Molecular Pathogenicity of Anti-NMDA Receptor Autoantibody From Patients With First-Episode Psychosis
To the Editor: Over the last decade, the detection of autoantibodies against neurotransmitter receptors in patients with neuropsychiatric disorders has raised hopes for a better understanding of the molecular cascades underlying these pathologies and for providing appropriate therapy (1). The link between psychotic disorders and autoimmunity has regained strong support following the discovery of anti–N-methyl-d-aspartate receptor (NMDAR) autoantibody encephalitis, which is associated with psychotic symptoms (2). However, the biological significance of circulating NMDAR autoantibodies from psychotic patients remains poorly understood (3). Indeed, most studies have focused on autoantibody detection assays and prevalence (3) but have hardly addressed whether NMDAR autoantibodies have the potency to alter the localization and, thus, the function of NMDAR, as predicted by the well-established model of NMDAR hypofunction in psychosis (4). Here, we directly addressed this important question by employing the innovative and sensitive single nanoparticle imaging method to examine the functional effect of NMDAR autoantibodies from patients with first-episode psychosis.
Schematically, NMDAR autoantibody seropositive patients with first-episode psychosis (4.7%), having minimal or no exposure to antipsychotics, were identified from the OPTIMIZE project (5). To determine whether these patients’ immunoglobulin G antibodies alter the synaptic function of NMDAR in live hippocampal neurons, we used single molecule imaging to localize and track membrane synaptic NMDAR exposed to NMDAR autoantibodies. Indeed, contrary to bulk imaging approaches, the development of single molecule imaging has provided unprecedented information about the dynamic organization of single NMDARs and their pathophysiological regulation.
As previously demonstrated (6), membrane NMDARs explore the dendritic and synaptic area, in which they specifically stabilize and contribute to the NMDAR-mediated transmission. When hippocampal neurons were acutely exposed to psychotic patients’ NMDAR autoantibodies, the synaptic NMDARs were clearly destabilized with increased dynamics that contrast with the behavior of receptors exposed to purified immunoglobulin G antibodies from healthy seronegative donors (Figure 1). It is noteworthy that the synaptic disorganization observed with NMDAR autoantibodies in patients with first-episode psychosis resembles the one previously described with NMDAR autoantibodies from anti-NMDA encephalitis patients (7). Thus, because of single molecule imaging, we could determine that NMDAR autoantibodies from patients with first-episode psychosis displaced synaptic NMDAR, leading to an NMDAR hypofunction that constitutes the core hypothesis of psychosis (4). The molecular pathogenicity of NMDAR autoantibodies in patients with first-episode psychosis, which would be relevant in vivo, should prompt clinicians to screen for these autoantibodies because it may open the possibility to use immunotherapy to treat patients with first-episode psychosis who are antibody-positive.
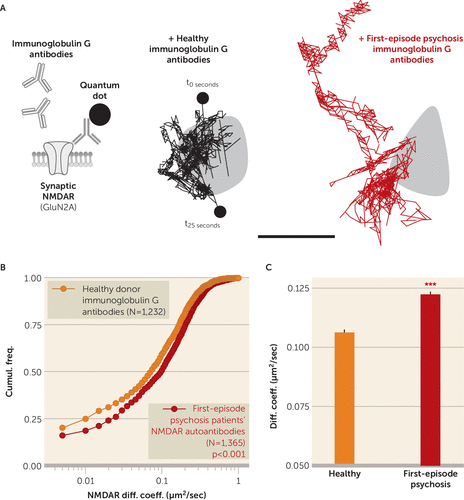
FIGURE 1. Single Nanoparticle Tracking of NMDA Receptors at the Surface of Hippocampal Neurons Exposed to Anti-NMDA Receptor Autoantibodies From Patients With First-Episode Psychosisa
a Schematic representation of the experimental design used to test the impact of immunoglobulin G antibodies of seropositive patients with first-episode psychosis compared with healthy seronegative donor immunoglobulin G antibodies. Cultured hippocampal neurons were first incubated for 30 minutes with immunoglobulin G antibodies (5 µg/mL), and then synaptic N-methyl-d-aspartate receptor (NMDAR) surface dynamics was measured through single nanoparticle (quantum dot) tracking of GluN2A-NMDAR (enriched in glutamatergic synapses). As shown in the right of panel A, representative single trajectories of surface GluN2A-NMDAR within synapses (gray area; 25-second trajectories) were exposed to immunoglobulin G antibodies from either healthy donors or seropositive patients with first-episode psychosis (scale bar, 500 nm). Panels B and C depict cumulative distributions of and mean synaptic NMDAR instantaneous diffusion coefficients from neurons exposed to healthy seronegative donors or seropositive patients (healthy donors, N=2, individual trajectories, N=1,232; positive patients with first-episode psychosis, N=5, individual trajectories, N=1,365; Mann-Whitney test).
***p<0.001.
1 : Autoimmune synaptopathies. Nat Rev Neurosci 2016; 17:103–117Crossref, Medline, Google Scholar
2 : Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74Crossref, Medline, Google Scholar
3 : Autoantibodies to central nervous system neuronal surface antigens: psychiatric symptoms and psychopharmacological implications. Psychopharmacology (Berl) 2015; 233:1605–1621Crossref, Medline, Google Scholar
4 : Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry 2004; 9:984–997Crossref, Medline, Google Scholar
5 : Cell- and single molecule-based methods to detect anti–N-methyl-d-aspartate receptor autoantibodies in patients with first-episode psychosis from the OPTiMiSE Project. Biol Psychiatry 2017; 82:766–772Crossref, Medline, Google Scholar
6 : Glutamate receptor dynamics and protein interaction: lessons from the NMDA receptor. Mol Cell Neurosci 2011; 48:298–307Crossref, Medline, Google Scholar
7 : Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 2012; 135:1606–1621Crossref, Medline, Google Scholar



