A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder
Abstract
Objective:
The oral formulation of the opioid antagonist naltrexone has shown limited effectiveness for treatment of opioid use disorder due to poor adherence. Long-acting injection naltrexone (XR-naltrexone), administered monthly, circumvents the need for daily pill taking, potentially improving adherence, and has been shown to be superior to placebo in reducing opioid use over 6 months of treatment. This open-label trial compared the outcomes of patients with opioid use disorder treated with XR-naltrexone or oral naltrexone in combination with behavioral therapy.
Method:
Sixty opioid-dependent adults completed inpatient opioid withdrawal and were transitioned to oral naltrexone. They were stratified by severity of opioid use (six or fewer bags versus more than six bags of heroin per day) and randomly assigned (1:1) to continue treatment with oral naltrexone (N=32) or XR-naltrexone (N=28) for 24 weeks. The first dose of XR-naltrexone (380 mg) was administered prior to discharge, with monthly doses thereafter, and oral naltrexone was given in a 50-mg daily dose. All participants received weekly behavioral therapy to support treatment and adherence to naltrexone.
Results:
A Cox proportional hazards model adjusting for race, gender, route of use, and baseline opioid use severity indicated that significantly more patients were retained in treatment for 6 months in the XR-naltrexone group (16 of 28 patients, 57.1%) than in the oral naltrexone group (nine of 32 patients, 28.1%) (hazard ratio=2.18, 95% CI=1.07, 4.43).
Conclusions:
Patients receiving XR-naltrexone had twice the rate of treatment retention at 6 months compared with those taking oral naltrexone. These results support the use of XR-naltrexone combined with behavioral therapy as an effective treatment for patients seeking opioid withdrawal and nonagonist treatment for preventing relapse to opioid use disorder.
Opioid use disorder continues to represent an unprecedented harm to public health, as rates of morbidity and mortality related to opioid abuse rise at an alarming rate (1). Agonist maintenance with methadone or buprenorphine remains the preferred treatment. When adequately dosed, buprenorphine and methadone reduce or eliminate opioid use and protect against overdose. However, not all patients will accept methadone or buprenorphine or respond well to it.
As a high-affinity opioid receptor antagonist, naltrexone represents an alternative treatment to agonist maintenance. However, its clinical utility, when taken orally, has been limited by poor adherence (2–4). Behavioral interventions, such as contingency management and involvement of significant others, have improved adherence to oral naltrexone, but to a limited extent (5–8). Extended-release (XR) parenteral formulations of naltrexone, as monthly injection (9, 10) or implants (11, 12), circumvent the daily pill requirement and have shown promising effectiveness. The intramuscular injection (marketed as Vivitrol) consists of polymer microspheres that dissolve slowly, releasing naltrexone at levels in the blood adequate for blocking the effects of exogenous opioids. Two large studies reported that these effects lasted about 4 weeks and produced 6-month retention of about 50% of participants, with patients predominantly abstinent while taking naltrexone (10, 13). Several implants, consisting of pellets surgically implanted under the skin, have been tested; one implant, produced in Russia, appears to produce adequate naltrexone blood levels for 2 to 3 months (11), and another, produced in Australia, produces adequate levels for up to 6 months (12, 14). The Russian implant, administered every 2 months, produced 53% retention in treatment over a 6-month trial, compared with 16% for a control group receiving oral naltrexone. The Australian (6-month) implant provides sustained naltrexone blood levels of ≥1 ng/mL (15) and has been shown to improve adherence, increase abstinence rates, and effectively reduce relapse to opioid dependence, compared with oral naltrexone (14).
To date, no randomized controlled studies have compared the efficacy of long-term treatment with oral relative to injectable naltrexone. One study found that a single administration of XR-naltrexone, at the outset of treatment with oral naltrexone, improved retention for low-severity users at 6 months (6). A quasi-experimental comparison demonstrated that, compared with oral naltrexone, injectable naltrexone yielded higher 8-week retention (16).
We conducted a randomized open-label trial of naltrexone for extended-release injectable suspension compared with oral naltrexone, both given in combination with “behavioral naltrexone therapy,” in participants with opioid dependence seeking opioid withdrawal and relapse-prevention treatment. Behavioral naltrexone therapy, a behavioral therapy developed to support treatment with naltrexone (7, 17), consists of incentives for adherence together with motivational and cognitive-behavioral counseling. We hypothesized that XR-naltrexone would demonstrate improved treatment retention compared with oral naltrexone.
Method
Participants
Individuals with opioid dependence seeking treatment were evaluated at an outpatient research clinic using the Structured Clinical Interview for DSM-IV and a clinical interview. Medical evaluation included history, physical examination, laboratory tests, and electrocardiogram. All participants gave written informed consent.
The target enrollment was 60 participants, ages 18–60, who met DSM-IV criteria for current opioid dependence. Individuals with unstable medical or psychiatric disorders were excluded. Other exclusion criteria included physiological dependence on alcohol or sedative-hypnotics, treatment with opioids for chronic pain, regular methadone use, psychotropic medications, and history of opioid overdose in the previous 3 years. Participants were drawn from across the greater New York area. This study was approved by the institutional review board of the New York State Psychiatric Institute.
Study Procedures
Randomization.
We conducted a randomized, parallel-group, open-label, 24-week pilot clinical trial from September 2007 to May 2012. Participants received an initial inpatient medication-assisted opioid detoxification and naltrexone induction (18, 19) on a state-funded inpatient clinical research unit at the New York State Psychiatric Institute. An outpatient induction option was offered for those who were already opioid abstinent, but only a few participants entered the trial by this route. After tolerating 50 mg of naltrexone, participants were stratified and randomly assigned to treatment with oral naltrexone (50 mg/day for those participants with a significant other to monitor daily ingestion at home; those without a significant other received, at the clinic, 100 mg on Monday and Wednesday and 150 mg on Friday) or XR-naltrexone (380 mg i.m. monthly), started prior to discharge. Stratification was organized by severity of opioid use (six or fewer bags of heroin per day compared with more than six bags per day, or morphine equivalence [mg] for prescription opioids), presence or absence of a participating significant other, and inpatient relative to outpatient induction. Starting in 2009, participants were no longer stratified by involvement of a significant other at study entry. Randomization was in a random block design.
Postinduction treatment phase.
All participants were asked to attend clinic visits three times per week for the first 2 weeks and then twice weekly for the remainder of the 24-week study. During each visit, on-site urine toxicology tests were performed, recent self-reported substance use was reviewed, and participants received a therapy session. XR-naltrexone was administered every 4 weeks (six injections over the course of the study). Oral naltrexone administration was clinic based for the first 2 weeks (dosed at 100, 100, and 150 mg on Monday, Wednesday, and Friday, respectively). Participants were asked to identify a monitor (a significant other or family member) who could oversee adherence to naltrexone and treatment. Criteria for an appropriate monitor included the absence of active substance abuse, being willing to participate in one weekly therapy session, and being willing to support adherence to medication and treatment. After providing two consecutive opioid-free urine tests, participants with a monitor transitioned to daily home-based self-administration. Participants unable to identify an involved monitor remained on clinic-based administration throughout the trial. Participants assigned to oral naltrexone were provided a 7-day supply of medication, in case of missed visits. Adherence was monitored in three ways: self-report, weekly naltrexone adherence logs submitted by the participant’s monitor, and riboflavin labeling of oral naltrexone to permit fluorescence testing of urine.
Relapse was suspected if the participant used opioids on 2 or more days and missed scheduled medication for >4 consecutive days in the oral naltrexone group or for >2 days following the date of a scheduled but missed injection. In these participants, a 0.8 mg i.m. naloxone challenge was performed; if the results were negative, naltrexone was resumed. If withdrawal symptoms were in the mild range following naloxone, a reduced dose of 12.5 mg of naltrexone was given, and the dose was increased over the following 2 days to the full 50-mg dose. If necessary, a second naloxone challenge was offered within 72 hours. Participants who failed two consecutive naloxone challenges were considered to have relapsed and were referred for alternative treatment. In the oral naltrexone group, patients who provided opioid-positive urine tests but had remained adherent to naltrexone treatment or had successfully resumed naltrexone returned to clinic-based administration of naltrexone until two consecutive opioid-negative urine tests were produced. Lastly, for individuals who had not returned to opioid use but had a nonfluorescent urine test result for naltrexone, 50 mg of naltrexone was provided on-site to reinforce and secure continued maintenance. Reinduction was attempted within 72 hours for patients in the process of relapsing who were using opioids and provided a positive challenge.
Participants who relapsed, according to either self-report or objective evidence, were offered referrals to local treatment programs. Participants were provided referrals for both agonist and detoxification/antagonist treatment available in the community.
Behavioral naltrexone therapy was developed at our clinic previously to support adherence to oral naltrexone for the treatment of opioid dependence, and in this study it was adapted to support oral or injectable forms. The major therapeutic components included contingency management for adherence to scheduled clinic visits, relapse prevention, community reinforcement approach, and network therapy (7, 16). The goals of behavioral naltrexone therapy are for patients to take naltrexone continuously and to abstain from opioids. These goals are reinforced by voucher incentives contingent on attendance, as well as the support of significant others who monitor medication ingestion and attend network therapy sessions. The target behavior for contingency management in behavioral naltrexone therapy is attendance at scheduled clinic visits to provide assessments. Individual treatment sessions assess and challenge motivation for treatment and abstinence and integrate cognitive-behavioral techniques to facilitate all of these goals. We employed an attendance-based prize bowl contingency management system (20, 21). Participants could earn up to approximately $500 in gift vouchers for assessments during the 24-week trial. All participants were eligible for prize draws on the same schedule: three visits per week during the first 2 weeks, and twice-weekly visits thereafter.
Behavioral naltrexone therapy, administered by master’s- or doctoral-level clinicians, is divided into three phases, induction, stabilization, and maintenance, based on recommendations by Kleber and Kosten (22) for establishing an effective treatment for opioid dependence with naltrexone. The goals of the induction phase are to educate, motivate, and support the patient through detoxification and naltrexone induction. The primary focus of the stabilization phase during the first month of treatment is to keep the patient in treatment, on naltrexone, and abstinent from opioids. During the maintenance phase in the second month through the sixth month, core modules include developing skills such as functional analysis of opioid and other drug use, coping with cravings, drug-refusal skills, and building a supportive network.
Assessments and Data Analysis
The primary aim of this study was to compare the retention (time to dropout) of participants across the two treatment arms during 24 weeks of treatment. Dropout, a typical failure mode for opioid dependence treatment, is most often accompanied by relapse to opioid use; this conservative assumption is consistent with the approach used in primary outcome analysis in other opioid antagonist trials. Date of dropout was defined as the date of last on-site clinical contact.
The effect of treatment arm on time to dropout was examined with Kaplan-Meier curves and log-rank tests. Cox proportional hazards regression was used to model time to dropout as a function of treatment while controlling for continuous age, binary gender, ethnicity (white compared with nonwhite), route of opioid administration, and baseline opioid use severity (bags of heroin per day or mg of morphine).
The secondary outcome of opioid use during the trial was also compared between treatment arms. Opioid use (positive if at least one of the following substances was detected: heroin, methadone, oxycodone, or buprenorphine) during the trial was evaluated by on-site immunoassay test. For each patient, the proportion of urine tests collected prior to dropout that was positive for an opioid was computed. The effect of treatment on the proportion of opioid use was examined using a Wilcoxon rank sum test.
Demographic and clinical differences between treatment arms at baseline were summarized and tabulated, and significant adverse event occurrences were tabulated.
A sample size of 30 per group (N=60) has at least 80% power to detect a difference of 25% significant versus 60% significant at a significance level of 5%. All analyses were conducted on the intent-to-treat sample. All statistical tests were two-tailed and used a statistical significance level of 5%.
Results
Sample Description
During the study, 608 persons were screened for eligibility; 110 consented to the study and entered the New York State Psychiatric Institute, a state-funded inpatient research unit, for a week-long medication-assisted opioid withdrawal and initiation of treatment with naltrexone (Figure 1). Fifty participants (45.5%) failed to complete the inpatient detoxification/naltrexone induction. Nine participants were opioid abstinent at study entry and able to undergo outpatient XR-naltrexone induction. Those who completed the induction procedure were subsequently seen for outpatient visits at the Substance Treatment and Research Service (STARS) of Columbia University.
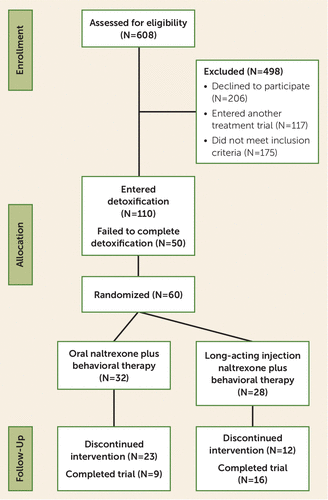
FIGURE 1. CONSORT Diagram for a Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder
Sixty participants received the 50-mg dose of oral naltrexone and were randomly assigned to continue treatment with oral naltrexone (N=32) or XR-naltrexone (N=28). Baseline demographic and clinical characteristics of the randomized participants are summarized in Table 1. The sample was predominantly male (83.3%), in their late 30s (mean age, 39.5 years, SD=11.1), and white (63.3%). Across both groups, the median amount of heroin use was five bags per day (interquartile range [IQR]=2.5–9.5), and the most common route of use was intranasal (46.7%).
| Characteristic | Oral Naltrexone Group (N=32) | XR-Naltrexone Group (N=28) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 39.5 | 12.5 | 39.4 | 9.4 |
| Age at onset of regular opioid use (years)a | 28.3 | 9.9 | 27.1 | 7.0 |
| N | % | N | % | |
| Male | 26 | 81.3 | 24 | 85.7 |
| Ethnicity | ||||
| White | 21 | 65.6 | 17 | 60.7 |
| Black | 2 | 6.3 | 5 | 17.9 |
| Hispanic | 9 | 28.1 | 4 | 14.3 |
| Other | 0 | 0.0 | 2 | 7.1 |
| Marital statusb | ||||
| Single | 17 | 63.0 | 16 | 57.1 |
| Divorced | 2 | 7.4 | 6 | 21.4 |
| Married | 8 | 29.6 | 6 | 21.4 |
| Employment statusc | ||||
| Full time | 8 | 29.6 | 4 | 14.3 |
| Part time | 4 | 14.8 | 3 | 10.7 |
| Unemployed | 7 | 25.9 | 17 | 60.7 |
| Student | 3 | 11.1 | 3 | 10.7 |
| Disabled | 3 | 11.1 | 1 | 3.6 |
| Other | 2 | 7.4 | 0 | 0.0 |
| Criminal justice involvementd | 4 | 21.1 | 10 | 35.7 |
| Mean | SD | Mean | SD | |
| Pattern of heroin use at baseline | ||||
| Amount of daily use (bags)e | 7.3 | 7.7 | 6.6 | 4.1 |
| N | % | N | % | |
| Route (heroin) | ||||
| Intravenous | 8 | 25.0 | 8 | 28.6 |
| Intranasal | 16 | 50.0 | 12 | 42.9 |
| Oral | 8 | 25.0 | 8 | 28.6 |
| Prescription opioid users | 8 | 25.0 | 8 | 28.6 |
| Mean | SD | Mean | SD | |
| Daily prescription opioid use (mg of morphine)e | 71.3 | 69.8 | 98.7 | 71.5 |
| Any drug or alcohol use (in last 30 days) | ||||
| Marijuana use (days)f | 18 | 62.1 | 8 | 34.8 |
| Cocaine use (days)g | 9 | 32.1 | 6 | 26.1 |
| Alcohol use (days)h | 20 | 69.0 | 17 | 73.9 |
| N | % | N | % | |
| Stratification factors | ||||
| Severity of use (more than six bags per day, or morphine equivalence [mg] for prescription opioids) | 12 | 37.5 | 11 | 39.3 |
| Inpatient induction | 28 | 87.5 | 26 | 92.9 |
| Participation by significant other | 10 | 31.2 | 7 | 25.0 |
| History of methadone usei | 5 | 16.1 | 9 | 34.6 |
| History of buprenorphine usej | 4 | 13.3 | 1 | 3.8 |
TABLE 1. Demographic Characteristics of Participants Receiving Oral Naltrexone or Extended-Release Injectable Suspension Naltrexone (XR-Naltrexone) (N=60)
Primary Outcome: Retention in Treatment
Figure 2 presents Kaplan-Meier survival curves for retention by treatment group. Time to dropout was significantly higher among individuals receiving XR-naltrexone compared with oral naltrexone, based on the Kaplan-Meier nonparametric log-rank test (χ2=4.64, df=1, p=0.03). Observed retention rates for those in the XR-naltrexone and the oral naltrexone groups, respectively, were 85.7% (24 of 28 participants) and 62.5% (20 of 32 participants) at 4 weeks; 60.7% (17 of 28 participants) and 43.8% (14 of 32 participants) at 12 weeks; and 57.1% (16 of 28 participants) and 28.1% (nine of 32 participants) at 24 weeks.
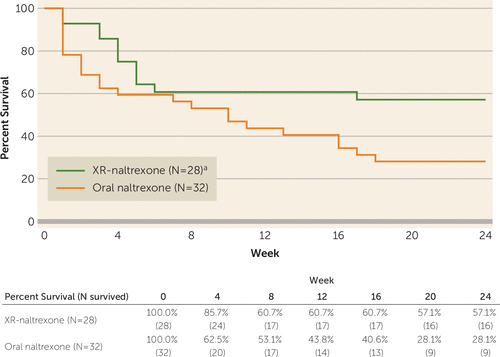
FIGURE 2. Time to Dropout for Participants Receiving Oral Naltrexone or Extended-Release Injectable Suspension Naltrexone (XR-Naltrexone)a
a Time to dropout was significantly higher in the XR-naltrexone group relative to the oral naltrexone group (Kaplan-Meier nonparametric log-rank test, χ2=4.64, df=1, p=0.03).
The Cox proportional hazards regression model similarly indicated significantly higher time to dropout in participants receiving XR-naltrexone (hazard ratio=2.18, 95% CI=1.07, 4.43) while adjusting for the following covariates: race (white or nonwhite), gender, route of use, and baseline opioid use severity. None of the covariates were significantly associated with time to dropout. Because severity of baseline opioid use was found to interact with treatment assignment in our previous study, where a single dose of XR-naltrexone was administered at the outset of 6 months of oral naltrexone treatment (XR-naltrexone produced superior retention when baseline severity was lower) (6), we also explored the interaction between baseline opioid use severity and treatment assignment, which was not significant (χ2=0.402, df=1, p=0.53).
Of 35 patients who did not complete the trial, 19 were nonadherent with attendance, five were nonadherent with study medication (prompting referral to agonist treatment, for patients’ safety), five relapsed to opioid use while still attending sessions but were unable to restart oral naltrexone or XR-naltrexone following an episode of drug use, four withdrew for adverse events (in the XR-naltrexone group, one had anxiety and alcohol use and another had hives; and in the oral naltrexone group, one experienced cannabis-induced psychosis and another died during sleep), and two withdrew consent.
Secondary Outcomes
Opioid use.
The median proportion of opioid-positive urine tests was 2.4% (IQR=0%−16.7%) in the XR-naltrexone group and 8.8% (IQR=0%−20%) in the oral naltrexone group. The Wilcoxon rank sum test revealed no significant difference in the proportion of opioid-positive urine tests between treatment groups (W=546.5, p=0.57). The percentage of urine tests collected at all scheduled visits prior to dropout was 53% in the oral naltrexone group and 51% in the XR-naltrexone group. Because time to dropout was the primary outcome, we did not impute opioid-positive status to a missed urine specimen; 61.5% of participants provided a positive urine test prior to an early discontinuation.
Attendance in therapy sessions.
All participants in both treatment arms were asked to attend twice-weekly therapy sessions for 6 months. The mean number of therapy sessions attended was 17.0 (SD=16.64) in the XR-naltrexone treatment arm and 12.3 (SD=13.54) in the oral naltrexone arm. The total amount earned in vouchers by each participant during the study was proportional to his or her retention in treatment. The mean monetary value of vouchers earned was $197.68 in the oral naltrexone group and $371.37 in the XR-naltrexone group.
Safety, Medication Tolerability, and Adverse Events
Twenty-one different adverse events occurred during the trial, and a total of 40 adverse events were reported. Insomnia, the most common adverse event (experienced by 21 of 40 participants, or by 52.5% overall), was reported with significantly (z=2.22, p=0.026) greater frequency in the oral naltrexone group (14 of 20 participants, 70.0%) than in the XR-naltrexone group (seven of 20 participants, 35.0%). In the oral naltrexone group, the most frequent adverse events were diarrhea and fatigue (six of 20 participants, 30%), and anxiety and increased or decreased appetite (five of 20 participants, 25%). In the XR-naltrexone group, the most common adverse events were anxiety, muscle or joint pain, diarrhea, and fatigue (five of 20 participants, 25%), and depression and increased or decreased appetite (four of 20 participants, 20%). None of the adverse events led to study discontinuation. Most were consistent with symptoms of opioid withdrawal and improved over time.
There were nine serious adverse events (Table 2), and five participants were removed from study participation as a result. One serious adverse event during detoxification and four postrandomization serious adverse events involved psychiatric or medical worsening following naltrexone induction and led to termination of study participation. Only one serious adverse event was determined to be related to a study drug. This participant developed pruritic hives after receiving XR-naltrexone; in light of this apparent allergic reaction, he was removed from the study. Three serious adverse events were considered possibly study related: one prior to randomization (severe hypertension) and two postrandomization (increased anxiety and alcohol use [in the XR-naltrexone group] and hospitalization for chest pain and dehydration [in the oral naltrexone group]). The first two participants were removed from the study; the third showed symptom resolution and continued participation. Five postrandomization serious adverse events were deemed not study related: two nonfatal motor vehicle accidents (in the XR-naltrexone group); a pregnancy and spontaneous abortion (in the XR-naltrexone group); and hospitalization for cannabis-induced psychosis (in the oral naltrexone group). One cardiac death occurred in a stable, abstinent patient (age 49) in the third month of adherence to oral naltrexone. There is no published evidence for a cardiotoxic or arrhythmogenic effect of naltrexone. The patient had multiple risk factors for cardiovascular disease, including a history of variable adherence with diltiazem for hypertension and a father who had died of a myocardial infarction in his 40s. A urine toxicology test obtained by the medical examiner was negative for drugs of abuse. Because this incident occurred prior to the rise in prevalence of novel synthetic opioids, fentanyl testing was not performed. The patient expired at home, and the family found no evidence of drug use.
| Participant Number | Treatment Group | Type of Adverse Event | Related to Study Drug? | Outcome |
|---|---|---|---|---|
| 084 | Oral naltrexone | Cardiac death during sleep | No | Termination of study participation |
| 088 | Oral naltrexone | Hospitalization for chest pain and dehydration | Possible; detoxification-related | Improvement of symptoms, continued participation |
| 104 | Oral naltrexone | Hospitalization for cannabis-induced psychosis | No | Removal from study |
| 008 | XR-naltrexone | Increased anxiety and alcohol use | Possible | Removal from study |
| 026 | Not randomized | Severe hypertension during naltrexone induction | Possible; detoxification-related | Removal from study |
| 052 | XR-naltrexone | Hives, pruritis | Yes | Removal from study |
| 054 | XR-naltrexone | Nonfatal motor vehicle accident | No | Continued study participation |
| 094 | XR-naltrexone | Pregnancy followed by spontaneous abortion | No | Continued study participation |
| 100 | XR-naltrexone | Nonfatal motor vehicle accident, then relapse to opioid dependence | No | Removal from study |
TABLE 2. List of Serious Adverse Events and Outcomes in Participants Receiving Oral Naltrexone or Extended-Release Injectable Suspension Naltrexone (XR-Naltrexone)
Discussion
This 24-week, open-label, randomized controlled trial provides a direct comparison of treatment outcome for participants with opioid dependence who underwent opioid withdrawal and were treated with oral naltrexone or XR-naltrexone. Participants receiving injection naltrexone were significantly more likely to remain in treatment. These results replicate and extend a previously reported quasi-experimental comparison (16) demonstrating superior retention at 8 weeks with XR-naltrexone compared with oral naltrexone. In addition, the results are consistent with the superior retention of naltrexone implant compared with oral naltrexone treatment (11). An earlier trial (6) combined oral naltrexone with single administration of XR-naltrexone at the outset of treatment and demonstrated that retention in treatment among those who received behavioral therapy combined with single-dose injection naltrexone was significantly greater with lower baseline opioid use severity, compared with an intervention of behavioral therapy combined with placebo injection. The present findings extend these earlier results by showing that retention rates are higher for XR-naltrexone when administered as monthly injections over 6 months, regardless of baseline opioid use severity.
At the time this trial began, oral naltrexone was the only opioid antagonist therapy in clinical use for the treatment of opioid dependence, and XR-naltrexone was pending approval from the Food and Drug Administration. Our goal was to compare the effectiveness of oral naltrexone and injection naltrexone in the setting of an intensive behavioral therapy, which had been shown to improve treatment outcomes with oral naltrexone. However, this trial shows that even in the presence of an intensive behavioral regimen aimed at supporting medication adherence, oral naltrexone is an inferior treatment and should be avoided clinically, other than perhaps for very select cases with a high likelihood of adherence.
This trial also replicates previous findings that retention rates of oral naltrexone range from 25.6% to 47.2% at 12 weeks (4–6) and from 9.0% to 22.0% at 24 weeks (7), with combined medication and behavioral therapy associated with higher retention (7, 23). In contrast, nearly 60% of participants receiving XR-naltrexone plus behavioral therapy were retained in treatment at 24 weeks. Laboratory studies have shown that injection naltrexone blocks the rewarding effects of heroin (24), and clinical trial data show low rates of opioid use while patients are adherent to naltrexone (25–27). Similarity in rates of opioid-positive toxicology results between the treatment groups in this trial, despite significant group differences in retention, is consistent with the observation that most patients remain opioid-abstinent while engaged in treatment and adherent to naltrexone (27). By contrast, long-term studies have shown that discontinuing medication for opioid use disorder predicts relapse (28–30).
The generalizability of the present findings is restricted to patients who are seeking a nonopioid medication treatment and who undergo full detoxification and succeed at initiating treatment with naltrexone—a group that may have above-average motivation. The high failure rate observed during detoxification highlights the need for further research to optimize the management of opioid withdrawal. A recent comparative effectiveness trial highlighted that induction can be a barrier to initiating XR-naltrexone treatment, although relapse rates appear similar among those who are successfully inducted onto buprenorphine or naltrexone (31). For individuals seeking to transition to antagonist treatment, the development of a well-tolerated regimen involving nonagonist medications could shorten the period of detoxification and induction and should reduce the risk of relapse prior to receiving XR-naltrexone. Recent studies have aimed to develop and refine such a regimen (25, 26, 31–33).
In addition, it should be noted that slightly more than half of the subjects screened either declined to participate or entered another treatment study. Patients often have strong preferences with respect to the type of treatment (agonist as opposed to antagonist) they are seeking for opioid use disorder (34, 35). At the time this study was initiated, XR-naltrexone was not yet approved as a treatment for opioid dependence, and oral naltrexone was unfamiliar to many patients. In addition, this study required a 7-day inpatient stay, which was not possible for many individuals with children at home or jobs that did not offer paid medical leave.
The most common adverse events in both treatment arms were consistent with protracted opioid withdrawal. There were four significant adverse events that could be attributed to naltrexone (hospitalization for chest pain and dehydration; increased anxiety and alcohol use; severe hypertension during naltrexone induction; and hives and pruritis); opioid withdrawal and rapid initiation of naltrexone can cause systemic stress. We excluded patients with unstable conditions, but hemodynamic or psychiatric instability may still occur during detoxification, and careful monitoring during induction is warranted.
The death of a participant in the oral naltrexone group was not considered to be related to the study, as the medical examiner found no evidence of opioids or other drugs on analysis. However, assays for fentanyl or carfentanyl were not performed at that time, and thus the presence of these drugs cannot be ruled out. The recent rapid rise in prevalence of novel synthetic opioids raises the possibility that recently synthesized drugs often go undetected, and thus current rates of opioid overdose may be underestimated.
These study findings have immediate clinical relevance for treatment of opioid use disorder, at a time when an opioid epidemic continues unabated in the United States. Antagonist treatment is a significantly underutilized treatment modality for opioid use disorder; 13.0% of a representative sample of U.S. treatment facilities report the use of naltrexone (36), and less than 0.3% of licensed opioid treatment programs offer XR-naltrexone (37). Given that postdetoxification outpatient treatment without pharmacotherapy yields poor completion rates, high (60%−90%) relapse rates (29, 30, 38), and heightened risk of overdose and death (39, 40), XR-naltrexone may be a viable alternative to prevent relapse in patients seeking treatment for opioid use disorder who do not prefer an agonist approach. The present data may call into question the current practice by some third-party payers of requiring a failed trial of oral naltrexone before approving XR-naltrexone, given the difference in effectiveness and the high risk of a failed treatment with oral naltrexone.
Our study had several potential limitations. The trial was designed as a pilot study to evaluate effect sizes of the differences between the two groups and was not powered for hypothesis testing of an anticipated specific difference in 6-month retention. We chose an open-label pragmatic design to evaluate regimens as they might be implemented in real-world practice. A masked design would have increased internal validity, but the use of placebo injections raises ethical concerns, as patients nonadherent to oral medication who falsely believe they have received an active XR-naltrexone injection may use opioids, putting them at risk for relapse and overdose. A high rate of screening failures reflected the fact that one-third (206 of 608) of individuals declined to participate in the study for the reasons cited above. A relatively high rate of missing urine samples was another limitation, as was the absence of postdiscontinuation urine data. The primary outcome in this study, retention in treatment, reflects adherence to naltrexone, but confirmation of avoidance of relapse depends on objective measures such as urine testing. Our sample size was relatively small. Clinical demands were similar between treatment arms; however, participants assigned to oral naltrexone who lacked a monitor needed to continue to report to the clinic three times per week to avail themselves of monitored ingestion. While participants in the oral naltrexone arm were supplied with 7 days of medication at all times, ensuring that adherence was possible, the additional weekly visit carried the burden of an additional time commitment for this group and thus represents a study limitation. In addition, payments for participation may have encouraged study visit attendance across both groups. After dropout from the study, participants were generally lost to follow-up. Future studies should employ robust tracking procedures, using patient navigators, for example, to locate and evaluate patients after discontinuation.
In summary, XR-naltrexone demonstrated long-term treatment retention that was twice as high as oral naltrexone following opioid withdrawal. These results support the use of XR-naltrexone combined with behavioral therapy as an effective treatment for patients seeking opioid withdrawal and nonagonist treatment for preventing relapse to opioid use disorder.
1 Centers for Disease Control and Prevention: Increases in Drug and Opioid-Involved Overdose Deaths: United States, 2010–2015. Morbidity and Mortality Weekly Report (MMWR) 65(50–51):1445–1452. https://www.cdc.gov/mmwr/volumes/65/wr/mm655051e1.htmGoogle Scholar
2 : Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction 2006; 101:491–503Crossref, Medline, Google Scholar
3 : Combining pharmacological antagonists and behavioural psychotherapy in treating addictions. Why it is effective but unpopular. Br J Psychiatry 1990; 157:34–40Crossref, Medline, Google Scholar
4 : Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend 1999; 54:127–135Crossref, Medline, Google Scholar
5 : Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry 2001; 58:755–761Crossref, Medline, Google Scholar
6 : Opioid use and dropout in patients receiving oral naltrexone with or without single administration of injection naltrexone. Drug Alcohol Depend 2015; 147:122–129Crossref, Medline, Google Scholar
7 : Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am J Drug Alcohol Abuse 2006; 32:503–517Crossref, Medline, Google Scholar
8 : Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Exp Clin Psychopharmacol 2013; 21:74–83Crossref, Medline, Google Scholar
9 : Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2006; 63:210–218Crossref, Medline, Google Scholar
10 : Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011; 377:1506–1513Crossref, Medline, Google Scholar
11 : Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry 2012; 69:973–981Crossref, Medline, Google Scholar
12 : A comparison of oral and implant naltrexone outcomes at 12 months. J Opioid Manag 2005; 1:249–256Crossref, Medline, Google Scholar
13 : Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med 2016; 374:1232–1242Crossref, Medline, Google Scholar
14 : Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry 2009; 66:1108–1115Crossref, Medline, Google Scholar
15 : Open label trial of naltrexone implants: measuring blood serum levels of naltrexone. Subst Abuse 2013; 7:75–84Medline, Google Scholar
16 : Long-acting injectable versus oral naltrexone maintenance therapy with psychosocial intervention for heroin dependence: a quasi-experiment. J Clin Psychiatry 2010; 71:1371–1378Crossref, Medline, Google Scholar
17 : Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J Subst Abuse Treat 2002; 23:351–360Crossref, Medline, Google Scholar
18 : Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse 2012; 38:187–199Crossref, Medline, Google Scholar
19 : Baseline characteristics of patients predicting suitability for rapid naltrexone induction. Am J Addict 2015; 24:258–264Crossref, Medline, Google Scholar
20 : Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000; 68:250–257Crossref, Medline, Google Scholar
21 : Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry 2005; 62:1148–1156Crossref, Medline, Google Scholar
22 : Naltrexone induction: psychologic and pharmacologic strategies. J Clin Psychiatry 1984; 45:29–38Medline, Google Scholar
23 : Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement magnitude. Exp Clin Psychopharmacol 2002; 10:54–63Crossref, Medline, Google Scholar
24 : Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology (Berl) 2006; 189:37–46Crossref, Medline, Google Scholar
25 : A placebo-controlled trial of memantine as an adjunct to injectable extended-release naltrexone for opioid dependence. J Subst Abuse Treat 2014; 46:546–552Crossref, Medline, Google Scholar
26 : The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend 2015; 154:38–45Crossref, Medline, Google Scholar
27 : Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade? Drug Alcohol Depend 2013; 133:80–85Crossref, Medline, Google Scholar
28 : Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry 2011; 68:1238–1246Crossref, Medline, Google Scholar
29 : Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J 2010; 103:176–179Medline, Google Scholar
30 : Inpatient versus other settings for detoxification for opioid dependence. Cochrane Database Syst Rev 2005; 2:CD004580Google Scholar
31 : Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet 2018; 391:309–318Crossref, Medline, Google Scholar
32 : Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: a very low dose naltrexone and buprenorphine open label trial. Drug Alcohol Depend 2014; 138:83–88Crossref, Medline, Google Scholar
33 : Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry 2017; 174:459–467Link, Google Scholar
34 : Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abuse Treat 2013; 45:302–305Crossref, Medline, Google Scholar
35 : Preferences for aftercare among persons seeking short-term opioid detoxification. J Subst Abuse Treat 2015; 59:99–103Crossref, Medline, Google Scholar
36 : Adoption of injectable naltrexone in U.S. substance use disorder treatment programs. J Stud Alcohol Drugs 2015; 76:143–151Crossref, Medline, Google Scholar
37 Substance Abuse and Mental Health Services Administration: National Survey of Substance Abuse Treatment Services (N-SSATS): 2013. Data on Substance Abuse Treatment Facilities. BHSIS Series S-73, HHS Publication No. (SMA) 14-489. Rockville, Md, 2014. http://www.samhsa.gov/data/sites/default/files/2013_N-SSATS/2013_N-SSATS_National_Survey_of_Substance_Abuse_Treatment_Services.pdfGoogle Scholar
38 : Relapse to opioid use disorder after inpatient treatment: protective effect of injection naltrexone. J Subst Abuse Treat 2018; 85:49–55Crossref, Medline, Google Scholar
39 : Mortality of those who attended drug services in Scotland 1996–2006: record-linkage study. Int J Drug Policy 2012; 23:24–32Crossref, Medline, Google Scholar
40 : 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 2003; 361:662–668Crossref, Medline, Google Scholar



