Randomized, Double-Blind, Placebo-Controlled Trial of Asenapine Maintenance Therapy in Adults With an Acute Manic or Mixed Episode Associated With Bipolar I Disorder
Abstract
Objective:
The authors determined the efficacy and safety of asenapine in preventing recurrence of any mood episode in adults with bipolar I disorder.
Method:
Adults with an acute manic or mixed episode per DSM-IV-TR criteria were enrolled in this randomized, placebo-controlled trial consisting of an initial 12- to 16-week open-label period and a 26-week double-blind randomized withdrawal period. The target asenapine dosage was 10 mg b.i.d. in the open-label period but could be titrated down to 5 mg b.i.d. After completing the open-label period, subjects meeting stabilization/stable-responder criteria were randomized to asenapine or placebo treatment in the double-blind period. The primary efficacy endpoint was time to recurrence of any mood event during the double-blind period. Kaplan-Meier estimation was performed, and 95% confidence intervals were determined. Safety was assessed throughout.
Results:
A total of 549 subjects entered the open-label period, of whom 253 enrolled in the double-blind randomized withdrawal period (127 in the placebo group; 126 in the asenapine group). Time to recurrence of any mood episode was statistically significantly longer for asenapine- than placebo-treated subjects. In post hoc analyses, significant differences in favor of asenapine over placebo were seen in time to recurrence of manic and depressive episodes. The most common treatment-emergent adverse events were somnolence (10.0%), akathisia (7.7%), and sedation (7.7%) in the open-label period and mania (11.9% of the placebo group compared with 4.0% of the asenapine group) and bipolar I disorder (6.3% compared with 1.6%) in the double-blind period.
Conclusions:
Long-term treatment with asenapine was more effective than placebo in preventing recurrence of mood events in adults with bipolar I disorder and was generally well-tolerated.
Bipolar I disorder is a serious chronic condition that requires acute and long-term management of mood episodes. In clinical practice, the focus during acute treatment of a mood episode is to reduce manic or depressive symptoms while aiming to avoid triggering new symptoms of the opposite polarity. After acute symptoms are stabilized, a major goal for long-term treatment is prevention of mood episodes of any polarity (1–3).
Although index episodes in bipolar I disorder may present as classic manic or depressive episodes, a substantial number of patients may experience symptoms of both polarities at the same time (e.g., mixed episodes, manic or depressive episodes with mixed features) (4). Moreover, available treatments for acute and long-term treatment appear to have different clinical efficacy profiles depending on the patient and the predominant symptom polarity (1). Acute manic symptoms can be treated by various antipsychotic or anticonvulsant medications, although few drugs have proven efficacy in the treatment of bipolar depression. In long-term treatment, clinical effects on the two polarities also appear to differ among compounds, indicating that some compounds may be more effective in controlling symptoms from the manic pole (e.g., aripiprazole) (5) and others from the depressive pole (e.g., lamotrigine) (6, 7). A polarity index has been proposed to characterize the profile of individual agents based on available data (8). Ideally, a compound would control symptoms of both polarities, both acutely and in the long term; however, few treatments have demonstrated this profile (9, 10). Many individuals are treated symptomatically with multiple compounds, as reflected in current treatment guidelines. There is a need for additional treatment options with empirical data from adequate clinical trials to guide clinical practice.
Asenapine—an atypical antipsychotic with a distinct pharmacological profile that differs from other approved compounds, formulated as a fast-dissolving, rapidly absorbed sublingual tablet—is approved by the U.S. Food and Drug Administration for acute treatment of adult and pediatric patients with bipolar mania and for acute and maintenance treatment of adults with schizophrenia (11). Asenapine exhibits high affinity for various serotonin, dopamine, α-adrenergic, and histamine receptors, with no appreciable affinity for muscarinic cholinergic receptors (12). The efficacy of asenapine monotherapy was demonstrated in acute trials in subjects with manic or mixed episodes (13, 14). Our objective was to investigate asenapine in preventing the recurrence of any mood episode in subjects with bipolar I disorder in a randomized withdrawal trial consisting of an initial open-label period followed by a randomized, placebo-controlled, double-blind withdrawal period; patients enrolled in double-blind treatment were stable asenapine responders based on prespecified criteria.
Method
Study Design
This phase 3b, randomized, placebo-controlled, double-blind, parallel-group trial evaluated the efficacy and safety of sublingually administered asenapine relative to placebo in the prevention of recurrent mood episodes. Subjects met diagnostic criteria for bipolar I disorder per DSM-IV-TR and were experiencing an acute manic or mixed episode. Following a 12- to 16-week open-label period (asenapine at 5 or 10 mg b.i.d., with a target dosage of 10 mg b.i.d.), patients meeting stable-responder criteria for 8 weeks were randomized to 26 weeks of double-blind treatment (asenapine at 5 or 10 mg b.i.d.) (Figure 1).
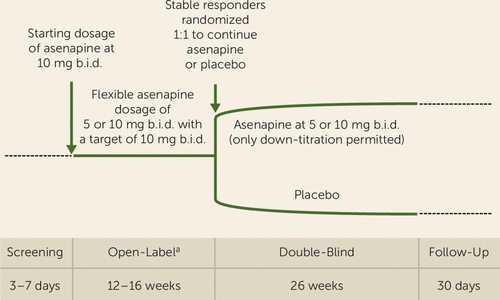
FIGURE 1. Study Design in a Trial of Asenapine for Relapse Prevention in Bipolar I Disorder
a During open-label treatment, subjects were required to be on asenapine as a monotherapy (i.e., previous psychotropic medications already were discontinued during cross-titration) for 4 weeks before a stabilization period of 8 weeks could be evaluated. Patients were required to meet stabilization criteria for 5 consecutive weeks (Young Mania Rating Scale [YMRS] and Montgomery-Åsberg Depression Rating Scale [MADRS] scores ≤12 at weeks 4, 6, 8, 10, and 12; or at weeks 6, 8, 10, 12, and 14; or at weeks 8, 10, 12, 14, and 16) or for 5 of 6 weeks with only one excursion event prior to the last visit in the series (YMRS and MADRS scores ≤12 at weeks 6 and 16 and also three out of four times at weeks 8, 10, 12, or 14).
Subjects were recruited from 87 centers in Bulgaria, Croatia, Romania, the Russian Federation, Serbia, Turkey, Ukraine, the Philippines, India, and the United States. Before trial initiation, documents were approved by appropriate institutional review boards and independent ethics committees. The study was conducted between Jan. 26, 2012, and April 30, 2015, in accordance with guidelines from the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, E6 Good Clinical Practice, and local laws. Written informed consent was obtained from subjects after procedures were explained.
Participants
Subjects were older than 18 years of age with a diagnosis of bipolar I disorder and a current manic (DSM-IV-TR 296.4x) or mixed (296.6x) episode, as determined by the Mini International Neuropsychiatric Interview, version 6.0.0, (15) at screening. The acute episode was confirmed by all of the following: no response (<50% improvement since the beginning of the episode per investigator judgment, based on available sources); marked or substantial change in current symptoms compared with the symptom state before the current episode; need for increased medical attention for worsening symptoms; and bipolar I disorder diagnosis for ≥2 years. Other inclusion criteria were the following: a Young Mania Rating Scale (YMRS) (16) score ≥18, and a score of at least moderately ill (≥4) in both the mania and overall bipolar illness subscales of the Clinical Global Impressions Scale for Bipolar Disorder–Severity (17).
To enter double-blind treatment, subjects were required to complete open-label treatment (12–16 weeks) and fulfill at least one of the following stable-responder criteria: YMRS and Montgomery-Åsberg Depression Rating Scale (MADRS) (18) scores ≤12 at the last five consecutive open-label visits or at five of six consecutive visits with only one missed visit before the last visit in the series.
Subjects were excluded if they had a clinically significant medical or psychiatric condition (other than bipolar I disorder) or abnormal laboratory or physical examination findings that may have interfered with interpretation of safety and efficacy evaluations. Body mass index <18.5 or >40.0, risk of self-harm or harm to others, substance abuse or dependence (in the prior 6 months), or a history of rapid cycling were also exclusionary. Subjects were completely tapered off prohibited psychotropic medications during up to 4 weeks of cross-titration in open-label treatment; benzodiazepines (i.e., lorazepam or diazepam in countries where lorazepam was not approved) were allowed for agitation, irritability, restlessness, insomnia, and hostility (in the open-label and double-blind phases).
Procedures
All subjects and staff were blind to treatment; placebo and asenapine tablets were indistinguishable in appearance. An interactive voice response telephone system was used to obtain a subject identification number, allocate medication kit numbers, randomize subjects to double-blind treatment, and register the end-of-treatment visit.
During open-label treatment, subjects received one placebo tablet and one asenapine tablet (5 mg or 10 mg) at the same time to reduce the risk of unblinding during double-blind treatment. Subjects were not blind to asenapine dose, but they were blind to which tablet was active or placebo. A protocol deviation related to the starting dose occurred during the first 13 months of the study. Namely, the interactive voice response system incorrectly dispensed a starting dosage of 5 mg b.i.d. instead of 10 mg b.i.d. to 207 subjects during open-label treatment; dosing during double-blind treatment was not affected. The potential effect of the dosing error was examined in sensitivity analyses, and it was concluded that the double-blind efficacy results were not affected by the dosing error (data on file). Clinical examination also determined that the dosing error had no impact on the safety of participants during the trial.
Subjects who met stabilization/stable-responder criteria were randomized 1:1 to continue treatment with asenapine or placebo for 26 weeks of double-blind treatment. During double-blind treatment, subjects received only a placebo or asenapine tablet per their randomization assignment.
Sample Size Considerations
A total of 734 subjects were expected to be screened, with 550 subjects treated during open-label treatment and 250 subjects randomized to asenapine or to placebo (1:1) for double-blind treatment. Based on the original assumption of 60% and 40% relapse rates for the placebo and asenapine groups, respectively, the study required 105 relapses and 250 randomized patients (assuming a 10% dropout rate for reasons other than relapse) to achieve 85% power with a 5% significance level by log-rank test (two-sided) to test the difference between the two survival curves.
Relapse rates from previously published bipolar recurrence prevention trials vary considerably and may reflect differences in stabilization and relapse criteria (19–22). In this trial, treatment-blind observation during the double-blind period suggested a substantially lower overall relapse rate than originally expected. Per protocol amendment, our relapse rate assumptions were updated to 34% for the placebo group (hazard ratio=0.45) and to 17% for the asenapine group. In this case, 56 recurrences were needed to achieve 85% power; the required number of randomized subjects remained at 250.
Study Outcomes
The primary efficacy outcome was time to recurrence of any mood event during double-blind treatment. Recurrence was defined as any one of the following: requirement or initiation of nonstudy medication to treat manic, mixed, or depressive symptoms; need for psychiatric hospitalization; study discontinuation because of a mood event; or a total YMRS or MADRS score ≥16. There was no key secondary efficacy endpoint during double-blind treatment; secondary outcomes of interest included rate of recurrence for manic, mixed, and depressive mood episodes and time to discontinuation for any reason.
Predefined safety events of interest based on drug class and previous asenapine studies included ≥7% weight increase from baseline to endpoint, extrapyramidal symptoms, akathisia, somnolence/sedation/hypersomnia combined, dizziness, insomnia, and oral hypoesthesia/dysgeusia. Treatment-emergent adverse events, lipid and metabolic parameters, and suicidality as measured by the Columbia–Suicide Severity Rating Scale (C-SSRS) (23) were also evaluated. Efficacy and tolerability were evaluated at regular study visits during open-label (days 1 and 3 and weeks 1, 2, 4, 6, 8, 10, 12, 14, and 16) and double-blind (weeks 2, 4, 6, 8, 10, 12, 16, 20, 24, and 26) treatment.
Statistical Analysis
Efficacy endpoints during the double-blind period were analyzed in the full analysis set (randomized patients who received ≥1 dose of the double-blind study drug). The Kaplan-Meier method was used to estimate time to first recurrence of any mood episode. The difference in survival curves was evaluated with a two-sided log-rank test with a 5% significance level; overall event rates per group were provided. Cox proportional hazard models with baseline YMRS and MADRS scores as covariates, stratified by country, were used to estimate hazard ratios and 95% confidence intervals (CI). Post hoc analyses were performed for time to first recurrence of manic, mixed, or depressive episodes using the Kaplan-Meier method and the Cox proportional hazard model as described for the primary analysis.
Time (in days from the first double-blind treatment dosage) to early discontinuation for any reason was analyzed using a similar Kaplan-Meier estimation as described for the primary analysis. No adjustment was made for multiplicity testing.
Predefined safety events of special interest (listed earlier) were subject to inferential tests with p values and 95% CIs provided for between-group comparisons. Point estimates with 95% CIs for between-group comparisons were provided for adverse events not prespecified but occurring in four or more subjects in any group or meeting predefined limits of change, including change from baseline to endpoint in fasting glucose, fasting triglycerides, fasting cholesterol, prolactin, fasting insulin, and glycosylated hemoglobin. Descriptive statistics were provided for all other treatment-emergent adverse events.
Results
Subject Disposition and Clinical Characteristics
Patient disposition is presented in Figure 2. During open-label treatment, the most common reasons for discontinuation were adverse events (16.6%) and protocol noncompliance (16.0%). Subjects meeting stabilization criteria were randomized (1:1) to double-blind treatment, and 126 subjects in each group received at least one dose of trial medication. One randomized subject discontinued due to an adverse event before receiving double-blind trial medication. Of the 252 subjects who received the study drug, 171 (67.9%) subjects completed double-blind treatment; the proportion of completers was higher in the asenapine group (80.2%) than in the placebo group (55.6%). The most common reasons for double-blind discontinuation were adverse events (19.8%) and recurrence (14.3%) for placebo-treated subjects, and adverse events (7.1%) and withdrawn consent (5.6%) for asenapine-treated subjects. Demographic and clinical characteristics were similar between groups (Table 1).
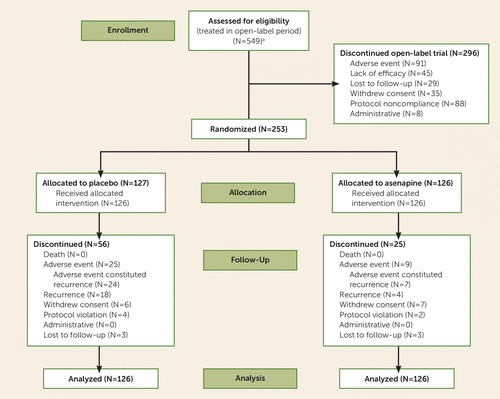
FIGURE 2. Participant Enrollment and Disposition in a Trial of Asenapine for Relapse Prevention in Bipolar I Disorder
a Because of a protocol deviation during open-label treatment, 207 subjects were incorrectly initiated on a starting dosage of asenapine at 5 mg b.i.d. instead of at 10 mg b.i.d. Of these 207 subjects, 82 (39.6%) completed open-label treatment; 28 (13.5%) were discontinued due to lack of efficacy; and 33 (15.9%) were discontinued due to an adverse event, of whom 17 (8.2%) discontinued due to worsening of disease under study.
| Characteristic | Open-Label Perioda | Double-Blind Periodb | ||||
|---|---|---|---|---|---|---|
| Asenapine (N=549) | Placebo (N=126) | Asenapine (N=126) | ||||
| N | % | N | % | N | % | |
| Sex | ||||||
| Male | 239 | 43.5 | 61 | 48.4 | 53 | 42.1 |
| Female | 310 | 56.5 | 65 | 51.6 | 73 | 57.9 |
| Race | ||||||
| White | 383 | 69.8 | 79 | 62.7 | 87 | 69.0 |
| Black | 73 | 13.3 | 14 | 11.1 | 14 | 11.1 |
| Other | 93 | 16.9 | 33 | 26.2 | 25 | 19.8 |
| Region | ||||||
| United States | 229 | 41.7 | 36 | 28.6 | 38 | 30.2 |
| Outside the United States | 320 | 58.3 | 90 | 71.4 | 88 | 69.8 |
| DSM-IV-TR diagnosis of current episodes | ||||||
| Manic | 383 | 69.8 | 103 | 81.7 | 94 | 74.6 |
| Mixed | 166 | 30.2 | 23 | 18.3 | 32 | 25.4 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 41.8 | 12.9 | 40.9 | 13.5 | 42.8 | 12.7 |
| Weight (kg) | 78.1 | 17.0 | 75.3c | 15.1 | 79.4c | 17.6 |
| Body mass index | 27.5 | 4.9 | 26.9c | 4.5 | 28.0c | 5.3 |
| Baseline scores | ||||||
| Young Mania Rating Scale | 27.6 | 5.5 | 3.7c | 2.9 | 4.0c | 2.9 |
| Montgomery-Åsberg Depression Rating Scale | 10.9 | 7.7 | 2.3c | 2.8 | 2.2c | 2.7 |
| Clinical Global Impressions Scale for Bipolar Disorder–Severity | 4.5 | 0.6 | 1.6c | 0.6 | 1.7c | 0.7 |
TABLE 1. Baseline Characteristics of Participants in a Trial of Asenapine for Relapse Prevention in Bipolar I Disorder
During open-label treatment, the mean daily asenapine dose was 15.2 mg (SD=4.9); mean medication compliance was 97.74%. During double-blind treatment, the mean daily asenapine dose was 16.6 mg (SD=4.6); mean medication compliance was 101.02% (more total doses taken than prescribed) and 99.58% in the placebo and asenapine groups, respectively.
Efficacy Outcomes
Kaplan-Meier and Cox regression analyses (Figure 3A) demonstrated that time to recurrence of any mood episode was significantly longer in asenapine-treated subjects relative to placebo-treated subjects (log-rank test p<0.0001; hazard ratio=0.22, 95% CI=0.11–0.43; number needed to treat=5) (Figure 3A). The most common predefined indicator of recurrence in both treatment groups was a YMRS and/or an MADRS score ≥16 (30.2% in the placebo group compared with 8.7% in the asenapine group). Other predefined indicators of recurrence among subjects treated with placebo compared with those treated with asenapine, respectively, were the requirement or initiation of any nonstudy medication to treat mixed, manic, or depressive symptoms (23.8% compared with 5.6%), discontinuation from the study due to mood event (23.0% compared with 5.6%), and the need for psychiatric hospitalization (4.8% compared with 1.6%).
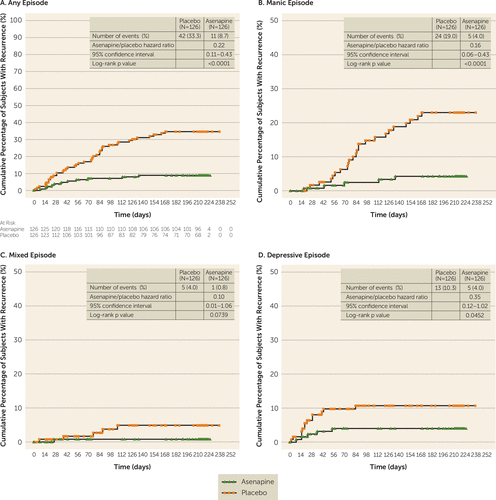
FIGURE 3. Efficacy Outcomes in a Trial of Asenapine for Relapse Prevention in Bipolar I Disordera
a Panel A depicts time to recurrence of any mood episode in the double-blind treatment period. Panel B depicts time to recurrence of a first manic episode in the double-blind treatment period. Panel C depicts time to recurrence of a first mixed episode in the double-blind treatment period. Panel D depicts time to recurrence of a first depressive episode in the double-blind treatment period.
Post hoc analyses demonstrated that time to recurrence of a manic or depressive episode was significantly longer in the asenapine group relative to the placebo group (hazard ratio for manic episode=0.16, 95% CI=0.06–0.43, p<0.0001, number needed to treat=7; hazard ratio for depressive episode=0.35, 95% CI=0.12–1.02, p=0.0452, number needed to treat=16) (Figure 3B and 3D). Time to recurrence of a mixed episode was also longer in asenapine-treated subjects relative to placebo-treated subjects, but the difference was not statistically significant (hazard ratio=0.10, 95% CI=0.01–1.06, p=0.0739, number needed to treat=32) (Figure 3C).
Based on Kaplan-Meier and Cox regression analyses, the time to early discontinuation for any reason was significantly longer among subjects who received asenapine compared with placebo (log-rank test p<0.0001, hazard ratio=0.34, 95% CI=0.21–0.55); approximately 20% of asenapine-treated patients discontinued by day 180, compared with 20% of placebo-treated patients by day 70.
Safety Outcomes
Open-label period.
Asenapine was generally well tolerated in the open-label period (Table 2). One 50-year-old woman (70.0 kg, 169.0 cm) had a fatal serious adverse event of cardiac arrest. No signs of electrocardiographic abnormality were detected at screening, and the event was not considered related to study medication.
| Adverse Event | Open-Label Period | Double-Blind Period | ||||
|---|---|---|---|---|---|---|
| Asenapine (N=549) | Placebo (N=126) | Asenapine (N=126) | ||||
| N | % | N | % | N | % | |
| Subjects with adverse events | ||||||
| ≥1 treatment-emergent adverse event | 358 | 65.2 | 64 | 50.8 | 60 | 47.6 |
| Serious adverse event | 30 | 5.5 | 11 | 8.7 | 6 | 4.8 |
| Discontinued due to adverse event | 92 | 16.8 | 32 | 25.4 | 9 | 7.1 |
| Treatment-emergent adverse event leading to death | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| Subjects with predefined treatment-emergent adverse events of interest | ||||||
| Somnolence/sedation/hypersomnia combined | 99 | 18.0 | 1 | 0.8 | 1 | 0.8 |
| Extrapyramidal symptoms | 55 | 10.0 | 1 | 0.8 | 3 | 2.4 |
| Oral hypoesthesia combined with dysgeusia | 53 | 9.7 | 0 | 0.0 | 0 | 0.0 |
| ≥7% increase in weight from baseline to endpoint | 51 | 10.3 | 9 | 7.1 | 10 | 7.9 |
| Akathisia | 42 | 7.7 | 1 | 0.8 | 2 | 1.6 |
| Dizziness | 26 | 4.7 | 0 | 0.0 | 1 | 0.8 |
| Insomnia | 25 | 4.6 | 6 | 4.8 | 2 | 1.6 |
| Treatment-emergent adverse events occurring in ≥2% of subjects | ||||||
| Somnolence | 55 | 10.0 | 1 | 0.8 | 0 | 0.0 |
| Akathisia | 42 | 7.7 | 1 | 0.8 | 2 | 1.6 |
| Sedation | 42 | 7.7 | 0 | 0.0 | 0 | 0.0 |
| Accidental overdose | 40 | 7.3 | 2 | 1.6 | 2 | 1.6 |
| Oral hypoesthesia | 33 | 6.0 | 0 | 0.0 | 0 | 0.0 |
| Headache | 32 | 5.8 | 2 | 1.6 | 3 | 2.4 |
| Dizziness | 26 | 4.7 | 0 | 0.0 | 1 | 0.8 |
| Insomnia | 25 | 4.6 | 6 | 4.8 | 2 | 1.6 |
| Dysgeusia | 24 | 4.4 | 0 | 0.0 | 0 | 0.0 |
| Nausea | 23 | 4.2 | 0 | 0.0 | 1 | 0.8 |
| Weight increased | 21 | 3.8 | 1 | 0.8 | 6 | 4.8 |
| Increased appetite | 19 | 3.5 | 0 | 0.0 | 1 | 0.8 |
| Mania | 18 | 3.3 | 15 | 11.9 | 5 | 4.0 |
| Vomiting | 17 | 3.1 | 0 | 0.0 | 0 | 0.0 |
| Fatigue | 16 | 2.9 | 0 | 0.0 | 0 | 0.0 |
| Agitation | 14 | 2.6 | 1 | 0.8 | 0 | 0.0 |
| Bipolar disorder | 13 | 2.4 | 4 | 3.2 | 0 | 0.0 |
| Depression | 13 | 2.4 | 6 | 4.8 | 3 | 2.4 |
| Oral paresthesia | 13 | 2.4 | 0 | 0.0 | 0 | 0.0 |
| Anxiety | 11 | 2.0 | 3 | 2.4 | 2 | 1.6 |
| Dry mouth | 11 | 2.0 | 0 | 0.0 | 0 | 0.0 |
| Nasopharyngitis | 7 | 1.3 | 2 | 1.6 | 3 | 2.4 |
| Upper respiratory tract infection | 5 | 0.9 | 5 | 4.0 | 1 | 0.8 |
| Bipolar I disorder | 4 | 0.7 | 8 | 6.3 | 2 | 1.6 |
| Weight decreased | 2 | 0.4 | 3 | 2.4 | 1 | 0.8 |
| Diabetes mellitus | 1 | 0.2 | 0 | 0.0 | 3 | 2.4 |
TABLE 2. Summary of Treatment-Emergent Adverse Events in a Trial of Asenapine for Relapse Prevention in Bipolar I Disorder
The most common adverse events leading to discontinuation were related to a worsening of the underlying condition (mania=13 subjects [2.4%]; bipolar disorder=12 subjects [2.2%]). The most common treatment-emergent adverse events were somnolence (55 [10.0%]), akathisia (42 [7.7%]), sedation (42 [7.7%]), accidental overdose (40 [7.3%]), oral hypoesthesia (33 [6.0%]), and headache (32 [5.8%]). Of the predefined treatment-emergent adverse events of interest, somnolence/sedation/hypersomnia was most frequent (99 [18.0%]), followed by clinically significant weight gain (≥7% increase) (51 [10.3%]), extrapyramidal symptoms (55 [10.0%]), oral hypoesthesia/dysgeusia (53 [9.7%]), and akathisia (42 [7.7%]). Relatively few subjects met predefined limits of change for lipid and endocrine parameters (prolactin, fasting total cholesterol, fasting triglycerides, fasting glucose, and glycosylated hemoglobin) during both the open-label and stabilization periods (see Table S1 in the data supplement that accompanies the online edition of this article). Fasting glucose was the only parameter in which ≥5% of subjects met predefined limits of change (6.4%).
Double-blind period.
The most common adverse events leading to discontinuation were mania, bipolar I disorder, and depression, all of which occurred more frequently in the placebo group than in the asenapine group (10.3% compared with 3.2%, 5.6% compared with 0.8%, and 4.0% compared with 1.6%, respectively). Mania and bipolar I disorder, the most commonly reported treatment-emergent adverse events, occurred in 11.9% and in 6.3%, respectively, of placebo-treated subjects, compared with 4.0% and 1.6% of asenapine-treated subjects. There were no statistically significant differences between the two groups for any of the predefined treatment-emergent adverse events of interest (extrapyramidal symptoms, insomnia, akathisia, somnolence/sedation/hypersomnia, dizziness, oral hypoesthesia/dysgeusia, ≥7% weight increase). Clinically significant weight gain (≥7% increase) was experienced by 7.1% and 7.9% of placebo- and asenapine-treated subjects, respectively. Few subjects met predefined limits of change criteria for lipid and endocrine parameters (see Table S1 in the data supplement), although 8.4% and 8.9% of placebo- and asenapine-treated subjects, respectively, met the glucose predefined limits of change.
Suicidality
During open-label treatment, suicidal ideation, as rated by the C-SSRS, was reported in 30 (5.5%) subjects, and suicidal behavior was reported in two (0.4%) subjects (one aborted and one interrupted suicide attempt). During double-blind treatment, suicidal ideation was reported in three (2.4%) asenapine- and six (4.8%) placebo-treated subjects; suicidal behavior was reported in one asenapine and one placebo subject (0.8% each).
Suicidality-related serious adverse events were reported during open-label (suicidal ideation=4 [0.7%], suicide attempt=1 [0.2%]) and double-blind treatment (placebo: suicidal ideation=1 [0.8%], suicide attempt=1 [0.8%; relapse event, discontinued]; asenapine: intentional overdose=1 [0.8%, completed the trial]).
Discussion
In patients with an acute bipolar I manic or mixed episode who were stable responders to 5 or 10 mg b.i.d. of asenapine over 12–16 weeks, asenapine was found to be statistically superior to placebo in preventing recurrences over 26 weeks of double-blind randomized withdrawal treatment. Kaplan-Meier curves and Cox regression analyses indicated that time to recurrence of any mood episode was significantly longer among subjects receiving asenapine compared with placebo. The calculated hazard ratio (0.22) indicated an approximate fourfold higher risk of recurrence for placebo-treated subjects relative to asenapine-treated subjects. Recurrence prevention results were similarly robust for asenapine in patients with bipolar mania or schizophrenia (24).
Although this study was not powered to detect differences in the recurrence rates of specific mood episodes, post hoc analyses showed that the time to recurrence of a manic or depressive episode was significantly longer in the asenapine group compared with the placebo group. While a statistically significant difference in favor of asenapine over placebo may be an expected outcome for time to recurrence of a manic episode, the significant difference in time to recurrence of a depressive episode is noteworthy because few atypical antipsychotics have demonstrated efficacy in bipolar depression (1). This finding is in line with a previous post hoc analysis that found a statistically significant difference in favor of asenapine over placebo on improvements in depressive symptoms in subjects with manic or mixed episodes associated with bipolar I disorder (25). Given the suggestion of positive treatment effects for asenapine in depressive symptoms, further investigation is warranted.
No new or emerging safety signals were reported. In the open-label period, 65.2% of subjects reported one or more treatment-emergent adverse events, the most common of which were somnolence, akathisia, sedation, accidental overdose, oral hypoesthesia, and headache. Among predefined treatment-emergent adverse events of interest, somnolence/sedation/hypersomnia, weight increase ≥7%, and extrapyramidal symptoms were most common (≥10%). Fasting glucose and ≥7% weight increase were the only metabolic parameters where ≥5% of subjects met predefined limits of change. Atypical antipsychotics, as a class, have a propensity for increased metabolic risk, and subjects should be monitored accordingly (26).
During double-blind treatment, similar percentages of placebo- and asenapine-treated subjects experienced treatment-emergent adverse events, although more placebo- than asenapine-treated subjects experienced serious adverse events and discontinuation. In addition, there were no significant between-group differences in predefined treatment-emergent adverse events of interest. Weight gain ≥7% was similar between groups. Of note, treatment-emergent adverse events may occur more frequently at the start of treatment, and subjects who entered the withdrawal period represent a selected population who maintained response with few adverse experiences.
Bipolar I disorder is a condition with high risk for recurrences and relapses (27–30). Of the 858 patients with bipolar I or bipolar II disorder who were symptomatic at entry into the Systematic Treatment Enhancement Program for Bipolar Disorder trial, 58% achieved recovery; however, during 2 years of follow-up, 49% experienced recurrences, with more than twice as many individuals developing depressive episodes (35%) as manic, hypomanic, or mixed episodes (14%) (31). Although some clinical evidence suggests that patients with a recent manic or mixed index episode have a higher likelihood of relapse into the same polarity (28, 30), relapse into the opposite polarity is also frequent (32). An ideal treatment of bipolar I disorder would prevent relapse into either pole. Results of the present study suggest that asenapine may reduce the risk of recurrence of mood episodes in this patient population.
Bipolar recurrence prevention trials have reported widely divergent relapse rates in placebo-treated patients (e.g., 43% when compared with aripiprazole [33], 80% when compared with olanzapine [34]). It should be noted that lower rates of recurrence than what were originally assumed were seen in both placebo- (33%) and asenapine-treated (9%) subjects during this trial. This finding may reflect the long duration of the stabilization period, specific inclusion and exclusion criteria, and the stringent criteria used to define mood stability, which may have resulted in greater mood stability than expected. To address the lower than expected recurrence rates observed during double-blind treatment, the protocol was amended to assume a recurrence rate of 34% for placebo (relapse hazard ratio of 0.45) and 17% for asenapine. Despite the low rate of recurrence in both treatment groups, the difference between placebo and asenapine was statistically significant for the primary outcome, suggesting that asenapine is effective in maintaining long-term efficacy in acute responders with an index manic or mixed episode associated with bipolar I disorder.
The randomized withdrawal design, as used in this trial, inherently selects for responders to the drug in the open-label period. Therefore, the results of this trial should be interpreted in view of the fact that they were generated in patients with manic or mixed episodes who initially responded to asenapine. Generalizability was further limited by the exclusion of subjects with rapid cycling, bipolar II disorder, and medical comorbidities that may be seen in clinical practice. In addition, the 6-month duration of this trial and strict open-label stabilization criteria may have limited the ability to fully characterize asenapine maintenance efficacy. It is also not known whether changes in the diagnostic criteria for manic episodes could have an effect on the translation of our results into practice. Our results regarding the prevention of depressive episodes should be viewed as exploratory because of the study design.
In conclusion, long-term asenapine therapy was more effective than placebo in preventing recurrence of mood events in stabilized adult subjects with bipolar I disorder. The known safety and tolerability profile for asenapine was confirmed in this trial, with no detection of new safety or tolerability signals.
1 : Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2015; 49:1087–1206Crossref, Medline, Google Scholar
2 : The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry 2013; 14:154–219Crossref, Medline, Google Scholar
3 : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15:1–44Crossref, Medline, Google Scholar
4 : The significance of mixed states in depression and mania. Curr Psychiatry Rep 2014; 16:486Crossref, Medline, Google Scholar
5 : Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry 2007; 68:1480–1491Crossref, Medline, Google Scholar
6 : A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression: Lamictal 602 Study Group. J Clin Psychiatry 1999; 60:79–88Crossref, Medline, Google Scholar
7 : Bipolar disorders and the effectiveness of novel anticonvulsants. J Clin Psychiatry 2002; 63(suppl 3):5–9Medline, Google Scholar
8 : Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur Neuropsychopharmacol 2012; 22:339–346Crossref, Medline, Google Scholar
9 Seroquel XR (package insert). Wilmington, Del., AstraZeneca Pharmaceuticals, 2009Google Scholar
10 Zyprexa (package insert). Indianapolis, Ind., Eli Lilly, 2009Google Scholar
11 Saphris (package insert). Saint Louis, Mo., Actavis, 2015Google Scholar
12 : Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 2009; 23:65–73Crossref, Medline, Google Scholar
13 : A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. Bipolar Disord 2009; 11:673–686Crossref, Medline, Google Scholar
14 : Asenapine versus olanzapine in acute mania: a double-blind extension study. Bipolar Disord 2009; 11:815–826Crossref, Medline, Google Scholar
15 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
16 : A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
17 : Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res 1997; 73:159–171Crossref, Medline, Google Scholar
18 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
19 : Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry 2009; 166:476–488Link, Google Scholar
20 : Aripiprazole in combination with lamotrigine for the long-term treatment of patients with bipolar I disorder (manic or mixed): a randomized, multicenter, double-blind study (CN138-392). Bipolar Disord 2012; 14:41–53Crossref, Medline, Google Scholar
21 : Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J Affect Disord 2008; 109:251–263Crossref, Medline, Google Scholar
22 : Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry 2010; 71:130–137Crossref, Medline, Google Scholar
23 : The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011; 168:1266–1277Link, Google Scholar
24 : A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry 2011; 72:349–355Crossref, Medline, Google Scholar
25 : Effects of asenapine on depressive symptoms in patients with bipolar I disorder experiencing acute manic or mixed episodes: a post hoc analysis of two 3-week clinical trials. BMC Psychiatry 2011; 11:101Crossref, Medline, Google Scholar
26 : Dopamine, striatum, antipsychotics, and questions about weight gain. JAMA Psychiatry 2016; 73:107–108Crossref, Medline, Google Scholar
27 : Course of illness and maintenance treatments for patients with bipolar disorder. J Clin Psychiatry 1995; 56:5–13Medline, Google Scholar
28 : Initial symptoms of manic relapse in manic or mixed-manic bipolar disorder: post hoc analysis of patients treated with olanzapine or lithium. J Psychiatr Res 2007; 41:616–621Crossref, Medline, Google Scholar
29 : The cost of relapse for patients with a manic/mixed episode of bipolar disorder in the EMBLEM study. Pharmacoeconomics 2010; 28:555–566Crossref, Medline, Google Scholar
30 : Affective temperaments are associated with specific clusters of symptoms and psychopathology: a cross-sectional study on bipolar disorder inpatients in acute manic, mixed, or depressive relapse. J Affect Disord 2013; 151:540–550Crossref, Medline, Google Scholar
31 : Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2006; 163:217–224Link, Google Scholar
32 : The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord 2009; 11:453–473Crossref, Medline, Google Scholar
33 : A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry 2006; 67:626–637Crossref, Medline, Google Scholar
34 : Randomized, placebo-controlled trial of olanzapine as maintenance therapy in patients with bipolar I disorder responding to acute treatment with olanzapine. Am J Psychiatry 2006; 163:247–256Link, Google Scholar



