fluid, and cord blood. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79(Pt B):213–219Crossref, Medline, Google Scholar



