Randomized Trial of an Integrated Behavioral Health Home: The Health Outcomes Management and Evaluation (HOME) Study
Abstract
Objective:
Behavioral health homes provide primary care health services to patients with serious mental illness treated in community mental health settings. The objective of this study was to compare quality and outcomes of care between an integrated behavioral health home and usual care.
Method:
The study was a randomized trial of a behavioral health home developed as a partnership between a community mental health center and a Federally Qualified Health Center. A total of 447 patients with a serious mental illness and one or more cardiometabolic risk factors were randomly assigned to either the behavioral health home or usual care for 12 months. Participants in the behavioral health home received integrated medical care on-site from a nurse practitioner and a full-time nurse care manager subcontracted through the health center.
Results:
Compared with usual care, the behavioral health home was associated with significant improvements in quality of cardiometabolic care, concordance of treatment with the chronic care model, and use of preventive services. For most cardiometabolic and general medical outcomes, both groups demonstrated improvement, although there were no statistically significant differences between the two groups over time.
Conclusions:
The results suggest that it is possible, even under challenging real-world conditions, to improve quality of care for patients with serious mental illness and cardiovascular risk factors. Improving quality of medical care may be necessary, but not sufficient, to improve the full range of medical outcomes in this vulnerable population.
People with serious mental disorders die years younger than the general population, with cardiovascular disease as the largest contributor to excess mortality (1). Compared with the general population, people with serious mental disorders are at risk for poor-quality medical care (2), and poor-quality care is likely an important determinant of adverse outcomes in this population (3, 4). These findings have led to a growing call from advocates and providers to improve access to and quality of general medical care for people with serious mental illness. However, only a handful of studies have examined these approaches using rigorous study designs (5).
One model that has attracted particular interest among providers and policy makers is the behavioral health home, a medical health home based in a community mental health center (6). Most of these programs are structured as partnerships between community mental health centers and Federally Qualified Health Centers, safety net clinics that receive federal funding to provide primary care services to poor and underserved populations. Since 2009, the Substance Abuse and Mental Health Services Administration's Primary and Behavioral Health Care Integration (PBHCI) program has funded a total of 185 community mental health centers to develop behavioral health homes for their enrollees, with Federally Qualified Health Centers representing approximately two-thirds of primary care partners (7). Behavioral health homes are being used in a wide range of settings nationwide, including state Medicaid Health Home waiver programs (8) and Center for Medicare and Medicaid Innovation demonstration projects (9).
Despite the widespread interest in and use of behavioral health homes, there are few data evaluating their impact on quality or outcomes of care. RAND’s 2013 evaluation of the PBHCI program (10) compared three grantees to three matched clinic sites from the same states. The study produced mixed results, with improvements in several but not most of the cardiometabolic outcomes and no difference in behavioral health indicators. The report concluded that clinics were able to successfully implement the programs but that there was a need for prospective randomized trials to better assess the impact of these care models on patient outcomes.
To fill these gaps in knowledge, the present study uses a randomized design to rigorously evaluate the impact of a behavioral health home on quality and outcomes of care in a sample of patients with comorbid serious mental illness and cardiometabolic risk factors. The program was implemented in a community setting under real-world conditions, with the intervention delivered by the Federally Qualified Health Center rather than research staff.
Method
The study was a single-blind randomized trial of a behavioral health home compared with usual care for patients at a community mental health center. The intervention lasted 12 months. The study protocol was approved by the Emory Institutional Review Board.
Recruitment, Eligibility, and Randomization
Participants were screened and enrolled using a two-stage process. During the first stage, potential subjects were identified from a list of active patients at the community mental health center or referred by mental health providers. Inclusion criteria included presence of a serious mental illness (schizophrenia, schizoaffective disorder, bipolar disorder, major depression, obsessive-compulsive disorder, or posttraumatic stress disorder, with or without comorbid substance use), by the Mini-International Neuropsychiatric Interview (11), and confirmed via the patient’s community mental health center chart. The exclusion criterion was cognitive impairment, as indicated by a score ≥3 on a 6-item validated screen (12).
Patients who met preliminary eligibility criteria were invited back to the community mental health center for a fasting screening test for metabolic abnormalities. To be eligible, patients had to have at least one of the following cardiometabolic risk factors: blood pressure ≥130/85 mmHg, glucose level ≥100 mg/dL, serum cholesterol level >240 mg/dL, and LDL level >160 mg/dL (13–15). Patients who met eligibility criteria and provided informed consent were randomly assigned to either the behavioral health home or usual care.
Intervention Groups
The behavioral health home was established on-site at the community mental health center by the Federally Qualified Health Center. The clinic provided care for participants’ cardiometabolic risk factors and comorbid medical problems. Clinic staff included a part-time nurse practitioner with prescribing authority and a full-time nurse care manager, both supervised by the Federally Qualified Health Center’s medical director. This is the most common staffing pattern used in behavioral health homes (7). A treat-to-target approach was used for the cardiometabolic risk factors, with weekly supervision meetings focusing on patients whose test results were not within normal range for blood pressure, glucose level, or cholesterol level (16). The care manager provided health education for lifestyle factors (e.g., smoking, diet) and logistical support to ensure that patients were able to attend their medical appointments. Both providers attended weekly rounds at the community mental health center to facilitate integration with the mental health team. After each medical or care management visit, the Federally Qualified Health Center provider entered a note remotely into the center’s chart, with a copy placed into the community mental health center chart. Patients with a usual source of care at baseline who were assigned to the intervention group had the choice of switching providers or receiving care from the behavioral health home as a supplement to their existing treatment.
For the usual care group, patients were provided with a summary of their laboratory tests and encouraged to make an appointment with a community medical provider. They were then given a list of providers near the community mental health center that provided discounted care and/or accepted Medicaid. This reflects typical care provided in the clinic, which provided services consistent with standards of care typically seen in community mental health centers, such as routine blood pressure testing and cardiometabolic laboratory tests for patients taking antipsychotic medications, but did not provide any wellness or primary care services.
Quality and Service Use Measures
The Service Use and Resource Form, a self-report service use instrument was used to identify and request charts from all medical and behavioral health facilities where a subject reported receiving care. Chart reviews were then used to assess health care utilization and quality of care. A full list of the quality and service use measures is provided in the data supplement that accompanies the online edition of this article.
Quality indicators for management of cardiometabolic risk factors were drawn from medical records using indicators from RAND’s Community Quality Index study (17–19). These indicators were developed based on literature reviews documenting reliability, construct validity, and linkage with clinical outcomes (i.e., identifiable health benefits to patients who receive guideline-concordant care) (20).
The study used seven indicators for hyperlipidemia, 13 for hypertension, and 11 for diabetes, each covering domains of screening, diagnosis, treatment, and follow-up (21). A quality score for each condition is generated by dividing all instances in which recommended care is delivered by the number of times a participant is eligible for the indicator. A total quality score across conditions is generated, representing the total number of services for which an individual is eligible that were received by that individual. Quality of preventive medical services and eligible populations were derived from the U.S. Preventive Services Task Force guidelines, with 11 indicators used for the study (22). All RAND and preventive service quality measures are listed in the online data supplement.
Participants also completed the Patient Assessment of Chronic Illness Care survey, which assesses the extent to which patients with chronic illness receive care that aligns with the chronic care model. Subscales document the degree to which care is patient-centered, proactive, and planned and the extent to which it includes collaborative goal setting, problem solving, and follow-up support. The instrument has been found to have good test-retest reliability and to be correlated with measures of primary care and patient activation (23).
Outcome Measures
Fasting blood levels of glucose, fractionated cholesterol, and hemoglobin A1c (HbA1c) were collected by the research interviewers every 6 months, using the Cholestech point-of-care fingerstick system, which has been shown to produce values that are comparable to those from gold-standard laboratory tests for cholesterol, glucose, and HbA1c (24, 25). Participants were asked to fast for 12 hours before these assessments. The Framingham risk score, an aggregate measure that predicts the 10-year risk of developing incident coronary heart disease (26), was calculated at baseline and 12-month follow-up using these values in conjunction with information about age, blood pressure, smoking status, and diabetes.
The 36-item Short-Form Health Survey (SF-36) is a measure of health-related quality of life constructed for use in the Medical Outcomes Study (27). For the present study, physical and mental component summary scores (28) were used to provide indicators of health-related quality of life. Patient activation, which reflects an individual’s capacity to manage his or her illness, was assessed using a validated brief version of the Patient Activation Measure (29, 30).
Data Analysis
Three time points were used for instruments administered during interviews (baseline, 6-month interview, and 12-month interview), and two time points were used for data collected via chart review (baseline, 12-month interview).
All analyses were conducted as intent to treat. First, to assess adequacy of randomization, bivariate analyses were used to examine differences between the intervention and usual care groups. All analyses were generated using SAS/STAT, version 9.4 (SAS Institute, Cary, N.C.). The PROC MIXED procedure was used for continuous variables, incorporating a compound symmetric covariance structure. For each outcome measure, the model assessed the outcome as a function of 1) randomization, 2) time since randomization, and 3) group-by-time interaction. The group-by-time interaction, which reflects the relative difference in change in the parameters over time, was the primary measure of statistical significance.
All hypotheses were two sided and tested at a significance threshold of 0.05. We prespecified a composite primary endpoint (overall quality of cardiometabolic care) to minimize type I error (i.e., false positives) and used a p threshold of 0.05 for secondary outcomes in order to minimize the potential for type II error (i.e., false negatives).
Additionally, mixed models were run separately for cases and controls to determine whether significant changes occurred within each randomized arm. For these models, a significant time effect indicated a change in the modeled outcome.
Results
Of approximately 2,000 active patients in the clinic, 970 were screened and 447 were enrolled in the study and randomly assigned to either the behavioral health home or usual care (Figure 1). The demographic and clinical characteristics of the study participants were similar to those of the overall clinic population.
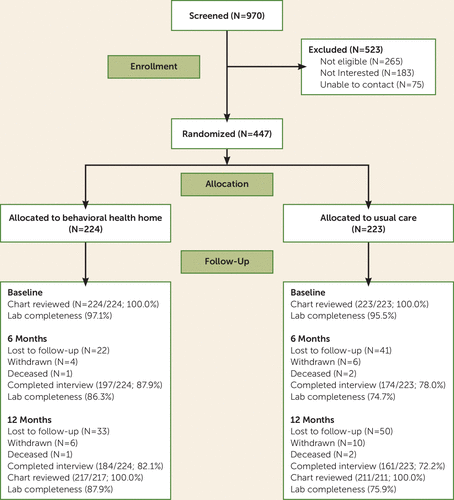
FIGURE 1. CONSORT Flow Diagram for a Randomized Trial of an Integrated Behavioral Health Home
The two study groups did not differ in any demographic or diagnostic characteristics (Table 1), which suggests that the randomization was successful. At the 12-month follow-up, a total of 77.2% patients completed interviews (82.1% in the behavioral health home group and 72.2% in the usual care group), 100% in both groups had chart review data, and 81.3% had fasting laboratory data (87.9% in the behavioral health home group and 75.9% in the usual care group).
| Measure | Behavioral Health Home Group (N=224) | Usual Care Group (N=223) | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 47.26 | 9.7 | 47.12 | 9.6 | 0.88 |
| Education (years) | 12.71 | 2.3 | 12.85 | 2.4 | 0.53 |
| Monthly income ($) | 492.48 | 519.3 | 532.50 | 722.2 | 0.50 |
| N | % | N | % | ||
| Male | 80 | 36 | 98 | 44 | 0.08 |
| Single | 70 | 34 | 81 | 39 | 0.29 |
| Race | |||||
| White | 122 | 54 | 129 | 58 | 0.47 |
| Black | 95 | 42 | 84 | 38 | 0.31 |
| Other race | 7 | 3 | 10 | 4 | 0.45 |
| Hispanic | 4 | 2 | 4 | 2 | 0.99 |
| Stable housing | 193 | 86 | 188 | 84 | 0.58 |
| Stable employment | 82 | 37 | 79 | 35 | 0.79 |
| Disability | 19 | 9 | 25 | 11 | 0.32 |
| Medicaid | 24 | 11 | 15 | 7 | 0.05 |
| Medicare | 18 | 8 | 18 | 8 | 0.99 |
| Private insurance | 4 | 2 | 10 | 4 | 0.1 |
| Usual source of care | |||||
| Other (not emergency department) | 90 | 40 | 80 | 36 | 0.35 |
| Emergency department | 50 | 22 | 41 | 18 | 0.30 |
| None | 84 | 38 | 101 | 45 | 0.09 |
| Medical diagnoses | |||||
| Diabetes | 91 | 41 | 86 | 39 | 0.66 |
| Heart disease | 11 | 5 | 19 | 9 | 0.13 |
| Hyperlipidemia | 141 | 63 | 137 | 61 | 0.74 |
| Hypertension | 184 | 82 | 177 | 79 | 0.46 |
| Mean | SD | Mean | SD | ||
| Number of medical diagnoses | 1.91 | 0.8 | 1.88 | 0.9 | 0.73 |
| N | % | N | % | ||
| Primary mental diagnosis | |||||
| Schizophrenia/schizoaffective disorder | 49 | 22 | 40 | 18 | 0.30 |
| Bipolar disorder | 108 | 48 | 122 | 55 | 0.17 |
| Depression | 66 | 29 | 58 | 26 | 0.41 |
| Anxiety | 1 | 0 | 2 | 1 | 0.56 |
| Substance abuse | 0 | 0 | 1 | 0 | 0.32 |
TABLE 1. Baseline Demographic and Clinical Characteristics of Patients in a Randomized Trial of an Integrated Behavioral Health Home
Quality of Care
The primary study outcome measure was a composite measure of quality of cardiometabolic care, based on RAND quality indicators representing the total number of services for which patients were eligible that they actually received (Table 2). Patients in the behavioral health home group demonstrated significant improvements in the proportion of indicated services they received (from 67% to 81% in the behavioral health home group and from 65% to 63% in the usual care group; p<0.001). This difference represents a Cohen’s d value of 0.7, a large effect size.
| Measure | Behavioral Health Home Group (N=224) | Usual Care Group (N=223) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | SD | F | df | p | |
| Composite RAND indicators | 32.95 | 421 | <0.001 | ||||
| Baseline | 0.67 | 0.2 | 0.65 | 0.3 | |||
| 12-Month interview | 0.81 | 0.2 | 0.63 | 0.3 | |||
| Diabetes indicators | 25.37 | 111 | <0.001 | ||||
| Baseline | 0.38 | 0.2 | 0.41 | 0.3 | |||
| 12-Month interview | 0.63 | 0.2 | 0.44 | 0.3 | |||
| Hyperlipidemia indicators | 3.55 | 101 | 0.062 | ||||
| Baseline | 0.62 | 0.5 | 0.55 | 0.5 | |||
| 12-Month interview | 0.96 | 0.2 | 0.74 | 0.4 | |||
| Hypertension indicators | 29.74 | 420 | <0.001 | ||||
| Baseline | 0.71 | 0.3 | 0.71 | 0.3 | |||
| 12-Month interview | 0.84 | 0.2 | 0.66 | 0.3 | |||
| Preventive services | 106.78 | 426 | <0.001 | ||||
| Baseline | 0.36 | 0.2 | 0.36 | 0.2 | |||
| 12-Month interview | 0.56 | 0.2 | 0.33 | 0.2 | |||
| Patient Assessment of Chronic Illness Care | 22.8 | 382 | <0.001 | ||||
| Baseline | 2.24 | 0.9 | 2.28 | 1.0 | |||
| 6-Month interview | 3.52 | 0.9 | 2.60 | 1.0 | |||
| 12-Month interview | 3.55 | 0.9 | 2.61 | 1.1 | |||
| Primary care provider visits | 9.94 | 708 | <0.001 | ||||
| Baseline | 0.93 | 1.7 | 0.65 | 1.2 | |||
| 6-Month interview | 2.01 | 2.7 | 0.74 | 1.4 | |||
| 12-Month interview | 1.73 | 1.8 | 0.86 | 1.2 | |||
| Mental health provider visits | 1.92 | 710 | 0.148 | ||||
| Baseline | 2.13 | 4.3 | 2.18 | 4.3 | |||
| 6-Month interview | 1.26 | 1.9 | 1.73 | 3.4 | |||
| 12-Month interview | 1.08 | 1.7 | 2.18 | 6.0 | |||
| Specialty provider visits | 0.86 | 709 | 0.423 | ||||
| Baseline | 0.27 | 1.3 | 0.17 | 0.7 | |||
| 6-Month interview | 0.31 | 1.1 | 0.41 | 1.4 | |||
| 12-Month interview | 0.49 | 1.4 | 0.56 | 1.4 | |||
| Emergency department visits | 0.52 | 420 | 0.472 | ||||
| Baseline | 0.87 | 1.5 | 0.71 | 1.7 | |||
| 12-Month interview | 0.97 | 2.2 | 0.70 | 1.5 | |||
| Hospitalization | 1.16 | 420 | 0.283 | ||||
| Baseline | 0.27 | 0.7 | 0.32 | 0.9 | |||
| 12-Month interview | 0.29 | 1.1 | 0.24 | 0.7 | |||
| N | % | N | % | χ2 | df | p | |
| Usual source of care | |||||||
| Baseline | 79 | 35.3 | 67 | 30.0 | 1.39 | 1 | 0.240 |
| 12 months | 162 | 88.0 | 75 | 47.0 | 68.63 | 1 | <0.001 |
TABLE 2. Quality of Care and Service Use in the Two Study Arms in a Randomized Trial of an Integrated Behavioral Health Home
For RAND quality measures for individual conditions, there was a significant difference in improvement for diabetes care (from 38% to 63% in the behavioral health home group and from 41% 44% for the usual care group; p<0.001 for the group-by-time interaction) and hypertension care (from 71% to 84% for behavioral health home group, compared with a drop from 71% to 66% for the usual care group; p<0.001). Care for dyslipidemia improved in both groups but the difference fell short of significance (from 62% to 96% for the behavioral health home group, and 55% to 74% in the usual care group; p=0.06). For the subset of questions specifically focused on quality of medication treatment, patients with diabetes in the intervention group were more likely to receive diabetes medication (81.0% compared with 67%; p=0.04), and those with hypertension were more likely to receive therapy for hypertension (92% compared with 75%; p<0.01).
There was also a significantly greater improvement on prevention services recommended by the U.S. Preventive Services Task Force in the behavioral health home group compared with the usual care group (from 36% to 56% and from 36% to 33%, respectively; p<0.001). The difference between the two groups is a Cohen’s d value of 1.2, a large effect size.
Scores on the Patient Assessment of Chronic Illness Care indicated that care for patients in the behavioral health home was more likely to align with the chronic care model than did care for those assigned to usual care. Out of a possible score of 5, the behavioral health home group improved from 2.2 to 3.6, compared with 2.3 to 2.6 for the usual care group (p<0.001 for the group-by-time interaction).
Service Use
For patients in the behavioral health home group, primary care visits increased from a mean of 0.93 during the 6 months prior to enrollment to a mean of 1.73 in the second 6 months of the program, as compared with an increase from 0.65 to 0.86 in the usual care group. The group-by-time interaction effect was statistically significant (p<0.001). None of the differences across the groups for any other service use measures were statistically significant (Table 2).
Outcomes of Care
Most clinical outcomes, including diastolic blood pressure, total and LDL cholesterol levels, blood glucose level, HbA1c level, Framingham risk score, patient activation, and the physical component summary of the SF-36, did not show a differential improvement between the behavioral health home and usual care groups (Table 3). For all variables except blood glucose level, the behavioral health home group showed a significant improvement between baseline and 12-month follow-up. However, the usual care group also showed improvements in all outcomes except blood pressure, blood glucose level, and HbA1c level, with the result that there was not a significant difference between groups in change in these outcomes over time. There were modest statistically significant differential improvements between the groups in the mental component summary (an improvement of 8.1 points in the behavioral health home group, compared with 7 points in the usual care group; p=0.03) and in systolic blood pressure (improvements of 4.9 points and 3.1 points, respectively; p=0.04) (Table 4).
| Behavioral Health Home Group (N=224) | Usual Care Group (N=223) | Analysis | |||||
|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | F | df | p |
| Diastolic blood pressure (mmHg) | 2.55 | 747 | 0.079 | ||||
| Baseline | 89.5 | 13.2 | 90.2 | 11.3 | |||
| 6-Month interview | 85.0 | 10.5 | 88.0 | 11.6 | |||
| 12-Month interview | 83.6 | 10.9 | 86.6 | 11.4 | |||
| Systolic blood pressure (mmHg) | 3.11 | 747 | 0.045 | ||||
| Baseline | 137.0 | 19.6 | 138.3 | 17.2 | |||
| 6-Month interview | 131.3 | 15.5 | 137.1 | 18.2 | |||
| 12-Month interview | 132.1 | 17.3 | 135.2 | 16.2 | |||
| Total cholesterol (mg/dL) | 0.6 | 712 | 0.548 | ||||
| Baseline | 202.6 | 44.2 | 201.4 | 42.5 | |||
| 6-Month interview | 197.5 | 39.0 | 199.5 | 40.7 | |||
| 12-Month interview | 193.8 | 43.8 | 195.2 | 40.3 | |||
| Blood glucose (mg/dL) | 0.46 | 726 | 0.629 | ||||
| Baseline | 113.7 | 42.0 | 114.9 | 47.7 | |||
| 6-Month interview | 112.8 | 46.9 | 112.4 | 45.3 | |||
| 12-Month interview | 111.9 | 41.2 | 108.9 | 39.9 | |||
| Hemoglobin A1c (%) | 1.46 | 447 | 0.233 | ||||
| Baseline | 6.4 | 2.2 | 6.2 | 2.3 | |||
| 6-Month interview | 6.5 | 1.6 | 6.6 | 2.1 | |||
| 12-Month interview | 6.5 | 1.6 | 6.3 | 1.8 | |||
| LDL (mg/dL) | 0.11 | 642 | 0.897 | ||||
| Baseline | 122.9 | 40.7 | 125.1 | 40.6 | |||
| 6-Month interview | 111.3 | 35.3 | 118.5 | 35.9 | |||
| 12-Month interview | 112.2 | 39.5 | 115.4 | 39.4 | |||
| Patient Activation Measure | 0.05 | 710 | 0.955 | ||||
| Baseline | 56.0 | 14.0 | 55.9 | 14.2 | |||
| 6-Month interview | 58.2 | 14.1 | 58.5 | 15.0 | |||
| 12-Month interview | 60.2 | 16.2 | 60.0 | 17.4 | |||
| SF-36 mental component summary | 3.55 | 708 | 0.029 | ||||
| Baseline | 29.9 | 13.5 | 29.3 | 13.9 | |||
| 6-Month interview | 33.3 | 14.8 | 35.4 | 14.7 | |||
| 12-Month interview | 38.0 | 14.3 | 36.3 | 15.2 | |||
| SF-36 physical component summary | 0.43 | 708 | 0.65 | ||||
| Baseline | 40.5 | 11.9 | 41.0 | 12.4 | |||
| 6-Month interview | 42.4 | 11.7 | 41.8 | 12.0 | |||
| 12-Month interview | 42.9 | 12.2 | 42.5 | 12.4 | |||
| Framingham coronary heart disease risk score | 0.69 | 634 | 0.504 | ||||
| Baseline | 10.4 | 8.3 | 11.0 | 8.2 | |||
| 6-Month interview | 8.4 | 6.5 | 10.2 | 7.1 | |||
| 12-Month interview | 9.2 | 7.8 | 10.3 | 7.2 | |||
TABLE 3. Clinical Outcomes in the Two Study Arms in a Randomized Trial of an Integrated Behavioral Health Homea
| Behavioral Health Home Group (N=224) | Usual Care Group (N=223) | |||||
|---|---|---|---|---|---|---|
| Measure | Mean | SD | p | Mean | SD | p |
| Diastolic blood pressure (mmHg) | <0.001 | 0.001 | ||||
| Baseline | 89.5 | 13.2 | 90.2 | 11.3 | ||
| 6-Month interview | 85.0 | 10.5 | 88.0 | 11.6 | ||
| 12-Month interview | 83.6 | 10.9 | 86.6 | 11.4 | ||
| Systolic blood pressure (mmHg) | <0.001 | 0.171 | ||||
| Baseline | 137.0 | 19.6 | 138.3 | 17.2 | ||
| 6-Month interview | 131.3 | 15.5 | 137.1 | 18.2 | ||
| 12-Month interview | 132.1 | 17.3 | 135.2 | 16.2 | ||
| Total cholesterol (mg/dL) | 0.002 | 0.021 | ||||
| Baseline | 202.6 | 44.2 | 201.4 | 42.5 | ||
| 6-Month interview | 197.5 | 39.0 | 199.5 | 40.7 | ||
| 12-Month interview | 193.8 | 43.8 | 195.2 | 40.3 | ||
| Blood glucose (mg/dL) | 0.750 | 0.123 | ||||
| Baseline | 113.7 | 42.0 | 114.9 | 47.7 | ||
| 6-Month interview | 112.8 | 46.9 | 112.4 | 45.3 | ||
| 12-Month interview | 111.9 | 41.2 | 108.9 | 39.9 | ||
| Hemoglobin A1c (%) | 0.043 | 0.915 | ||||
| Baseline | 6.4 | 2.2 | 6.2 | 2.3 | ||
| 6-Month interview | 6.5 | 1.6 | 6.6 | 2.1 | ||
| 12-Month interview | 6.5 | 1.6 | 6.3 | 1.8 | ||
| LDL (mg/dL) | <0.001 | <0.001 | ||||
| Baseline | 122.9 | 40.7 | 125.1 | 40.6 | ||
| 6-Month interview | 111.3 | 35.3 | 118.5 | 35.9 | ||
| 12-Month interview | 112.2 | 39.5 | 115.4 | 39.4 | ||
| Patient Activation Measure | <0.001 | 0.002 | ||||
| Baseline | 56.0 | 14.0 | 55.9 | 14.2 | ||
| 6-Month interview | 58.2 | 14.1 | 58.5 | 15.0 | ||
| 12-Month interview | 60.2 | 16.2 | 60.0 | 17.4 | ||
| SF-36 mental component summary | <0.001 | <0.001 | ||||
| Baseline | 29.9 | 13.5 | 29.3 | 13.9 | ||
| 6-Month interview | 33.3 | 14.8 | 35.4 | 14.7 | ||
| 12-Month interview | 38.0 | 14.3 | 36.3 | 15.2 | ||
| SF-36 physical component summary | 0.003 | 0.036 | ||||
| Baseline | 40.5 | 11.9 | 41.0 | 12.4 | ||
| 6-Month interview | 42.4 | 11.7 | 41.8 | 12.0 | ||
| 12-Month interview | 42.9 | 12.2 | 42.5 | 12.4 | ||
| Framingham coronary heart disease risk score | <0.001 | 0.024 | ||||
| Baseline | 10.4 | 8.3 | 11.0 | 8.2 | ||
| 6-Month interview | 8.4 | 6.5 | 10.2 | 7.1 | ||
| 12-Month interview | 9.2 | 7.8 | 10.3 | 7.2 | ||
TABLE 4. Changes in Clinical Outcomes Within Each Study Arm in a Randomized Trial of an Integrated Behavioral Health Homea
To better understand whether differential loss to follow-up could have affected the study findings, we compared the baseline clinical and demographic characteristics of participants with follow-up data to those who dropped out of the study. Participants who dropped out were significantly younger on average than those who completed 12 months of follow-up (mean age, 44.8 years compared with 47.7 years; p=0.01) and were less likely to have a diagnosis of schizophrenia (11% compared with 22%). There were no other significant differences in any demographic or baseline cardiometabolic parameters between the two groups. (See Table S2 in the online data supplement for full results.)
Discussion
To our knowledge, this is the first randomized study of a behavioral health home, and it is one of only a handful of randomized trials examining medical homes that have been conducted to date (31). For patients with serious mental illness and cardiovascular risk factors, the behavioral health home was associated with significant benefits with regard to quality of general medical care, cardiometabolic care, and concordance with the chronic care model. However, there was not a significant effect on most clinical outcomes, including cardiometabolic parameters.
Across a range of indicators, participants with serious mental illness and cardiovascular risk factors who were enrolled in the behavioral health home received care of a significantly higher quality compared with those assigned to usual care. It is notable that the findings were stronger and more consistent than the effect of medical homes in the general medical sector (31, 32), which have found a more limited impact of medical homes on quality of medical care. This positive impact on quality of care is notable given the fact that the behavioral health home delivered care under real-world conditions, with broad inclusion criteria and use of Federally Qualified Health Center staff rather than research staff to deliver the intervention. It demonstrates that it is possible to deliver high-quality care even under the challenging circumstances seen in community mental health centers, with high levels of comorbidity, functional disability, and social disadvantage.
However, the behavioral health home did not have an impact on the majority of cardiometabolic and medical outcomes. Both study groups demonstrated significant improvements in cardiometabolic outcomes between baseline and 12-month follow-up, and it is possible that screening and community referral were enough to partially improve outcomes in the usual care group. For patients who were unaware of their conditions or were not motivated to seek care, a recommendation to seek care from a community provider might help engage patients and motivate their mental health providers to refer them to treatment. However, caution should be used in interpreting within-group improvements, as they are based on observational data and may reflect factors unrelated to the study.
Poor quality of care is only one factor underlying adverse medical outcomes in patients with serious mental illness and cardiometabolic risk (33). Behavioral factors such as poor diet, smoking, and lack of physical activity also raise cardiovascular risk and early mortality in this group (34, 35). Several recent studies have demonstrated that it is possible to improve diet (36, 37), physical activity (38, 39), weight (36, 37, 39), and smoking (40) in patients with serious mental illness. However, there is more limited evidence that lifestyle programs improve cardiometabolic outcomes or overall cardiovascular risk in populations with serious mental illness (41, 42). In general medical populations with coronary heart disease, intensive multifactor risk models that combine medication with lifestyle change have demonstrated promise in improving aggregate outcomes, including risk of fatal and nonfatal cardiovascular events (43). Future research should examine the potential benefits of programs combining medical care and lifestyle factors, tailored to the clinical needs and socioeconomic challenges faced by patients with serious mental illness (44).
There are several limitations that should be considered in interpreting the study findings. First, there were more dropouts from the usual care group than the behavioral health home group. While we did not find any significant between-group differences in baseline clinical or demographic characteristics among study dropouts, we cannot rule out the possibility that this group improved more or less than those who remained in the study. Second, it is possible that larger improvements in quality of care or a longer intervention duration would have resulted in better clinical outcomes. Further research should be conducted to assess how best to optimize medical quality under real-world conditions in behavioral health homes. Third, the Framingham risk score is not an optimal outcome measure, since it includes factors such as age that are not sensitive to change, and it does not factor in characteristics that are unique to patients with serious mental illness. There is a need to develop high-quality aggregate outcome measures that can meaningfully capture the full range of modifiable risk factors that contribute to early mortality in patients with serious mental illness (45). Finally, the study was conducted in a single behavioral health home. It is possible that other clinics might have better or worse outcomes based on differences in patient factors (e.g., differing diagnostic case mix), organizational factors (e.g., Federally Qualified Health Center versus other primary care partners), or community resources (e.g., nearby specialty medical clinic options for referral).
The results suggest that it is possible, even under challenging, real-world conditions, to improve the quality of medical care for patients with serious mental illness and cardiovascular risk factors treated in community mental health settings. However, better quality alone may be insufficient to improve more distal medical outcomes. Addressing the poor clinical health and early mortality in this population will require multimodal strategies addressing the full range of risk factors that underlie these problems.
1 : Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015; 72:334–341Crossref, Medline, Google Scholar
2 : Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009; 194:491–499Crossref, Medline, Google Scholar
3 : Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol 2010; 24(Suppl):69–80Crossref, Medline, Google Scholar
4 : Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry 2001; 58:565–572Crossref, Medline, Google Scholar
5 : An evidence synthesis of care models to improve general medical outcomes for individuals with serious mental illness: a systematic review. J Clin Psychiatry 2013; 74:e754–e764Crossref, Medline, Google Scholar
6 : Behavioral health homes for persons with mental and substance use conditions: the core clinical features. Washington, DC, SAMHSA/HRSA Center for Integrated Heatlh, 2012Google Scholar
7 : Integrating primary care into community behavioral health settings: programs and early implementation experiences. Psychiatr Serv 2013; 64:660–665Link, Google Scholar
8 : About half of the states are implementing patient-centered medical homes for their Medicaid populations. Health Aff (Millwood) 2012; 31:2432–2440Crossref, Medline, Google Scholar
9 Centers for Medicare and Medicaid Services (CMS): Health Care Innovation Awards Round One Project Profiles. Baltimore, CMS, 2012Google Scholar
10 Scharf D, Eberhart N, Schmidt N, et al: Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program. Santa Monica, Calif, RAND, 2014Google Scholar
11 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
12 : Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002; 40:771–781Crossref, Medline, Google Scholar
13 : Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239Crossref, Medline, Google Scholar
14 : Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203Crossref, Medline, Google Scholar
15 : The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572Crossref, Medline, Google Scholar
16 : Treating complexity: collaborative care for multiple chronic conditions. Int Rev Psychiatry 2014; 26:638–647Crossref, Medline, Google Scholar
17 : The quality of health care delivered to adults in the United States. N Engl J Med 2003; 348:2635–2645Crossref, Medline, Google Scholar
18 Brook R: The RAND/UCLA appropriateness method, in Clinical Practice Guideline Development: Methodology Perspectives. Edited by McCormick KA, Moore SR, Siegel RA. Rockville, Md, Agency for Healthcare Policy and Research 1994, pp 59–70Google Scholar
19 : The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med 1998; 338:1888–1895Crossref, Medline, Google Scholar
20 (eds): Quality of Care for Cardiopulmonary Conditions: A Review of the Literature and Quality Indicators. Edited by RAND. Santa Monica, Calif, RAND, 2000Google Scholar
21 : Hypertension, in Quality of Care for Cardiopulmonary Conditions: a review of the literature and quality indicators. Edited by Kerr EA, Asch SM, Hamilton EG, . Santa Monica, Calif, RAND, 2000, pp 217–231Google Scholar
22 : Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Med Care 2006; 44:187–191Crossref, Medline, Google Scholar
23 : Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005; 43:436–444Crossref, Medline, Google Scholar
24 : Accuracy and precision of the Cholestech LDX system in monitoring blood lipid levels. Am J Health Syst Pharm 2002; 59:1774–1779Crossref, Medline, Google Scholar
25 : Agreement between laboratory results and on-site pathology testing using Bayer DCA2000+ and Cholestech LDX point-of-care methods in remote Australian Aboriginal communities. Clin Chim Acta 2006; 367:69–76Crossref, Medline, Google Scholar
26 : Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry 2004; 49:753–760Crossref, Medline, Google Scholar
27 : The MOS 36-item Short-Form Health Survey (SF-36), III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32:40–66Crossref, Medline, Google Scholar
28 : Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995; 33(suppl):AS264–AS279Crossref, Medline, Google Scholar
29 : Development and testing of a short form of the Patient Activation Measure. Health Serv Res 2005; 40:1918–1930Crossref, Medline, Google Scholar
30 : The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res 2010; 118:264–270Crossref, Medline, Google Scholar
31 : Improving patient care: the patient centered medical home: a systematic review. Ann Intern Med 2013; 158:169–178Crossref, Medline, Google Scholar
32 : The patient-centered medical home and associations with health care quality and utilization: a 5-year cohort study. Ann Intern Med 2016; 164:395–405Crossref, Medline, Google Scholar
33 : Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care 2011; 49:599–604Crossref, Medline, Google Scholar
34 : Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry 2009; 31:555–563Crossref, Medline, Google Scholar
35 Bartels S, Desilets R, Dartmouth Health Promotion Research Team: Health Promotion Programs for People With Serious Mental Illness: What Works? A Systematic Review and Analysis of the Evidence Base in Published Research Literature on Exercise and Nutrition Programs. Washington, DC, SAMHSA-HRSA Center for Integrated Health Solutions, 2012Google Scholar
36 : A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013; 368:1594–1602Crossref, Medline, Google Scholar
37 : The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry 2015; 172:71–81Link, Google Scholar
38 : Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv 2013; 64:729–736Link, Google Scholar
39 : Pragmatic replication trial of health promotion coaching for obesity in serious mental illness and maintenance of outcomes. Am J Psychiatry 2015; 172:344–352Link, Google Scholar
40 : Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2013; 2:CD007253Medline, Google Scholar
41 Gierisch JM, Nieuwsma JA, Bradford DW, et al: Interventions to Improve Cardiovascular Risk Factors in People With Serious Mental Illness (Comparative Effectiveness Review No 105). Rockville, Md, Agency for Healthcare Research and Quality, 2013Google Scholar
42 : The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016; 15:155–165Crossref, Medline, Google Scholar
43 : Lifestyle interventions in patients with coronary heart disease: a systematic review. Am J Prev Med 2013; 45:207–216Crossref, Medline, Google Scholar
44 : A meta-review of lifestyle interventions for cardiovascular risk factors in the general medical population: lessons for individuals with serious mental illness. J Clin Psychiatry 2015; 76:e477–e486Crossref, Medline, Google Scholar
45 : Cardiovascular risk prediction models for people with severe mental illness: results from the Prediction and Management of Cardiovascular Risk in People With Severe Mental Illnesses (PRIMROSE) research program. JAMA Psychiatry 2015; 72:143–151Crossref, Medline, Google Scholar



