Early Variations in White Matter Microstructure and Depression Outcome in Adolescents With Subthreshold Depression
Abstract
Objective:
White matter microstructure alterations have recently been associated with depressive episodes during adolescence, but it is unknown whether they predate depression. The authors investigated whether subthreshold depression in adolescence is associated with white matter microstructure variations and whether they relate to depression outcome.
Method:
Adolescents with subthreshold depression (N=96) and healthy control subjects (N=336) drawn from a community-based cohort were compared using diffusion tensor imaging and whole brain tract-based spatial statistics (TBSS) at age 14 to assess white matter microstructure. They were followed up at age 16 to assess depression. Probabilistic tractography was used to reconstruct white matter streamlines spreading from the regions identified in the TBSS analysis and along bundles implicated in emotion regulation, the uncinate fasciculus and the cingulum. The authors searched for mediating effects of white matter microstructure on the relationship between baseline subthreshold depression and depression at follow-up, and then explored the specificity of the findings.
Results:
Lower fractional anisotropy (FA) and higher radial diffusivity were found in the anterior corpus callosum in the adolescents with subthreshold depression. Tractography analysis showed that they also had lower FA in the right cingulum streamlines, along with lower FA and higher mean diffusivity in tracts connecting the corpus callosum to the anterior cingulate cortex. The relation between subthreshold depression at baseline and depression at follow-up was mediated by FA values in the latter tracts, and lower FA values in those tracts distinctively predicted higher individual risk for depression.
Conclusions:
Early FA variations in tracts projecting from the corpus callosum to the anterior cingulate cortex may denote a higher risk of transition to depression in adolescents.
First episodes of major depression have been associated with changes in white matter diffusion tensor imaging–derived indices in adolescents, mainly in the corpus callosum, the cingulum, and the uncinate bundles (1–5). Evidence for white matter tract alterations in the frontal-thalamic-striatal circuit and in the default mode network nodes has also been found (6). Although the involvement of white matter alterations in depressive episodes is recognized, it is still unclear whether the alterations predate the onset of the disease, indicating vulnerability, or whether they indicate ongoing pathology. Studies in individuals at familial risk for depression have addressed this issue and found that white matter microstructure alterations may indicate genetic risk for affective disorders (7).
Subthreshold depression in adolescence is another at-risk condition for depressive disorders, with an estimated risk of 67% for escalation to depression (8). It has been defined as an episode of clinically relevant depressive symptoms that do not meet enough criteria for a diagnosis of a major depressive episode, either in number of symptoms, duration, or impact on functioning (9). In adolescence, subthreshold depression has a high lifetime prevalence, ranging between 20% and 30% (9, 10).
We recently reported (11) that adolescents with subthreshold depression had lower white matter volumes in the internal capsule, a region involved in frontal striatal networks, and in the forceps minor and the cingulum, which are part of the default mode network. Also, they had lower gray matter volumes in the medial prefrontal cortex and the striatum, and they exhibited functional striatal deficits when anticipating reward (12).
In the present study, we hypothesized that adolescents with subthreshold depression would also have white matter microstructure differences within the frontal striatal-limbic and default mode networks, both of which are implicated in depression. To investigate white matter microstructure, we used tract-based spatial statistics (TBSS) to search for regional differences in indices derived from diffusion tensor imaging (DTI) within white matter tracts. We then performed probabilistic diffusion tractography to explore cortical projections from the regions identified in the TBSS approach (13) and to assess tractography-derived measures within the cingulum and the uncinate fasciculus, two bundles reported to be altered in adolescent depression (2, 3). Finally, we investigated whether the observed differences in DTI-derived indices would mediate the relationship between subthreshold depression at age 14 and the occurrence of depression at age 16.
Method
Participants
The study was approved by the ethics committees of all participating institutions. All adolescents and their parents provided written informed assent and consent, respectively, after receiving a complete description of the study.
Neuroimaging and clinical data were obtained from a large sample of community adolescents recruited around age 14 in middle schools from eight sites in four European countries (www.imagen-europe.com). A detailed description of recruitment and assessment procedures, with exclusion and inclusion criteria, has been published elsewhere (14). Presence of any obvious psychopathology at baseline (e.g., bipolar disorder, schizophrenia, and major neurodevelopmental disorders) was an exclusion criterion.
Baseline Assessment
Psychiatric symptoms were assessed with the Development and Well-Being Assessment (DAWBA), a self-administered diagnostic questionnaire consisting of open and closed questions (15). The DAWBA provides computer-generated probabilities of having DSM-IV diagnoses (the DAWBA bands), and definitive diagnoses are generated by clinical raters. The open comments from participants and their families provided in the DAWBA were taken into account to assess potential past history.
The assessment battery was self-administered both in participants’ homes and at the neuroimaging facilities using the Psytools software program (Delosis, Middlesex, U.K.), via its web-based platform (14). Data on substance use were obtained with the Alcohol Use Disorders Identification Test (AUDIT) (16) and the European School Survey Project on Alcohol and Drugs (www.espad.org). Other assessments included personality dimensions, based on the Substance Use Risk Profile Scale (17) and the NEO Five-Factor Inventory (18); handedness; pubertal status, based on the Pubertal Development Scale questionnaire (19); parental psychiatric history (14; see also the online supplement); and life events, using the Life Events Questionnaire (20). Participants with any psychiatric diagnosis, any lifetime history of drug use, or any symptoms of alcohol abuse or dependence (an AUDIT score >4) were excluded from the present analysis, as these disorders have neural correlates that would have biased our neuroimaging investigation. None of the participants or their parents reported having received prescriptions for antidepressants, mood stabilizers, anxiolytics, antipsychotics, or hypnotics.
Adolescents were included in the subthreshold depression group if, in the past 4 weeks, they had experienced at least three depressive symptoms, including at least one core symptom (abnormally depressed, irritable mood, or loss of interest) and two or more other DSM-IV depressive symptoms, without fulfilling criteria for a major depressive episode in terms of duration, symptom number, or significant impact on functioning (11, 12). The control group, which was matched on sex with the subthreshold depression group, included adolescents who had fewer than three symptoms of depression and a probability of less than 0.1% for having a diagnosis of major depression according to the DAWBA. Participants with fewer than three symptoms constitute the standard in the IMAGEN community-based sample of adolescents, with a prevalence of 85% (11).
At baseline, 2,223 IMAGEN adolescents completed the DAWBA (see Figure S1 in the online supplement). Of these, 37 (1.7%) had depression and 301 (13.5%) had subthreshold depression. After exclusion criteria were applied, the sample for the present study included 96 adolescents with subthreshold depression and 336 healthy control subjects.
Data from 81% of the subthreshold depression group and 73% of the control group were included in our previous report on regional brain volumes (11).
Follow-Up Assessment
Two years after baseline, at age 16, participants were followed up using the same web-based questionnaires, but they did not undergo neuroimaging. The presence of a depression diagnosis was assessed using the DAWBA. Among the 1,717 IMAGEN participants who completed the DAWBA, 42 (2.45%) had depression and 214 (12.46%) had subthreshold depression.
The follow-up assessment included 84.4% (N=81) of the initial subthreshold depression group and 84.5% (N=284) of the initial control group (χ2=0.00, df=1, 432, p=1) (Table 1). Participants who were lost to follow-up were more likely to be non-Caucasian than those who were followed up (see the Method section in the online supplement).
| Baseline | Follow-Up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Subthreshold Depression Group (N=96) | Control Group (N=336) | Testa | Subthreshold Depression Group (N=81) | Control Group (N=284) | Testa | ||||||
| Mean | SD | Mean | SD | F | p | Mean | SD | Mean | SD | F | p | |
| Age (years) | 14.47 | 0.38 | 14.41 | 0.40 | 2.26 | 0.13 | 16.47 | 0.49 | 16.43 | 0.52 | 0.42 | 0.52 |
| Pubertal Development Scale (0–5) | 3.03 | 0.52 | 2.95 | 0.54 | 0.90 | 0.34 | 3.45 | 0.40 | 3.41 | 0.40 | 0.34 | 0.56 |
| N | % | N | % | χ2 | p | N | % | N | % | χ2 | p | |
| Female | 62 | 64.58 | 217 | 64.58 | 0.00 | 1 | 52 | 64.20 | 182 | 64.08 | 0.00 | 1 |
| Right-handed | 80 | 83.33 | 298 | 88.69 | 1.50 | 0.22 | 67 | 82.72 | 253 | 89.08 | 1.81 | 0.18 |
| Parental history of depression | 8 | 8.33 | 24 | 7.14 | 0.03 | 0.86 | 6 | 7.41 | 19 | 6.69 | 0.00 | 0.98 |
| Any parental psychiatric history | 15 | 15.63 | 46 | 13.69 | 0.12 | 0.73 | 12 | 13.38 | 38 | 14.81 | 0.02 | 0.88 |
| Non-Caucasian ethnicity | 21 | 21.88 | 27 | 8.04 | 13.11 | <0.001 | 13 | 16.05 | 68 | 5.28 | 8.85 | 0.003 |
| Adverse childhood experienceb | 12 | 12.50 | 30 | 8.93 | 0.77 | 0.38 | 4 | 4.94 | 28 | 9.86 | 1.34 | 0.25 |
| Familial financial difficulties | 36 | 37.50 | 82 | 24.40 | 5.81 | 0.02 | 29 | 35.80 | 73 | 25.70 | 2.71 | 0.10 |
| Subthreshold depression at age 16 | 22 | 27.16 | 29 | 10.21 | 13.69 | <0.001 | ||||||
| Depression at age 16 | 8 | 9.88 | 7 | 2.46 | 7.01 | 0.008 | ||||||
| Self-harm since age 14 | 24 | 29.63 | 24 | 8.51 | 22.65 | <0.001 | ||||||
| Anxiety disorder at age 16c | 1 | 1.23 | 5 | 1.76 | 1 | |||||||
| Externalizing disorder at age 16d | 1 | 1.23 | 1 | 0.35 | 0.40 | |||||||
| Psychosis at age 16 | 0 | 0 | 0 | 0 | ||||||||
| Mania at age 16 | 0 | 0 | 1 | 0.35 | 1 | |||||||
| Monthly cannabis use at age 16 | 6 | 7.41 | 18 | 6.33 | 0.01 | 0.93 | ||||||
| Alcohol abuse at age 16e | 15 | 23.81 | 26 | 12.38 | 4.10 | 0.04 | ||||||
| Mean | SD | Mean | SD | U | p | Mean | SD | Mean | SD | U | p | |
| NEO Five-Factor Inventory | ||||||||||||
| Neuroticism | 29.11 | 7.46 | 20.97 | 6.76 | 6,802 | <0.001 | 27.16 | 6.42 | 20.67 | 7.28 | 5,483 | <0.001 |
| Extraversion | 27.83 | 6.68 | 30.75 | 5.30 | 19,924 | <0.001 | 27.58 | 6.33 | 30.26 | 5.59 | 13,961 | <0.001 |
| Openness | 26.47 | 6.55 | 26.30 | 5.57 | 16,070 | 0.96 | 28.21 | 6.47 | 27.46 | 6.03 | 10,610 | 0.47 |
| Agreeableness | 26.44 | 5.67 | 30.91 | 5.07 | 23,380 | <0.001 | 27.31 | 5.11 | 31.03 | 5.55 | 15,633 | <0.001 |
| Conscientiousness | 26.57 | 7.35 | 29.19 | 6.45 | 19,572 | 0.001 | 26.73 | 7.62 | 29.65 | 6.79 | 13,635 | 0.003 |
| Substance Use Risk Profile Scale | ||||||||||||
| Hopelessness | 14.89 | 3.93 | 12.42 | 2.54 | 10,079 | <0.001 | 14.18 | 3.98 | 12.44 | 3.06 | 8,174 | <0.001 |
| Anxiety-sensitivity | 11.86 | 2.43 | 11.05 | 2.24 | 13,320 | 0.009 | 11.35 | 2.53 | 10.95 | 2.38 | 9,920 | 0.14 |
| Impulsivity | 13.04 | 2.37 | 11.42 | 2.01 | 9,924 | <0.001 | 12.03 | 2.06 | 10.95 | 2.11 | 7,815 | <0.001 |
| Sensation seeking | 16.21 | 3.03 | 15.58 | 3.13 | 14,362 | 0.10 | 13.83 | 2.61 | 13.80 | 2.80 | 11,306 | 0.82 |
| Life Events Questionnaire score (−78 to +78) | –1.90 | 4.75 | –0.23 | 4.44 | 19,319 | 0.003 | 0.61 | 5.45 | 2.11 | 4.33 | 12,526 | 0.045 |
TABLE 1. Clinical Characteristics in Adolescents With Subthreshold Depression Compared With Control Subjects at Baseline and Follow-Up
Diffusion MRI Acquisition and Preprocessing
Diffusion images were acquired on 3-T scanners using an echo planar imaging sequence adapted to tensor measurements and tractography analysis (see the online supplement).
Diffusion data preprocessing was performed using the FMRIB Software Library (FSL) (www.fmrib.ox.ac.uk/fsl) (21) to obtain tensor-based parameters such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) (see the online supplement).
Tract-Based Spatial Statistic
Voxel-wise statistical analysis of the FA data was carried out using the TBSS procedure in FSL (22). All participants’ FA data were aligned into a common space using the nonlinear registration tool FNIRT (23, 24). Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the group. This skeleton was then thresholded to FA>0.2 to keep only the main tracts. Each adolescent’s aligned FA, MD, AD, and RD data were then projected onto the skeleton, and the resulting data were fed into voxel-wise cross-individual statistics. All DTI images, for both the control and subthreshold depression adolescents, underwent a quality control procedure in order to discard images with excessive head movement, poor tensor computation, and defective spatial normalization (see Figure S1 in the online supplement).
Probabilistic Diffusion Tractography
The result from the between-group comparison of FA was used to create a seed region to perform probabilistic diffusion tractography. The warp fields of nonlinear registration and their inverses were used for the translation between the original space and the Montreal Neurological Institute–152 standard space. Briefly, diffusion data were used to estimate a probability distribution function of fiber direction allowing the modeling of multiple fiber orientations at each voxel. Tractography then proceeds by drawing 5,000 streamlines through these probability distributions from each seed voxel to target 45 cortical and 15 subcortical regions based on the Harvard-Oxford atlas. For each target region, we extracted the number of samples reaching it from all seed voxels. Only pathways demonstrating a high number of streamlines to the target (mean samples >1,000) were considered for the statistical analyses. In addition, we used the same probabilistic tractography algorithm to reconstruct, in each participant, the uncinate fasciculus and the cingulum bundle based on seed and target region described by Wakana et al. (25). A mean streamline map was created for each pathway of interest and thresholded to 1,000 samples to create a mask. Mean samples, FA, MD, RD, and AD were computed within each mask for each participant.
Statistical Analysis
Cross-sectional analyses.
Voxel-wise group comparisons on FA and MD maps were tested in the framework of the general linear model using a randomization-based method (5,000 permutations). AD and RD were compared when differences in FA values were observed. Analyses were conducted in which FA and MD were included as the dependent variables, group (subthreshold depression or control) and sex as main factors, and sex-by-group interaction to explore a potential sex effect. If the interaction term was not statistically significant, only group was considered as main factor and sex was added as confounding factor. Age and DTI acquisition type (i.e., scanner manufacturer and/or software level to account for interscanner variance) were entered as confounding covariates. Statistical thresholds were set at a p value of 0.05, family-wise error corrected and threshold-free cluster enhancement corrected (26). The ICBM-DTI-81 white matter labels atlas (27) and the Johns Hopkins University tractography atlas (25) were used within FSLView to locate the tracts that displayed significant differences.
Group comparisons for sociodemographic and clinical characteristics, global diffusion values, and regional tractography measures were performed within the framework of the general linear model using the R software package (http://cran.r-project.org). As in the voxel-based analyses, sex-by-group interactions were tested before considering group as main factor and sex as confounding factor. Age and site were entered as confounding covariates. AD and RD were compared when differences were observed in FA values.
Longitudinal analyses.
Binomial logistic regression analyses were used to examine the association between subthreshold depression at age 14, entered as the predictor of interest, and depression at age 16, entered as the dependent variable. Age, sex, and site were entered as confounding covariates.
Causal mediation analyses were then conducted to determine whether FA and MD measures identified in the TBSS and tractography analyses could mediate the relation between subthreshold depression at age 14 and depression at age 16. They were performed with a validated algorithm using a set of general linear models to derive the mediation and direct effects from the total effect (28). Depression at age 16 was entered as dependent factor, and group (subthreshold depression or control) as independent factor within a logistic regression model. FA and MD values were entered as mediator variable. Age, sex, and DTI acquisition type were entered as confounding covariates. This mediation model was performed using 5,000 Monte Carlo draws for nonparametric bootstrap. In causal mediation analysis, a significant mediating effect is defined as a 95% confidence interval that does not include zero.
General linear models were used to examine the associations between the clinical characteristics that were associated with subthreshold depression at age 14 and a diagnosis of depression at age 16. We also examined the associations between these clinical characteristics and FA and MD measures that we identified in the TBSS and tractography analyses.
Post hoc analyses.
The specificity of the neuroimaging and clinical findings was investigated in the study sample (N=365), and the reproducibility and specificity of the findings were explored in a distinct sample of 686 healthy adolescents at baseline (see the Method section in the online supplement). For those analyses, the probabilities of having a diagnosis, measured with the DAWBA bands, were used as risk levels (see Tables S3 and S6 in the online supplement).
Finally, a machine-learning analysis was performed in the study sample, aimed at individually predicting depression diagnosis at age 16, based on FA values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex at age 14 (see the Method section in the online supplement).
Results
Demographic and Clinical Characteristics
At baseline, compared with the control group, adolescents in the subthreshold depression group had significantly higher scores on dimensions of hopelessness, anxiety-sensitivity, impulsivity, and neuroticism, and lower scores on extraversion, agreeableness, and conscientiousness (Table 1). They also had a higher level of negative life events and were more likely to have at least one nonwhite parent and to have more familial financial difficulties. No sex-by-group interaction was found.
At follow-up, the subthreshold depression group similarly differed from the control group except in anxiety-sensitivity (Table 1). In addition, participants in the subthreshold depression group reported significantly more self-harm since baseline and were more likely to have subthreshold or full depression and alcohol abuse.
DTI Whole Brain Analyses
The subthreshold depression group compared with the control group globally had lower FA values and higher RD values (Table 1). No sex-by-group interaction was found regarding DTI global measures.
Compared with the control group, the subthreshold depression group had lower FA and higher RD within the anterior body and genu of the corpus callosum, including the forceps minor and the anterior thalamic radiation (Table 2, Figure 1A).
| Subthreshold Depression Group (N=96) | Control Group (N=336) | Test | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | F (df=1, 425) | p |
| DTI global measures | ||||||
| Global FA values (×10–1) | 4.47 | 0.17 | 4.50 | 0.20 | 3.96 | 0.047 |
| Global MD values (×10–4) | 7.35 | 0.24 | 7.26 | 0.20 | 2.38 | 0.12 |
| Global AD values (×10–3) | 1.13 | 0.34 | 1.12 | 0.29 | 0.28 | 0.60 |
| Global RD values (×10–4) | 5.40 | 0.23 | 5.31 | 0.22 | 3.89 | 0.049 |
| DTI measures within the cluster region identified in voxel-wise comparisonb | ||||||
| FA values (×10–1) | 6.23 | 0.38 | 6.39 | 0.39 | 19.41 | <0.001 |
| RD values (×10–4) | 5.38 | 0.24 | 5.20 | 0.24 | 30.84 | <0.001 |
| Tractography analysesc | ||||||
| Tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex | ||||||
| FA values (×10–1) | 4.51 | 0.31 | 4.60 | 0.34 | 10.06 | 0.01 |
| MD values (×10–4) | 8.04 | 0.46 | 7.85 | 0.36 | 8.56 | 0.03 |
| AD values (×10–3) | 1.24 | 0.06 | 1.22 | 0.05 | 1.58 | 0.21 |
| RD values (×10–4) | 5.85 | 0.49 | 5.66 | 0.42 | 10.93 | 0.001 |
| Right cingulum bundle | ||||||
| FA values (×10–1) | 3.45 | 0.34 | 3.59 | 0.39 | 9.13 | 0.03 |
| MD values (×10–4) | 8.16 | 0.54 | 7.98 | 0.45 | 0.01 | 1 |
| AD values (×10–3) | 1.13 | 0.07 | 1.12 | 0.06 | 0.02 | 0.88 |
| RD values (×10–4) | 6.60 | 0.54 | 6.40 | 0.48 | 7.29 | 0.007 |
| Left cingulum bundle | ||||||
| FA values (×10–1) | 3.71 | 0.39 | 3.80 | 0.41 | 5.17 | 0.24 |
| MD values (×10–4) | 8.24 | 0.54 | 8.08 | 0.45 | 2.39 | 1 |
| Right uncinate bundle | ||||||
| FA values (×10–1) | 3.99 | 0.39 | 3.99 | 0.35 | 0.02 | 1 |
| MD values (×10–4) | 7.85 | 0.40 | 7.73 | 0.29 | 0.45 | 1 |
| Left uncinate bundle | ||||||
| FA values (×10–1) | 4.01 | 0.49 | 3.99 | 0.48 | 0.42 | 1 |
| MD values (×10–4) | 8.01 | 0.47 | 7.88 | 0.29 | 0.12 | 1 |
TABLE 2. Neuroimaging Characteristics in Adolescents With Subthreshold Depression Compared With Control Subjects at Baselinea
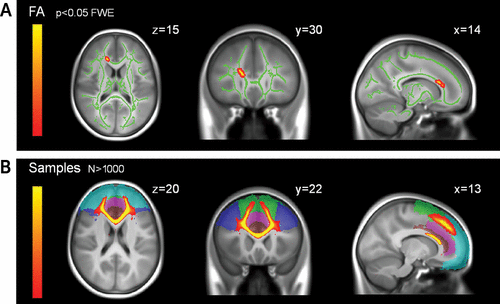
FIGURE 1. Fractional Anisotropy and Diffusion Tractography Results for Adolescents With Subthreshold Depression and Healthy Comparison Subjectsa
a Panel A highlights regions with lower fractional anisotropy (FA) in 96 adolescents with subthreshold depression compared with 336 control subjects. Green indicates white matter skeleton. FA images are displayed using the “tbss_fill” script, which allows better visualization of the regions with significant between-group differences. Results are superimposed on the average T1-weighted MRI of the adolescent brains of the IMAGEN database. FWE=family-wise error corrected. Panel B presents probabilistic diffusion tractography from the anterior corpus callosum seed thresholded to 1,000 samples. The middle frontal gyrus (dark blue), frontal pole (light blue), superior frontal gyrus (green), paracingulate gyrus (pink), and anterior cingulate gyrus (brown) are from the Harvard-Oxford atlas. Results are superimposed on the average T1-weighted MRI of the adolescent brains of the IMAGEN database.
Tractography Analysis
Using the anterior corpus callosum cluster as a seed region for probabilistic tractography, we found a high number of cortical streamlines (mean samples >1,000) reaching the anterior cingulate cortex (Brodmann’s areas [BA] 24, 25, 32, and 33), middle frontal (BA 9, 10, and 46), frontal pole (BA 9, 10, 11, and 12), superior frontal (BA 4, 6, and 8), and paracingulate regions (BA 24 and 32, corresponding to the dorsal part of the anterior cingulate cortex) (Figure 1B). There was no between-group difference in the number of samples to any of these target regions (see Table S1 in the online supplement).
After correction for multiple comparisons, lower FA and higher MD and RD were found in adolescents with subthreshold depression within tracts reconstructed between the anterior corpus callosum cluster and the anterior cingulate cortex (Table 2), which included the forceps minor, the cingulum, the anterior thalamic radiation, the superior longitudinal fasciculus, and the inferior fronto-occipital fasciculus.
In the tractography analyses of the cingulum and uncinate fasciculus based on a priori hypotheses, the adolescents with subthreshold depression had a higher number of samples and lower FA in the right cingulum, and no difference in MD (Table 2). They also had higher RD in this region, and no difference in AD.
No sex-by-group interaction effects were found in any of the voxel-based or the tractography-based analyses.
Longitudinal Analyses
Subthreshold depression at age 14 was associated with an increased risk of a depression diagnosis at age 16 (odds ratio=3.96, 95% CI=1.27, 12.38).
Higher scores on neuroticism and hopelessness at age 14 were also associated with an increased risk of a depression diagnosis at age 16 (β=6.61, t=1.88, p<0.001, and β=3.43, t=0.78, p<0.001, respectively) but not with any DTI measure (see Table S2 in the online supplement).
Causal mediation analyses showed that lower FA values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex accounted for 21% (p=0.01) of the total variance in the relationship between subthreshold depression at baseline and depression at age 16 (Table 3; Figure 2A,B). Although short of statistical significance, higher MD values also had a mediation effect (p=0.06) (Table 3).
| Effect Type | Point Estimate | 95% CI | p |
|---|---|---|---|
| Mediator variable: FA values | |||
| Mediation effect (subthreshold depression at age 14–FA–depression at age 16) | 0.018 | 0.004, 0.044 | 0.01 |
| Direct effect (subthreshold depression at age 14–depression at age 16) | 0.067 | –0.001, 0.162 | 0.06 |
| Total effect | 0.085 | 0.016, 0.184 | 0.01 |
| Proportion of total effect via mediation | 0.206 | 0.042, 0.902 | |
| Mediator variable: MD values | |||
| Mediation effect (subthreshold depression at age 14–MD–depression at age 16) | 0.010 | 0.000,0.027 | 0.06 |
| Direct effect (subthreshold depression at age 14–depression at age 16) | 0.073 | 0.008, 0.016 | 0.02 |
| Total effect | 0.083 | 0.016, 0.177 | 0.01 |
| Proportion of total effect via mediation | 0.017 | –0.002, 0.512 |
TABLE 3. Causal Mediation Analysis of the Relationship Between Subthreshold Depression at Age 14 and Depression at Age 16 With Fractional Anisotropy (FA) and Mean Diffusivity (MD) Values in Tracts Spanning From the Anterior Corpus Callosum Cluster to the Anterior Cingulate as Mediator
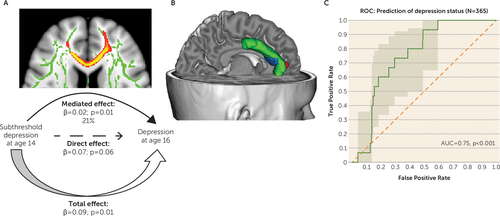
FIGURE 2. Mediation of the Relationship Between Subthreshold Depression at Age 14 and Depression at Age 16a
a Panel A illustrates the mediation of FA values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate on the relationship between subthreshold depression at age 14 and depression at age 16, using causal mediation analysis. Panel B is a three-dimensional representation (http://brainvisa.info) of mediation results projected onto a T1-weighted MRI scan of an adolescent brain from the IMAGEN database. Green indicates anterior cingulate cortex; blue indicates probabilistic diffusion tractography from the anterior corpus callosum white matter cluster to the anterior cingulate cortex; and red indicates the medial prefrontal cortex gray matter cluster, the lower volume of which was previously found (11) to mediate the relation between subthreshold depression at age 14 and depression at age 16. Panel C presents the receiver operating characteristic (ROC) curve illustrating individual prediction of depression diagnosis at age 16 from fractional anisotropy values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex at age 14 in a sample of 365 adolescents. The area under the curve (AUC) can vary between 0 and 1; an uninformative classifier would yield an AUC of 0.5.
Post Hoc Analyses
In the study sample, subthreshold depression at age 14 was associated with a higher risk for various DSM-IV disorders at age 16 (see the Results section in the online supplement). Lower FA values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex at age 14 predicted a higher risk at age 16 for depression but not for any other diagnosis, either in the study sample or in the distinct sample of 686 adolescents (see the Results section in the online supplement).
In contrast, in the study sample, higher scores on neuroticism and hopelessness at age 14 mediated the association between subthreshold depression at age 14 and risk for both depression and other diagnoses at age 16 (see the Results section and Tables S4 and S5 in the online supplement).
Finally, individual prediction of a depression diagnosis at age 16 using FA values in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex at age 14 resulted in an area under the curve of 0.75, with a p value <0.001 (Figure 2C), denoting the likelihood of FA values in those tracts in predicting the onset of depression for an individual adolescent.
Discussion
Regional variations in white matter microstructure were identified for the first time in 14-year-old community adolescents with subthreshold depression and no familial risk for depression. These differences involved the anterior body and genu of the corpus callosum. Tractography analyses revealed variations in the right cingulum and within fibers between the anterior corpus callosum and the anterior cingulate cortex. Lower FA values in those prefrontal tracts mediated transition to depression at age 16. Furthermore, in a larger sample of healthy adolescents, we found that lower FA in these fibers indicated risk for depression but not for other diagnoses.
Alterations in similar tracts have previously been reported in depressed adolescents and adults (1–4, 29) and in adolescents at familial risk (7).
The anterior part of the corpus callosum, frequently implicated in adolescent depression (2–4), connects to the anterior cingulate cortex, a core region of the pathophysiology of depression whose volume we previously reported to be smaller (11) and to mediate depression outcome 2 years later (Figure 2B).
Our tractography analysis also revealed white matter microstructural variations in the cingulum bundle in adolescents with subthreshold depression, consistent with our previous report that this group had smaller white matter volume than the control subjects in the right cingulum (11). The cingulum contains fibers connecting the cingulate, prefrontal cortices, and temporal limbic regions and is involved in functional connectivity of the default mode network (30).
In the present study, lower FA values were also observed in the right anterior thalamic radiation that runs in close proximity to the superolateral branch of the medial forebrain bundle. Both tracts may be difficult to distinguish and are not differentiated in the atlas used in this study (25, 31). Converging both in the anterior limb of the internal capsule and in the prefrontal cortex (31), they may be differentially involved in the pathophysiology of depression. The anterior thalamic radiation would convey negative affective states, such as sadness, whereas the superolateral branch of the medial forebrain bundle would be involved in reward seeking and appetitive motivation (31), as it connects core regions of the reward system. Also, lower FA in this tract has been associated with higher anhedonia (32). In previous studies in almost the same group of 14-year-olds with subthreshold depression, we consistently found functional striatal deficits during reward anticipation (12) and smaller gray matter volumes in the caudates (11). Our findings also indicate that FA measures at age 14 in tracts spanning from the anterior corpus callosum cluster to the anterior cingulate cortex hold a significant individual predictive value for later depression, with a fair accuracy. Altogether, these complementary findings suggest that a particular developmental pathophysiology involving these white matter tracts and adjacent gray matter regions may account for the vulnerability to developing depression.
White matter changes have also been associated with other psychiatric disorders in adolescents, notably generalized anxiety disorder and attention deficit hyperactivity disorder (33, 34). In the present study, subthreshold depression at age 14 was also associated with risk for such disorders at follow-up, in line with the literature (35), and higher neuroticism and hopelessness scores at age 14 were found to mediate the association between subthreshold depression at age 14 and risk for both depression and other diagnoses at age 16. However, lower FA values did not predict higher risk for any diagnosis other than depression at follow-up, either in the study sample or in the distinct sample of 686 adolescents who were healthy at baseline. Thus, our imaging findings appear to be specific to increased risk for depression.
In line with the literature, the participants with subthreshold depression in this study had higher scores on neuroticism, hopelessness, and negative life events. It has been suggested that such characteristics may alter sensitivity to stressful events and increase the risk of depression (36). In the adolescent brain, these factors may interact with hormonal and maturational changes. Indeed, adolescence is a critical period for white matter maturation, as white matter volumes and FA values show a clear increase throughout adolescence, particularly in some tracts involved in emotion regulation (37, 38). Thus, in the present study, lower FA may indicate white matter microstructure alteration as well as delayed white matter maturation.
The study has several limitations. The first of these is inherent to DTI, which is currently the only method capable of mapping the fiber architecture of tissue in vivo (39) but whose results must be interpreted with caution, as it does not allow the uncovering of the mechanisms underlying variations in diffusion tensor measures. Second, because of the study’s inclusion and exclusion criteria and given the cultural differences, the proportions of subthreshold depression and control adolescents differed between sites. For instance, fewer adolescents were eligible as control subjects in London. Even though we controlled for DTI acquisition type in the analyses, the between-scanner variability in our measurements may have underpowered the detection of the effects of interest and must be considered a limitation of our study. Third, as discussed elsewhere (11), the self-administered symptom rating could have biased the diagnostic ratings. However, the rate of IMAGEN participants with subthreshold depression and the rate of participants who transitioned to depression are consistent with rates reported in the literature. Finally, DTI was not repeated at age 16, at which time only psychometric data were available.
In summary, variations in DTI measures in brain regions involved in emotion regulation were detected in 14-year-old community adolescents with subthreshold depression and no familial risk. The results suggest that these adolescents may have delayed white matter maturation or early alterations in tracts spanning from the anterior corpus callosum to the anterior cingulate cortex, which in turn may denote high risk for transition to depression.
1 : Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry 2010; 49:173–83.e1Crossref, Medline, Google Scholar
2 : Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med 2014; 44:2287–2298Crossref, Medline, Google Scholar
3 : White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry 2014; 53:899–909, 909.e1–909.e7Crossref, Medline, Google Scholar
4 : White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behav 2014; 8:531–541Crossref, Medline, Google Scholar
5 : A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry 2013; 4:152Crossref, Medline, Google Scholar
6 : Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry 2014; 76:567–574Crossref, Medline, Google Scholar
7 : White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology 2011; 36:684–691Crossref, Medline, Google Scholar
8 : Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome depressive disorders. J Am Acad Child Adolesc Psychiatry 2009; 48:703–710Crossref, Medline, Google Scholar
9 : Adolescent subthreshold depression and anxiety: psychopathology, functional impairment, and increased suicide risk. J Child Psychol Psychiatry 2013; 54:670–677Crossref, Medline, Google Scholar
10 : Major and minor depression in female adolescents: onset, course, symptom presentation, and demographic associations. J Clin Psychol 2009; 65:1339–1349Crossref, Medline, Google Scholar
11 : Subthreshold depression and regional brain volumes in young community adolescents. J Am Acad Child Adolesc Psychiatry 2015; 54:832–840Crossref, Medline, Google Scholar
12 : The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172:1215–1223Link, Google Scholar
13 : Frontal networks in adults with autism spectrum disorder. Brain 2016; 139:616–630Crossref, Medline, Google Scholar
14 : The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 2010; 15:1128–1139Crossref, Medline, Google Scholar
15 : The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–655Crossref, Medline, Google Scholar
16 : Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction 1993; 88:791–804Crossref, Medline, Google Scholar
17 : The Substance Use Risk Profile Scale: a scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav 2009; 34:1042–1055Crossref, Medline, Google Scholar
18 : Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1992Google Scholar
19 : A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988; 17:117–133Crossref, Medline, Google Scholar
20 : A multidimensional assessment of stressful life events among adolescents: derivation and correlates. J Health Soc Behav 1981; 22:400–415Crossref, Google Scholar
21 : Fast robust automated brain extraction. Hum Brain Mapp 2002; 17:143–155Crossref, Medline, Google Scholar
22 : Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505Crossref, Medline, Google Scholar
23 Andersson J, Jenkinson M, Smith S: Non-Linear Registration, aka Spatial Normalisation (FMRIB technical report TR07JA2). Oxford, UK, FMRIB Centre, 2007Google Scholar
24 : Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999; 18:712–721Crossref, Medline, Google Scholar
25 : Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007; 36:630–644Crossref, Medline, Google Scholar
26 : Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence, and localisation in cluster inference. Neuroimage 2009; 44:83–98Crossref, Medline, Google Scholar
27 : Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008; 40:570–582Crossref, Medline, Google Scholar
28 : A general approach to causal mediation analysis. Psychol Methods 2010; 15:309–334Crossref, Medline, Google Scholar
29 : Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry 2016; 79:293–302Crossref, Medline, Google Scholar
30 : The structural-functional connectome and the default mode network of the human brain. Neuroimage 2014; 102:142–151Crossref, Medline, Google Scholar
31 : Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci 2012; 24:223–236Crossref, Medline, Google Scholar
32 : Hedonic tone is associated with left supero-lateral medial forebrain bundle microstructure. Psychol Med 2015; 45:865–874Crossref, Medline, Google Scholar
33 : White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 2014; 14:41Crossref, Medline, Google Scholar
34 : Common white matter microstructure alterations in pediatric motor and attention disorders. J Pediatr 2014; 164:1157–1164.e1Crossref, Medline, Google Scholar
35 : Minor depression during adolescence and mental health outcomes during adulthood. Br J Psychiatry 2009; 195:264–265Crossref, Medline, Google Scholar
36 : Depression in adolescence. Lancet 2012; 379:1056–1067Crossref, Medline, Google Scholar
37 : Imaging brain development: the adolescent brain. Neuroimage 2012; 61:397–406Crossref, Medline, Google Scholar
38 : Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008; 40:1044–1055Crossref, Medline, Google Scholar
39 : Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia 2013; 51:67–78Crossref, Medline, Google Scholar



