Reduced Structural Connectivity in Frontostriatal White Matter Tracts in the Associative Loop in Schizophrenia
Abstract
Objective:
The striatum receives segregated and integrative white matter tracts from the cortex facilitating information processing in the cortico-basal ganglia network. The authors examined both types of input tracts in the striatal associative loop in chronic schizophrenia patients and healthy control subjects.
Method:
Structural and diffusion MRI scans were acquired on a 3-T system from 26 chronic schizophrenia patients and 26 matched healthy control subjects. Using FreeSurfer, the associative cortex was parcellated into ventrolateral prefrontal cortex and dorsolateral prefrontal cortex subregions. The striatum was manually parcellated into its associative and sensorimotor functional subregions. Fractional anisotropy and normalized streamlines, an estimate of fiber counts, were assessed in four frontostriatal tracts (dorsolateral prefrontal cortex-associative striatum, dorsolateral prefrontal cortex-sensorimotor striatum, ventrolateral prefrontal cortex-associative striatum, and ventrolateral prefrontal cortex-sensorimotor striatum). Furthermore, these measures were correlated with a measure of cognitive control, the Trail-Making Test, Part B.
Results:
Results showed reduced fractional anisotropy and fewer streamlines in chronic schizophrenia patients for all four tracts, both segregated and integrative. Post hoc t tests showed reduced fractional anisotropy in the left ventrolateral prefrontal cortex-associative striatum and left ventrolateral prefrontal cortex-sensorimotor striatum and fewer normalized streamlines in the right dorsolateral prefrontal cortex-sensorimotor striatum and in the left and right ventrolateral prefrontal cortex-sensorimotor striatum in chronic schizophrenia patients. Furthermore, normalized streamlines in the right dorsolateral prefrontal cortex-sensorimotor striatum negatively correlated with Trail-Making Test, Part B, time spent in healthy control subjects but not in chronic schizophrenia patients.
Conclusions:
These findings demonstrated that structural connectivity is reduced in both segregated and integrative tracts in the striatal associative loop in chronic schizophrenia and that reduced normalized streamlines in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum predicted worse cognitive control in healthy control subjects but not in chronic schizophrenia patients, suggesting a loss of a “normal” brain-behavior correlation in chronic schizophrenia.
In neuropsychiatric conditions, cortical-basal ganglia distributed circuits are of interest because the output from corticostriatal-thalamic circuits targets prefrontal cortical cognitive and limbic regions, as well as frontal motor regions, areas that allow the basal ganglia to modulate nonmotor, cognitive, and emotional functions (1–3). The striatum can be divided into three functional zones—limbic striatum, associative striatum, and sensorimotor striatum—all defined by their cortical connections. Each of these functional zones receives topographically organized tracts from the cortex forming three spatially and functionally segregated corticostriatal-thalamic feedback subloops. Furthermore, these striatal zones also receive spatially overlapping, functionally integrative tracts from the cortex (4, 5). Abnormalities in any one of the gray matter core components, or in the various white matter tracts connecting the gray matter components of the three subloops, can disrupt the functional output of the entire subloop and thus directly alter cognitive, affective, or sensorimotor function (6–10).
Recent evidence highlights deficits in the associative subloop of the corticostriatal-thalamic circuits in schizophrenia (11–14). Our study of frontostriatal white matter pathways in first-episode schizophrenia that showed abnormal diffusion measures of white matter microstructure in tracts connecting the prefrontal cortex and the striatum (15) further supports such a deficit in the associative loop in schizophrenia. Given the role of the associative loop in schizophrenia, it is worth emphasizing that the associative striatum has been implicated in goal-directed behavior in both rodents and humans (16–18). It is noteworthy here that avolition and executive dysfunction are core features of schizophrenia (19, 20), both of which can be understood in terms of deficits in goal-directed behavior.
The present study focuses on white matter tracts in chronic schizophrenia patients and healthy control subjects that connect both the associative striatum and the sensorimotor striatum to the lateral surface of the cognitive associative cortex, key components of the associative and sensorimotor basal ganglia loops. The corticostriatal circuitry in normal brains is parsed into segregated versus integrative white matter pathways that project from the cortex to the striatum. Segregated tracts are those tracts that topographically connect functionally corresponding regions in the cortex and striatum (e.g., associative cortex to associative striatum), whereas integrative tracts connect functionally noncorresponding regions between the cortex and striatum (e.g., associative cortex to sensorimotor striatum) (Figure 1A). Such an organization is strongly supported by both animal tract tracing studies and human imaging studies (4, 5, 21). The distinction may have clinical importance because it has been proposed that the function of segregated tracts and their striatal target regions is to refine a skill already learned, whereas the role of the integrative tracts and their target regions is to allow for new, reward-based learning (5, 22). More broadly, striatal subregions that receive both segregated and integrative inputs from the prefrontal cortex may play a role in integrating diverse prefrontal cognitive functions (23–25).
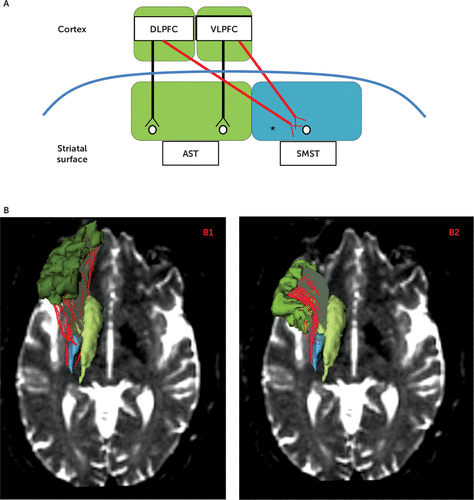
FIGURE 1. Frontostriatal Tracts for One Hemisphere and Three-Dimensional Renderings of Cortical Regions of Interesta
a Panel A shows a diagram of corticostriatal connections for one hemisphere. Parallel, segregated tracts (two black arrows) are shown, as well as convergent, integrative tracts (two red arrows). Panel B shows three-dimensional renderings of cortical regions of interests (dorsolateral prefrontal cortex [DLPFC] in dark green and ventrolateral prefrontal cortex [VLPFC] in light green) and striatal regions of interest (associative striatum [AST] in light green and sensorimotor striatum [SMST] in blue), plus three-dimensional renderings of segregated frontostriatal tracts (in gray) and integrative frontostriatal tracts (in red) overlaid on an axial view slice of a diffusion-weighted image. Panel B1 shows tracts emanating from the prefrontal DLPFC, specifically, DLPFC-AST in gray tracts and integrative DLPFC-SMST in red tracts. Panel B2 shows tracts emanating from the prefrontal VLPFC, specifically, segregated VLPFC-AST in gray tracts and integrative VLPFC-SMST in red tracts. The asterisk indicates that the streamline count residuals in the right-hemisphere DLPFC-SMST tract inversely correlated with the Trail-Making Test, Part B, and Part B minus Part A time scores in healthy control subjects but not in chronic schizophrenia patients.
Here, we seek to replicate and extend our prior study (15) by importantly adding the additional step of manual parcellation of the striatum based on a priori anatomic criteria using our published method (26). This allows us to analyze, separately, functionally segregated and integrative tracts from the cortex to the striatum, which is not possible without this additional step. Furthermore, in addition to estimating diffusion measures of white matter microstructural integrity such as fractional anisotropy, in this study we also estimated axonal fiber counts corrected for intracranial contents, using the number of streamlines, as a measure of anatomical, macrostructural connectivity.
Using diffusion-weighted imaging tractography in chronic schizophrenia patients and healthy control subjects, we investigated fractional anisotropy and streamline counts, measures of brain region structural connectivity, in associative loop tracts connecting the prefrontal cortex and the striatum. Additionally, in all participants, we investigated the association between these measures of structural connectivity and executive function as assessed using the Trail-Making Test, Part B, a measure of executive function cognitive control (i.e., the ability to resist interference while pursuing a goal-directed task). First, we hypothesized reduced fractional anisotropy and number of streamlines, reflecting reduced corticostriatal connectivity in tracts connecting the prefrontal associative cortex with the associative and somatosensory subregions of the striatum in chronic schizophrenia patients. Second, we hypothesized an association between reduced structural connectivity in these tracts in chronic patients and in healthy control subjects and worse performance on the Trail-Making Test, Part B. Third, we hypothesized an imbalance in the type of corticostriatal tract input in chronic schizophrenia patients compared with healthy control subjects, with more pronounced group differences in integrative compared with segregated type tracts. We base this last hypothesis on the idea that schizophrenia has been described as a condition with impaired integration of thought, emotion, and behavior (27), for example, as demonstrated by classic symptoms such as flat or inappropriate affect. Furthermore, since cognitive impairments, including learning new material, are characteristic of schizophrenia (e.g., see reference 28), we hypothesized that a deficit in integrative tracts that might underlie such impairments would be even more pronounced in the schizophrenia patient group than a deficit in segregated tracts.
Method
Participants
Twenty-six male patients diagnosed with chronic schizophrenia were recruited from the VA Boston Healthcare System in Brockton, Mass. Assessments with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) (29) were used to make DSM-IV diagnoses. The nonpatient edition of SCID (30) was completed for 26 healthy male control subjects who were recruited from the general community and were group-matched on age, sex, handedness (31), and parental socioeconomic status (32) to the patient sample. Inclusion criteria were right-handedness, 17 to 55 years of age, no history of ECT shock treatment, no history of neurological illness, no alcohol or drug abuse in the last 5 years, no medication with known effects on MR (such as steroids), and a verbal IQ above 75. Additionally, healthy control subjects were screened to exclude individuals who have a first-degree relative with an axis I disorder. Written, informed consent was obtained from all participants after they received a complete description of the study. Institutional review board approval for this study was granted by the local institutional review board committees at the VA Boston Healthcare System and at Brigham and Women’s Hospital.
Mean age did not differ between schizophrenia patients and healthy control subjects (43.81 years [SD=9.60] compared with 39.65 years [SD=11.10], p=0.16). Parental socioeconomic status also showed no significant difference between the two groups (p=0.36). However, as anticipated, there were significant differences in years of education (mean=13.3 [SD=1.83] compared with mean=15.11 [SD=1.91], p=0.003), verbal IQ (mean=98.35 [SD=13.90], N=26, compared with mean=112.78 [SD=16.26], N=18, p=0.003), and participant socioeconomic status (mean=3.31 [SD=1.05], N=26, compared with mean=2.04 [SD=0.81], N=24, p<0.001). Used as a proxy measure of premorbid IQ, the Wide Range Achievement Test reading score did not significantly differ between the two groups (p=0.09). Patients received a mixture of typical and atypical neuroleptic medications and were taking a mean dosage of 425.71 mg of chlorpromazine-mg equivalents. The schizophrenia patients had a mean duration of illness of 20.1 years (SD=10.4) (N=22). The clinical Positive and Negative Syndrome Scale (PANSS) scores, as well as other information relevant to demographic characteristics and medication, are provided in Table 1.
| Characteristic | Male Schizophrenia Patients (N=26) | Male Healthy Control Subjects (N=26) | Analysis by Student’s t Test (Two-Tailed) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | dfa | t | p | |
| Age (years) | 43.81 | 9.60 | 39.65 | 11.10 | 50 | –1.44 | 0.16 |
| Handednessb | 0.72 | 0.25 | 0.79 | 0.17 | 50 | 1.33 | 0.19 |
| Socioeconomic statusc | |||||||
| Participant’s own | 3.31 | 1.05 | 2.04d | 0.81 | 48 | –4.65 | <0.001* |
| Parental | 2.52e | 1.12 | 2.22f | 1.17 | 46 | –0.91 | 0.36 |
| Education (years) | 13.3 | 1.83 | 15.11 | 1.91 | 42 | 3.13 | 0.003* |
| Wechsler Adult Intelligence Scale-III verbal IQ | 98.35 | 13.90 | 112.78g | 16.26 | 42 | 3.16 | 0.003* |
| Wide Range Achievement Test Reading score | 97.88d | 12.51 | 104.50h | 10.2 | 38 | 1.76 | 0.086 |
| Trail-Making Test | |||||||
| Part A (seconds) | 52.5e | 20.4 | 29.9i | 12.2 | 39.5 | –4.50 | <0.001* |
| Part B (seconds) | 125.1e | 62.9 | 64.0i | 19.5 | 30.4 | –4.55 | <0.001* |
| Part B minus Part A (seconds) | 72.6e | 56.4 | 34.1i | 17.9 | 30.7 | –3.18 | 0.003* |
| Symptom onset (age) | 23.59j | 5.81 | NA | ||||
| Duration of illness (years) | 20.09j | 10.44 | NA | ||||
| Antipsychotic medication dosagek | 425.71 | 328.76 | NA | ||||
| Positive and Negative Syndrome Scale | |||||||
| Sum of positive scores | 20.52e | 8.96 | NA | ||||
| Sum of negative scores | 20.40e | 10.01 | NA | ||||
| Sum of general scores | 41.76e | 16.51 | NA | ||||
| Sum of all scores | 82.68e | 28.58 | NA | ||||
TABLE 1. Demographic Characteristics and Neuropsychological and Clinical Measures
Neuropsychological and Clinical Measures
We assessed goal-directed behavior using the Trail-Making Test, Part B, time spent and the Trail-Making Test, Part A, time spent (33). To control for processing speed, we subtracted scores on Part A from scores on Part B to yield a Trail-Making time-change score. In addition, to assess clinical severity of psychopathology in patients diagnosed with schizophrenia, we used the PANSS total positive, total negative, general, and total psychopathology measures (34).
Structural and Diffusion-Weighted Image Acquisition and (Initial) Postprocessing
All images were acquired on a 3.0 Tesla whole-body General Electric MRI scanner (General Electric Medical Systems, Milwaukee, Wisc.). The MRI sequences included a high-resolution three-dimensional T1 (inversion recovery-prepared fast-spoiled gradient-recalled sequence, time to repeat=7.79 ms, echo time=2.98 ms, T1=600 ms, flip angle=10°, field of view matrix size=256×256, 176 slices, 1-mm slice thickness). Diffusion-weighted images were acquired using a twice refocused echo-planar imaging sequence (time to repeat=17 seconds, echo time=80 ms, flip angle=90°, field of view matrix size=144×144, 85 slices, 1.7-mm slice thickness, 51 gradient directions with b=900 seconds/mm2 and eight baseline scans with b=0).
Intracranial contents were defined to include all white matter, gray matter, and CSF found above the most inferior axial slice that contains cerebellum. FreeSurfer software was used to parcellate cortical regions of interest (35). Subcortical striatal regions of interest were based on our manual parcellation approach (see below). Left and right striatal regions of interest were divided into associative striatum, as well as sensorimotor striatum.
Regions-of-Interest Acquisition
Cortical regions of interest.
The FreeSurfer software package (version 4.0.2) was used to parcellate T1-weighted spoiled gradient echo images into cortical gray and white matter regions for each participant (35). High-resolution three-dimensional T2-weighted images were also acquired (time to repeat=3 seconds, echo time=90 ms, flip angle=90°, field of view=256×256 mm, matrix size=256×256, 176 slices, and 1-mm slice thickness). The T2 image of the same participant in the same coordinate space as the spoiled gradient echo image was then registered to the baseline diffusion-weighted image using the FSL (FMRIB Software Library) nonlinear image registration tool (FNIRT) algorithm in the FSL software (36). We used pars opercularis, pars triangularis, and pars orbitalis for the inferior frontal gyrus gray matter regions of interest, which most likely represent the ventrolateral prefrontal cortex, and we used the rostral middle frontal gyrus as the gray matter region of interest that most likely represents the dorsolateral prefrontal cortex based on previous studies (37, 38).
Striatal regions of interest.
For manual regions of interest tracing the striatum, we employed 3D Slicer, an image editing tool developed in the Surgical Planning Laboratory, Department of Radiology, Brigham and Women's Hospital (http://www.slicer.org). Briefly, the caudate and putamen nuclei were delineated bilaterally, using three orthogonal planes, in all slices in which they appeared. The ventral striatum was bounded superiorly by an oblique line from a calculated designated point on the ventrolateral putamen to a calculated designated point on the ventromedial caudate. The vertical plane passing through the anterior commissure formed the boundary between the anterior and posterior dorsal caudate and dorsal putamen. Per hemisphere, this approach yields five striatal anatomic subregions that can be grouped into three functional subregions defined by their cortical connections. Specifically, the limbic striatum is comprised of the ventral striatum, the associative striatum is comprised of the pre- and postcommissural caudate and precommissural putamen, and the sensorimotor striatum is comprised of the postcommissural putamen. Intraclass correlation intrarater reliability was high for striatal subregions, which was based on five cases. For the left-hemisphere precommissural and postcommissural caudate and precommissural putamen (which together comprise the associative striatum), intraclass correlation coefficients were 0.920, 0.904, and 0.989, respectively. For the left-hemisphere postcommissural putamen (sensorimotor striatum) and ventral striatum (limbic striatum), intraclass correlation coefficients were 0.980 and 0.966, respectively. For the right-hemisphere precommissural and postcommissural caudate and precommissural putamen (which together comprise the associative striatum), intraclass correlation coefficients were 0.859, 0.953, and 0.996, respectively. For the right-hemisphere postcommissural putamen (sensorimotor striatum) and right-hemisphere ventral striatum (limbic striatum), intraclass correlation coefficients were 0.989 and 0.984, respectively. (For further details, see Levitt et al. [26, 39] and the data supplement accompanying the online version of this article.).
Two-Tensor Tractography Postprocessing
The Unscented Kalman Filter-based two-tensor tractography algorithm was used to trace fiber paths throughout the brain (40, 41). Seeding was done in all the voxels where single-tensor fractional anisotropy was greater than 0.18. Each voxel was randomly seeded 10 times, and each generated streamline, interpreted as an estimate of fibers, was traced from seed to termination, with the termination criteria fractional anisotropy as 0.15 for the primary tensor component, which is most consistent with the tracking direction. We then separately extracted from the whole-brain tractography only those streamlines connecting two distinct frontal regions of interest—the rostral middle frontal gyrus representing the dorsolateral prefrontal cortex and the inferior frontal gyrus representing the ventrolateral prefrontal cortex—with two distinct striatal regions of interest—the associative striatum and sensorimotor striatum—yielding four corticostriatal tracts per hemisphere (see Figure 1A and 1B).
We used the diffusion index, fractional anisotropy, to represent the three-dimensional character of the diffusion tensor (42). Fractional anisotropy describes the degree of asphericity of diffusion and is believed to measure white matter microstructural integrity (42–44), as well as representing an indirect measure of the strength of structural connectivity (45, 46). It was computed at each point along the streamline for all selected tract bundles yielding a mean fractional anisotropy for each tract. In addition to estimating the microstructural diffusion measure fractional anisotropy, we also estimated fiber counts correcting for intracranial contents using normalized streamline counts, as a measure of anatomical, macrostructural connectivity. To correct for head motion, we have carefully designed an in-house script that utilizes the FSL software framework (47) to spatially register (rigid registration) each of the diffusion-weighted images to the first nondiffusion-weighted (b=0) image and subsequently updated the corresponding gradient directions to account for head motion. We also conducted a statistical test with the motion parameters obtained for both the groups and found no significant difference between the head motion parameters. Thus, we believe that motion is unlikely to be a contributor to group differences observed in our analysis. The multitensor tractography algorithm (40) was tested on several data sets, and the tracts obtained were manually inspected to ensure that the fibers enter into the neocortical and subcortical gray matter regions. Based on these tests, the termination criterion was set to fractional anisotropy <0.15 for the primary tensor as described in detail by Malcolm et al. (40).
Statistical Analysis
A mixed-model analysis of variance (ANOVA) was used, with diagnostic group as the between-subjects factor and hemisphere (left, right) and tract as the within-subjects factors, using standardized residuals to control for head size for streamline counts but not for fractional anisotropy. Because we performed two mixed-model ANOVAS, we used the false-discovery rate correction for multiple comparisons. Higher standardized residual streamline counts represent higher streamline counts relative to intracranial contents. Because streamline counts are influenced by the overall number of seed points and larger intracranial contents have more seed points, it is important to control for intracranial contents when calculating streamline counts. Conversely, fractional anisotropy represents a mean number for each streamline and thus is not influenced by overall intracranial contents. Tract-specific streamline count standardized residuals and tract-specific measures of white matter integrity (fractional anisotropy) were the dependent measures used in this model. In the case of a significant main effect or interaction, post hoc t tests were used with significance set at a p value <0.05 (two-tailed) to compare group mean differences, separately for the left and right hemispheres. Post hoc t tests based on the mixed-model ANOVAs were performed to explore the source of the significant fractional anisotropy and normalized streamline effects in schizophrenia. Corrections for multiple comparisons for the post hoc t tests were not used, since these analyses were considered “justified” based on the mixed-model ANOVAs being significant and their consistency with a priori hypotheses. Associations between normalized streamline counts and fractional anisotropy and cognitive, clinical, and demographic measures were evaluated using Spearman’s rho correlation coefficients, with two-tailed p values. To minimize the occurrence of multiple testing, we tested correlations only in those frontostriatal tracts (for fractional anisotropy, two tracts; for normalized streamline count, three tracts) that showed significant group differences in the post hoc protected t tests (p<0.05).
Results
Measures of Diffusivity and Streamline Counts in Associative Loop Tracts
In order to test for group differences in fractional anisotropy in the four tracts of the associative striatum loop, a mixed-model ANOVA was performed with group as the between-subjects factor and side (left, right) and tract (dorsolateral prefrontal cortex-associative striatum, ventrolateral prefrontal cortex-associative striatum, dorsolateral prefrontal cortex-sensorimotor striatum, ventrolateral prefrontal cortex-sensorimotor striatum) as the within-subjects factors. Results showed a main effect for group for fractional anisotropy (F=5.5, df=1, 50, p=0.023). We did not find a significant group-by-tract type (segregated compared with integrative) interaction. Post hoc t tests showed reduced fractional anisotropy in the left ventrolateral prefrontal cortex-associative striatum (t=2.0, df=50, p=0.047) and left ventrolateral prefrontal cortex-sensorimotor striatum (t=2.4, df=50, p=0.019) in chronic schizophrenia patients compared with healthy control subjects (Figure 2A).
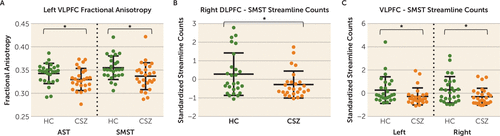
FIGURE 2. Scatter Plots of Fractional Anisotropy and Streamline Counts in Frontostriatal Tractsa
a Panel A shows a scatter plot of fractional anisotropy in the healthy control group (HC) and the patients with chronic schizophrenia (CSZ) in the left-hemisphere ventrolateral prefrontal cortex-associative striatum (LVLPFC-AST) and left-hemisphere ventrolateral prefrontal cortex-sensorimotor striatum (LVLPFC-SMST) tracts. Panel B shows a scatter plot of standardized streamline counts (i.e., count residuals) in the control subjects and patients in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum (DLPFC-SMST) tract. Panel C shows a scatter plot of standardized streamline counts (i.e., count residuals) in the control subjects and patients in left- and right-hemisphere VLPFC-SMST tracts. Higher standardized residual streamline counts represent higher streamline counts relative to intracranial contents. The wide bars represent means. Error bars represent standard deviations. Asterisks represent significant group differences (p<0.05).
Additionally, we performed the same analyses for streamline count standardized residuals. There was no significant group difference in intracranial contents in schizophrenia patients compared with healthy control subjects (mean=1545.1 [SD=132.3] compared with mean=1572.1 [SD=128.9]; t=0.75, df=50, p=0.46). Results showed a main effect for group (F=4.65, df=1, 50, p=0.036), with chronic schizophrenia patients showing fewer streamline counts. Again, we did not find a significant group-by-tract type (segregated compared with integrative) interaction. Post hoc t tests showed fewer streamlines, corrected for intracranial contents, in the right dorsolateral prefrontal cortex-sensorimotor striatum (t=2.1, df=42.36, p=0.042) (Figure 2B) and in the left and right ventrolateral prefrontal cortex-sensorimotor striatum (t=2.0, df=50, p=0.048; t=2.2, df=50, p=0.030) in chronic schizophrenia patients compared with healthy control subjects (Figure 2C). We applied the false-discovery rate correction for multiple comparisons, since we performed two mixed-model ANOVA comparisons, one for fractional anisotropy (p=0.023) and one for streamline counts (p=0.036). With a false-discovery rate p value criterion of 0.05, we found that both group differences remained statistically significant.
Cognitive and Clinical Correlations With Associative Striatum Loop Fractional Anisotropy and Streamline Count Residuals
For the neuropsychological tests, in order to test for group differences in the Trail-Making Test Part A and Part B performance times, a mixed-model ANOVA was performed with group as the between-subjects factor and task (Part A and Part B) as within-subjects factors. Results showed a main effect for group for performance times (F=19.5, df=1, 40, p≤0.001) with a group-by-task interaction (F=7.4, df=1, 40, p=0.010). Post hoc t tests showed that chronic schizophrenia patients performed more poorly than healthy control subjects, with longer time scores on both the Trail-Making Test, Part A (mean=52.5 [SD=20.4], N=25, compared with mean=29.9 [SD=12.2], N=17, t=–4.50, df=39.5, p<0.001) and Part B (mean=125.1 [SD=62.9], N=25, compared with mean=64.0 [SD=19.5], N=17, t=–4.55, df=30.4, p<0.001). Additionally, chronic schizophrenia patients, compared with healthy control subjects, showed significantly greater time-change scores when Trail-Making Test, Part A, scores were subtracted from scores on Part B (mean=72.6 [SD=56.4], N=25, compared with mean=34.1 [SD=17.9], N=17, t=–3.18, df=30.7, p=0.003) (Table 1).
We then correlated streamline count residuals in both groups in the three tracts showing post hoc t test group differences with Trail-Making Test Part A and Part B time-spent scores and Part B minus Part A time-change scores. We found in healthy control subjects that only the right-hemisphere streamline count residuals in the dorsolateral prefrontal cortex-sensorimotor striatum integrative tract negatively correlated with any measure. Specifically, we found that in healthy control subjects, it correlated with Part B time spent (rho=−0.51, p=0.037; N=17) (Figure 3) but not with Part A time spent (p=0.52). Furthermore, when we subtracted Part A scores from Part B scores, adjusting for processing speed, we found in healthy control subjects that Part B minus Part A change scores, again, negatively correlated only with right-hemisphere streamline count residuals in the dorsolateral prefrontal cortex-sensorimotor striatum integrative tract (rho=−0.50, p=0.04; N=17). Conversely, in schizophrenia patients, streamline count residuals in none of the three above tracts correlated with either Part A or Part B time spent or with Part B minus Part A time-spent change scores.
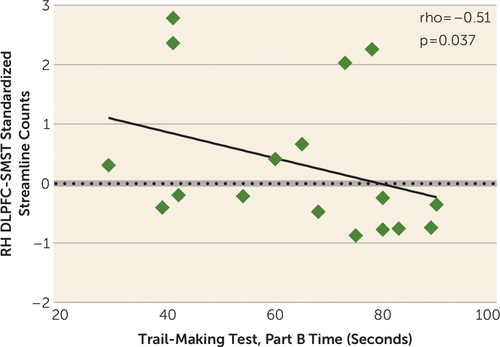
FIGURE 3. Scatter Plot of the Correlation Between the Right-Hemisphere Dorsolateral Prefrontal Cortex-Sensorimotor Striatum (RH DLPFC-SMST) Standardized Streamline Counts (i.e., Count Residuals) and Trail-Making Test, Part B, Time Scores in Healthy Control Subjectsa
a The black line represents the best linear-fit for the data. (Although Spearman’s correlations were used for testing statistical significance, a regression line has been plotted for the convenience of the reader.)
We also correlated fractional anisotropy in the two tracts (left ventrolateral prefrontal cortex-associative and left ventrolateral prefrontal cortex-sensorimotor striatum) that showed post hoc group differences with our Trail-Making Test time scores. In both healthy control subjects (N=17) and schizophrenia patients (N=25), fractional anisotropy in neither tract significantly correlated with either Part A or Part B time spent or with Part B minus Part A time-spent change scores.
For chronic schizophrenia patients, we did not find significant correlations of the clinical PANSS positive, negative, general, or overall scores (N=25) or duration of illness (N=22) with the post hoc fractional anisotropy and streamline counts showing group differences.
Discussion
There are three principal findings in this study. First, we show reduced structural connectivity between the dorsolateral and ventrolateral associative cortex and the striatum in chronic schizophrenia as manifested by reduced fractional anisotropy, a measure of microstructural white matter integrity, and reduced normalized streamline counts, a measure of anatomical, macrostructural connectivity. Specifically, we show reduced fractional anisotropy in the left-hemisphere ventrolateral prefrontal cortex-associative striatum segregated tract and in the left-hemisphere ventrolateral prefrontal cortex-sensorimotor striatum integrative tract and a reduction in normalized streamline counts in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum segregated tract and in the left- and right-hemisphere ventrolateral prefrontal cortex-sensorimotor striatum integrative tracts. Second, in healthy control subjects, but not in chronic schizophrenia patients, we show that reduced streamline counts, corrected for intracranial contents, in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum integrative tract predicted worse performance on a measure of cognitive control, the Trail-Making Test, Part B. Third, against our prediction, we did not show a more pronounced group difference in integrative compared with segregated tracts (i.e., an imbalance in the degree of disruption in the type of corticostriatal tract input in chronic schizophrenia patients compared with healthy control subjects).
Our finding of reduced striatal structural connectivity in the associative loop in schizophrenia is noteworthy because the associative loop has gained in importance in understanding the pathophysiology of schizophrenia. For example, it has been demonstrated with positron emission tomography studies that there is excessive presynaptic release of dopamine both in acutely exacerbated chronic schizophrenia patients and in prodromal schizophrenia patients, which occurs, in particular, in the associative striatum subregion of the striatum (12–14).
It is also noteworthy that an important glutamatergic-dopaminergic interaction occurs at corticostriatal terminals in the brain. It has been shown that dopamine release at corticofugal glutamatergic synapses in the striatum decreases corticostriatal glutamate release via D2 receptors, which serve as inhibitory presynaptic heteroreceptors on corticostriatal terminals (48). Thus, decreased frontostriatal input from glutamatergic tracts projecting to the striatum in schizophrenia combined with excessive presynaptic dopamine release at the striatum in schizophrenia might compromise information processing via diminished corticostriatal glutamatergic neurotransmission in frontostriatal pathways in schizophrenia. Moreover, since increased dopamine release in schizophrenia targets the associative striatum in particular (13, 14), one could speculate further that disrupted processing of information through corticostriatal loops would especially affect the associative loop and cognitive function influenced by this loop, such as executive function cognitive control. Further confirmation of this potential interaction between glutamate and dopamine is needed in future studies.
Behaviorally, we found poorer performance on both the Trail-Making Test, Part A, and Trail-Making Test, Part B, and in the change scores, in schizophrenia patients compared with healthy control subjects. By subtracting Part A time scores from Part B time scores, we adjusted for an individual’s processing speed and isolated the extra time it took an individual to perform a similar sensorimotor task but with the added cognitive complexity of Part B. Part B represents a dual-attention task shifting between numbers and letters. It requires the use of cognitive control with resisting interference. In order to perform the task speedily, the participant needs to rapidly inhibit the default response to go from a number to the next highest number and similarly to go from a letter to the next highest letter. Our data show that schizophrenia patients had even more difficulty performing the more cognitively challenging Part B task because there was a group-by-task interaction. Of importance regarding our brain-behavior correlations, it is the Part B cognitive control task and not the Part A processing speed task that correlates with frontostriatal connectivity in healthy control subjects.
This brain-behavior relationship is consistent with anatomic studies in rodents and humans linking the associative striatum to goal-directed behavior (16–18), which requires cognitive control. That we found this correlation in healthy control subjects but not in schizophrenia patients may suggest that the disrupted frontostriatal connectivity that we demonstrate in schizophrenia may also disrupt brain-behavior associations that should be present. That is, there is a loss of a brain-behavior association in schizophrenia that should be present were there not diminished connectivity in this key neural pathway for goal-directed behavior.
Moreover, that we found a correlation between normalized streamline counts in the dorsolateral prefrontal cortex-sensorimotor striatum integrative tract and performance on the Trail-Making Test, Part B, suggests that the dorsolateral prefrontal cortex mediates its effect on this cognitive control task through its projections to the sensorimotor subregion of the striatum, an integrative tract. That our correlation occurred with this tract emphasizes the potential behavioral importance of functionally integrative, corticostriatal connections. Integrative tracts project to striatal subregions that also receive tracts from other functional cortical areas. For example, the sensorimotor striatum receives functionally segregated tracts from the sensorimotor cortex and functionally integrative tracts from the associative cortex (Figure 1A). Indeed, Averbeck et al. (23) have suggested that portions of the striatum that receive input from multiple prefrontal cortical regions might serve as connection hubs where higher-level cognitive processing and integration of information from multiple systems can occur. In a similar vein, Haber and Calzavara (25) have noted that a requirement for learning is the “integration of inputs related to emotional, cognitive, and motor cortical functions.” Furthermore, they have proposed that parallel and integrative tracts in combination permit “coordinated behaviors to be maintained and focused (via parallel networks), but also to be modified and changed according to the appropriate external and internal stimuli (via integrative networks).” That is, maintaining acquired skills might occur in striatal regions receiving parallel, segregated corticostriatal inputs, whereas adaptation and learning new skills might occur in hub regions receiving converging, integrative corticostriatal inputs. Lastly, that our finding was in the right hemisphere is logical in that Parts A and B of the Trail-Making Test are visual scanning attention tasks, and visuospatial attention is believed to be a right-hemisphere function (33).
Furthermore, the frontoparietal cognitive control network, which is a neural substrate for cognitive control tasks such as the Trail-Making Test, Part B, is comprised of nodes that include the dorsolateral prefrontal cortex and the inferior parietal cortex based on resting-state functional MRI (fMRI) functional connectivity analyses (49). Additionally, Choi et al. (50), again based on resting-state fMRI, showed that the dorsolateral prefrontal cortex is functionally connected (i.e., temporally correlates, or coactivates) with both the associative striatum in the dorsal caudate and with the sensorimotor striatum in the posterior putamen. Because the dorsolateral prefrontal cortex and the sensorimotor striatum (the posterior putamen) are both components of the frontoparietal control network, disrupting structural connectivity between them (i.e., in the dorsolateral prefrontal cortex-sensorimotor striatum tract) would be expected to impair cognitive control performance, which is what we show in our healthy control subjects. Moreover, in a small, longitudinal study of individuals at ultra-high risk for psychosis who were scanned twice, it was reported that those ultra-high risk individuals who developed psychosis by scan 2 showed an increase in dopamine synthesis capacity in the sensorimotor striatum (51). This study additionally suggests that the sensorimotor striatum, as well as associative striatum, is a potential region of interest in schizophrenia for further investigation.
It is noteworthy that in the present study, tracts projecting from the sensorimotor cortex to the sensorimotor striatum were not explored, since we previously showed diffusion abnormalities in pathways projecting from the associative cortex to the whole, undivided striatum in first-episode schizophrenia (15). Here, we therefore similarly focused on associative, but not sensorimotor, cortical projections to the striatum. Nonetheless, considering the present findings that show diffusion abnormalities in associative cortical projections to the sensorimotor striatum in chronic schizophrenia, it would be of interest in a future study to also examine tracts from the sensorimotor cortex to the sensorimotor striatum.
Limitations of this study include the potential confound of neuroleptic medication, including that of cumulative antipsychotic exposure, on our measurements of frontostriatal tracts. However, since axons do not express receptors targeted by neuroleptic medication, such as dopamine and 5-HT receptors (52), the structural effect of neuroleptic medication on white matter pathways is not readily apparent. Future studies of white or gray matter in schizophrenia patients should differentiate between those taking atypical neuroleptics and those taking typical neuroleptics, although we point out that not all atypical neuroleptic medications resemble one another in their effect on gray matter brain structures (26, 53, 54). Moreover, to lessen the confounds of medication and illness chronicity, these studies should be replicated in early-onset and neuroleptic-naive prodromal schizophrenia patients, as well as in longitudinal studies that enable the assessment of the trajectory of brain changes in schizophrenia over the course of the illness.
A further potential limitation of our study is that in order not to reduce power, we did not explore all potential non-illness-related variables as covariates unless they reached a p value <0.05, which prevented us from fully exploring all potential covariates.
In summary, we found reduced frontostriatal structural connectivity in the associative loop of the striatum in chronic schizophrenia as reflected by a reduction in fractional anisotropy and streamline counts in both functionally segregated and integrative pathways. Such diminished frontostriatal connectivity in the associative loop in combination with hyperdopaminergia targeting the associative loop may especially compromise associative loop information processing in schizophrenia. In addition, we report in healthy control subjects, but not schizophrenia patients, the novel brain-behavior correlation that increased streamline counts in the right-hemisphere associative loop dorsolateral prefrontal cortex-sensorimotor striatum integrative tract predicted better performance on the Trail-Making Test, Part B, cognitive control measure. This finding is consistent with numerous studies in both rodents and humans demonstrating a role for the associative striatum in goal-directed behavior.
1 : Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990; 13:266–271Crossref, Medline, Google Scholar
2 : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
3 : Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Crossref, Medline, Google Scholar
4 : Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 2008; 28:7143–7152Crossref, Medline, Google Scholar
5 : The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 2003; 26:317–330Crossref, Medline, Google Scholar
6 : The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859–876Crossref, Medline, Google Scholar
7 : The neostriatum beyond the motor function: experimental and clinical evidence. Neuroscience 1997; 78:39–60Crossref, Medline, Google Scholar
8 : Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
9 : A diffusion tensor imaging study of the anterior limb of the internal capsule in schizophrenia. Psychiatry Res 2010; 184:143–150Crossref, Medline, Google Scholar
10 : MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry 2002; 159:1190–1197Link, Google Scholar
11 : Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry 2013; 74:106–112Crossref, Medline, Google Scholar
12 : Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 2011; 168:1311–1317Link, Google Scholar
13 : Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 2009; 66:13–20Crossref, Medline, Google Scholar
14 : Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67:231–239Crossref, Medline, Google Scholar
15 : White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res 2013; 145:1–10Crossref, Medline, Google Scholar
16 : Control of goal-directed and stimulus-driven attention in the brain. Nature Rev 2002; 3:201–215Crossref, Medline, Google Scholar
17 : Habits, rituals, and the evaluative brain. Annu Rev Neurosci 2008; 31:359–387Crossref, Medline, Google Scholar
18 : Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nature Rev 2010; 11:760–772Crossref, Medline, Google Scholar
19 : Schizophrenia, in Schizophrenia, 3rd ed. Edited by Weinberger DR, Harrison PJ. Chichester, West Sussex, United Kingdom, Blackwell Publishing, 2011, pp 3–8Google Scholar
20 : Schizophrenia. Lancet 2009; 374:635–645Crossref, Medline, Google Scholar
21 : Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 2004; 55:522–529Crossref, Medline, Google Scholar
22 : Convergence of Limbic, Cognitive, and Motor Cortico-Striatal Circuits with Dopamine Pathways in Primate Brain, in Dopamine Handbook. Edited by Iversen LL, Iversen SD, Dunnett SB, . Oxford, United Kingdom, Oxford University Press, 2010, pp 38–48Google Scholar
23 : Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci 2014; 34:9497–9505Crossref, Medline, Google Scholar
24 : The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J Neurosci 2013; 33:5718–5727Crossref, Medline, Google Scholar
25 : The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 2009; 78:69–74Crossref, Medline, Google Scholar
26 : A volumetric MRI study of limbic, associative and sensorimotor striatal subregions in schizophrenia. Schizophr Res 2013; 145:11–19Crossref, Medline, Google Scholar
27 : Dementia Praecox and Paraphrenia. Edinburgh, Scotland, Livingstone, 1919Google Scholar
28 : Neuropsychology of reward learning and negative symptoms in schizophrenia. Schizophr Res 2014; 159:506–508Crossref, Medline, Google Scholar
29 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, Biometrics Research, New York State Psychiatric Institute, 2002Google Scholar
30 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, Biometrics Research, New York State Psychiatric Institute, 2002Google Scholar
31 : The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
32 : Two-Factor Index of Social Position. New Haven, Conn, Yale Station, 1965Google Scholar
33 : Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
34 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
35 : Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14:11–22Crossref, Medline, Google Scholar
36 : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1):S208–S219Crossref, Medline, Google Scholar
37 : An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–980Crossref, Medline, Google Scholar
38 : Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res 2010; 123:153–159Crossref, Medline, Google Scholar
39 : Fractional anisotropy and radial diffusivity: Diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr Res 2012; 136:55–62Crossref, Medline, Google Scholar
40 : Filtered multitensor tractography. IEEE Trans Med Imaging 2010; 29:1664–1675Crossref, Medline, Google Scholar
41 : Tensor kernels for simultaneous fiber model estimation and tractography. Magn Reson Med 2010; 64:138–148Crossref, Medline, Google Scholar
42 : Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed 2002; 15:456–467Crossref, Medline, Google Scholar
43 : A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 2007; 41:15–30Crossref, Medline, Google Scholar
44 : Diffusion tensor imaging findings and their implications in schizophrenia. Curr Opin Psychiatry 2014; 27:179–184Crossref, Medline, Google Scholar
45 : Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108:3–10Crossref, Medline, Google Scholar
46 : Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry 2013; 26:172–187Crossref, Medline, Google Scholar
47 : Neuroimage 2012; 62:782–790Crossref, Medline, Google Scholar
48 : Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 2004; 42:653–663Crossref, Medline, Google Scholar
49 : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106:1125–1165Crossref, Medline, Google Scholar
50 : The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 2012; 108:2242–2263Crossref, Medline, Google Scholar
51 : Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry 2011; 16:885–886Crossref, Medline, Google Scholar
52 : Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 2014; 344:319–324Crossref, Medline, Google Scholar
53 : Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68:128–137Crossref, Medline, Google Scholar
54 : A Selective Review of Volumetric and Morphometric Imaging in Schizophrenia. Edited by Swerdlow N. New York, Springer, 2010Crossref, Google Scholar



