Disambiguating the Psychiatric Sequelae of Parkinson’s Disease, Deep Brain Stimulation, and Life Events: Case Report and Literature Review
“Mr. R,” a 66-year-old white man with a 15-year history of Parkinson’s disease, was evaluated for surgical implantation of electrodes for deep brain stimulation (DBS). Initial neuropsychological testing showed nearly normal cognitive functioning with an isolated mild to moderate impairment on one measure of semantic verbal fluency. Mr. R’s history and presurgical evaluation revealed no contraindications for surgery; he did not meet criteria for mild cognitive impairment, dementia, or any Parkinson-plus syndromes, such as Lewy body dementia. No psychiatric concerns were noted by his neurology providers, so he was not scheduled for additional psychological or psychiatric evaluation prior to surgery. Because of his motor fluctuations and medication-induced dyskinesia, he was considered to be a good candidate for DBS.
Mr. R underwent staged bilateral DBS surgery targeting the subthalamic nucleus (STN). Leads were placed using microelectrode recording. The left STN and right STN leads were implanted a week apart, and coordinates were consistent with acceptable lead placement. The indirect coordinates relative to the midcommissural point were as follows: for the left STN, 10.95 mm lateral, 3.38 mm posterior, and 7.86 mm inferior; for the right STN, 10.92 mm lateral, 1.66 mm posterior, 6.88 mm inferior (consistent with the right electrode being anterior, slightly medial, and superior relative to the left electrode). Relative to consensus coordinates for the STN (11–13 mm lateral to midline, 3–4 mm posterior to the midcommissural point, and 4–5 mm ventral to the intercommissural plane) (1), the left electrode was therefore medial and inferior, and the inferior contacts would be expected to be ventral to the STN and therefore situated in the substantia nigra on both sides. Direct visualization confirmed this (Figure 1).
The programmable implantable pulse generator was placed 11 days later, and initial programming was completed 11 days after placement of the generator. (For initial programming settings, see Table S1 in the online data supplement that accompanies the online edition of this article.) Over the next 3 months, the patient had an excellent response to DBS, and no changes were made to his stimulation settings. His carbidopa-levodopa dosage was decreased by 80%. Regarding his behavior, Mr. R reported having more energy, his wife noted that he was more helpful with chores, and his daughter said, “It’s like having a teenage boy in the house.” This initial “hypomanic” state was the first indication of developing adverse behavioral effects. The patient and family were asked to watch his behavior and report any concerns immediately.
Four and a half months after initial programming of the pulse generator, Mr. R reported more dyskinesia, prompting a decrease in his regular carbidopa-levodopa dosage and discontinuation of his entacapone and extended-release carbidopa-levodopa.
Two weeks later, he was seen emergently because his wife reported “acting-out and angry behavior.” On the way to his appointment, he became very agitated and crushed his eyeglasses, resulting in multiple facial lacerations. Given that turning off stimulation completely would have a profound negative impact on motor function, his DBS device was reprogrammed to use a more dorsal left electrode contact. This change was effected in order to shift stimulation away from the ventromedial aspect of the STN and substantia nigra. Unfortunately, this change worsened his motor control; bradykinesia and tremor were more pronounced. Therefore, pulse width and frequency of stimulation were increased (Figure 2). Two days later, Mr. R reported that his anger had resolved. Over the next 8 months, Mr. R did well and made only minor changes to the amplitude using the patient programmer (see Table S1 in the online data supplement). (In this clinic, most patients are provided the opportunity to increase stimulation by 0.1–0.2 V per day for rigidity, bradykinesia, or tremor and to decrease stimulation as much as needed to mitigate dyskinesia or problematic side effects. Patients are encouraged to make gradual adjustments, and the programmer determines the range within which a patient can make changes.)
Seventeen months after initial programming, Mr. R developed irritability, anger outbursts, cognitive decline, paranoid delusions, and visual hallucinations. He also complained of worsening memory and verbal fluency. His wife described him as disinhibited and “not himself” and reported that the formerly reticent man was now voluble. He exhibited paranoia, believing that his neighborhood was suddenly unsafe and that teenagers were breaking into his home. He tried to get his gun to protect his family. His behavior became reckless and unpredictable. His daughter reported that while she was driving along a cliff road with no guardrail, he swung his cane at her in an attempt to “get a rise out of her.” His family became so concerned for the patient’s and others’ safety that they hid his firearms.
Mr. R was seen immediately in the DBS clinic. As there were no recent programming changes to correlate with his behavioral changes, he was referred to psychiatry. Three weeks later, prior to his psychiatric evaluation, he began to note an improvement in mood and behavior despite there having been no programming changes at the previous visit. He was experiencing more pronounced bradykinesia, so he underwent reprogramming in the off-medication state, with retesting of each contact for symptomatic benefit and side effect profile. The electrode configuration was changed to double monopolar bilaterally (Figure 3) (also see Table S1 in the online data supplement).
Mr. R was evaluated by psychiatry 1 week after reprogramming, 4 weeks after he was referred. As noted, his mood and behavior had already improved prior to reprogramming. During the psychiatric evaluation, Mr. R and his wife denied any new symptoms other than cognitive decline. He denied a history of mood symptoms or formal psychiatric history except for mild untreated depressive symptoms while in college. Mr. R and his wife disclosed that 4 months before starting DBS, Mr. R had been involved in a motor vehicle collision at work. After the collision, he disclosed to his supervisor his diagnosis of Parkinson’s disease, which he had hidden for more than a decade. Working in a physically demanding profession, he requested accommodations for his Parkinson’s disease. He reports that his supervisor subjected him to increased job stress, forcing him into retirement against his wishes. Soon afterward, he filed a lawsuit against his employer. The transition to retirement was very difficult for the couple. Mr. R’s wife worked from home, and they fought over many minor domestic issues, such as whether or not the blinds could be opened during the day. During this period, he was having difficulty coping with the stress of his job loss, his lawsuit against his employer, and daily conflict with his wife. On the day when Mr. R swung his cane at his daughter while she was driving, he had been drinking alcohol excessively. Mr. R had continued to be agitated and difficult to manage for a few weeks after that incident. His wife said that he had been so difficult to manage that the family was considering placing him in a nursing home.
On mental status examination, Mr. R demonstrated a flat affect and limited verbal interaction. No other abnormalities were noted. His wife explained that this was normal, “how he is.” He was given the diagnoses of adjustment disorder with disturbance of emotions and conduct; alcohol use disorder; and mild cognitive disorder.
He underwent repeat neuropsychological testing 2 weeks later. His overall performance on cognitive function was average, with slight declines noted in the domains of perceptual reasoning, working memory, processing speed, executive function, phonemic fluency, and nondominant hand fine motor coordination. In contrast, his verbal comprehension improved, and he showed stable performance in tasks of attention, confrontation naming, semantic fluency, and visuospatial and visual memory.
With this second episode of behavior change, there was no direct correlation between the changes in his mood and behavior and the timing of DBS programming changes. His behavior change was therefore attributed primarily to the psychological impact of multiple stressors, including worsening Parkinson’s disease symptoms, his job loss and the lawsuit he was filing against his employer, and the difficult transition to being at home, where his wife worked full-time. Additionally, at least one behavioral incident could be attributed to alcohol intoxication.
Mr. R restricted his alcohol use, developed new hobbies, and he and his wife began to compromise at home. Both Mr. R and his wife reported that communication between them significantly improved. His depressive symptoms improved, and there were no residual behavioral concerns. His wife described him as “so much better.” The improvement persisted, and he has been doing well at all follow-up appointments.
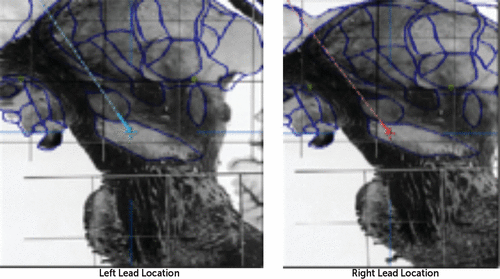
FIGURE 1. Lead Locations for Deep Brain Stimulation in a Patient With a 15-Year History of Parkinson’s Diseasea
a Sagittal views of left and right lead locations superimposed on the Schaltenbrand and Wahren atlas (2), lateral 10.5 slice. The center and tip of the leads were determined on coregistration of a preoperative T2-weighted MRI and a postoperative CT scan; a deformable atlas was then coregistered to the MR images.
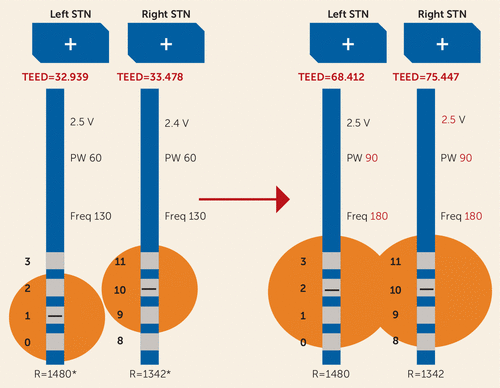
FIGURE 2. Deep Brain Stimulation Settings 5 Months After Initial Programminga
a This diagram presents total electrical energy delivered (TEED) by size as a visual aid, but TEED does not always equate to radius stimulated; it depends on variables such as number of active contacts, impedance of different tissues, frequency, and so on. For instance, increasing the number of active contacts decreases the impedance, resulting in higher flow of electrical current and greater surface area of the electrical contacts (3). STN=subthalamic nucleus; V=volts; PW=pulse width; Freq=frequency; R=impedance (an asterisk indicates estimated impedance—not measured before programming).
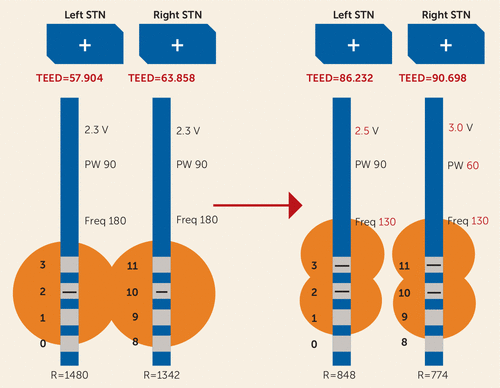
FIGURE 3. Deep Brain Stimulation Settings 18 Months After Initial Programminga
a As in Figure 2, this diagram presents total electrical energy delivered (TEED) by size as a visual aid, but TEED does not always equate to radius stimulated. STN=subthalamic nucleus; V=volts; PW=pulse width; Freq=frequency; R=impedance.
Discussion
Deep brain stimulation (DBS) is an effective treatment for motor symptoms in advanced Parkinson’s disease (4). Most commonly, DBS electrodes are placed in the subthalamic nucleus (STN) but can also be placed in the globus pallidus pars interna (GPi). The STN comprises three main functional subterritories. The dorsolateral region is the motor territory, the ventromedial portion is the associative territory, and the anteromedial region subserves limbic function (5, 6).
Adverse Behavioral Effects Associated With DBS for Parkinson’s Disease.
Adverse behavioral effects of DBS in Parkinson’s patients are recognized but not well understood. In a systematic review of 82 published reports (1,398 patients), Temel et al. (7) noted impaired cognition (41%), depression (8%), hypomania (4%), anxiety disorders (2%), attempted suicide (0.4%), and personality changes, hypersexuality, apathy, and aggression (<0.5%). In another study (8), 17 Parkinson’s patients treated with STN DBS were compared with 22 Parkinson’s patients treated with medical therapy. At baseline, the only statistically significant between-group difference was that the DBS group had a higher rate of depression. After implantation, there were no significant between-group differences in the physical symptoms of depression, but the DBS group experienced increased cognitive-emotional symptoms of depression. There was one suicide in the DBS group and none in the non-DBS group. Case reports suggest that stimulation of the ventromedial aspect of the STN (5, 9, 10) and of the underlying substantia nigra may result in manic behavior (11, 12) or acute depression (13). York et al. (14) reported an association between decline in mood and placement of the leads inferiorly and laterally to the STN.
Stimulation of the STN or GPi has comparable benefit for motor symptoms, although behavioral side effects vary (15–18). A prospective 4-year study (15) of 49 patients found that adverse behavioral effects were more common in patients treated with STN DBS (53%) than in those treated with GPi DBS (35%). The patients who developed behavioral effects had, on average, a longer illness duration, more gait disturbance, and more psychiatric symptoms at baseline. In contrast, a randomized controlled trial (16) across five centers in the Netherlands (65 patients with GPi DBS and 63 with STN DBS) found no differences between groups in cognition, mood, or behavior after 1 year. Likewise, in a prospective blind trial, Okun et al. (17) found no differences in mood or cognition between STN and GPi with optimized DBS stimulation parameters. However, a meta-analysis of six trials (563 patients) comparing STN and GPi DBS (18) found equal improvement in motor symptoms at 1 year and a significantly greater improvement in depression symptoms in the GPi DBS group, as measured by the Beck Depression Inventory. Additionally, STN DBS was significantly more effective in reducing dopaminergic medication, which can have the additional benefit of improving impulse control.
Impulsive behaviors are present in almost one-third of patients with Parkinson’s disease (19), and they are also reported as adverse behavioral effects of STN DBS (20). However, the data are inconclusive, with studies showing that preoperative impulse control disorders may resolve, improve, worsen, or show no change after STN DBS (21).
Mood changes, such as increased anger, have also been reported. In a prospective open-label study, Burdick et al. (22) compared the anger subscore of the Visual Analog Mood Scales in patients undergoing unilateral DBS of the STN (N=195) or GPi (N=56) for Parkinson’s disease and of the ventral intermediate nucleus of the thalamus (N=71) for essential tremor. At 1 to 3 months after stimulation, both the STN and GPi DBS groups exhibited significantly more anger. For every year of illness duration, the anger subscore increased by 0.24. A higher number of electrode passes during surgery was associated with increased anger scores, and anger subscores did not change when the DBS was turned off, supporting a lesion effect rather than a stimulation-induced effect.
Premorbid psychiatric conditions increase the risk of psychiatric adverse effects. Significant active residual symptoms are a contraindication to DBS (23–25). Dementia is also a contraindication. A decrease greater than one standard deviation from the premorbid IQ suggests a neurodegenerative process and high susceptibility for further decline after surgery (26).
Stimulation parameters and electrode configuration can contribute to adverse behavioral effects. The goals of DBS programming are to select contact(s) for stimulation and to determine optimal stimulation parameters. This is accomplished by adjusting amplitude, pulse width, and frequency (3). Use of higher amplitudes (>3 V) has been associated with an increased risk of mania (27). The amount of the applied stimulation can be calculated using the total electrical energy delivered (TEED) formula (28):

The mode of stimulation can be either monopolar or bipolar. In monopolar mode, the implantable pulse generator is selected as the anode, and one or several contacts are chosen as the cathode; in bipolar mode, both anode(s) and cathode(s) are within the DBS electrode (3). Monopolar configurations generate a weaker electrical field but the largest volume of stimulation. Bipolar configurations are therefore often used to minimize side effects. Changes in the active contact (or contacts) that shift electrical field away from limbic and associative circuits within the STN and underlying substantia nigra, as with the selection of a more dorsal contact, can reduce adverse behavioral effects.
In summary, behavioral changes after DBS are associated with several factors: the trajectory of electrodes during placement, the number of electrode passes, the specific target chosen, the stimulation parameters used, the electrode configuration, concurrent dopaminergic medication use, premorbid psychiatric illness, and the natural course of Parkinson’s disease.
Our Patient.
Mr. R’s mood and behavioral changes did not consistently correlate with DBS programming parameter changes. The psychiatric examination revealed an adjustment disorder, alcohol misuse in the context of multiple stressors, and increased cognitive decline, all of which can cause mood and behavioral changes. Although adverse behavioral effects are common after DBS, not all behavioral and mood changes are related to DBS. The differential diagnosis for mood and behavior changes in the context of DBS includes complications of DBS, mood and behavioral changes due to Parkinson’s disease, substance use disorders, medication side effects, dementia, a primary mood or psychotic disorder, and psychosocial conflict.
Conclusions
DBS surgery is a dramatic and effective treatment for Parkinson’s disease–related motor symptoms. DBS does not improve cognition or other nonmotor symptoms of Parkinson’s disease, and it has been associated with significant adverse behavioral effects in some patients. In Mr. R’s case, adjustment of DBS programming parameters seemed directly related to symptom improvement after his first episode of behavior changes. Total electrical energy delivered (TEED) actually increased, but the left STN contact had been changed to a more dorsal contact, likely pulling stimulation away from the substantia nigra as well as from limbic and associative circuits. However, his second episode of increased anger, impulsivity, and irritability did not correspond to programming changes. A careful consideration of the location of active contacts and TEED did not explain the patient’s sudden change in mood and behavior or the resolution of his mood and behavior symptoms. During Mr. R’s second episode of increased irritability, depression, and mood lability, he had been drinking alcohol excessively in the context of marital conflict and life stress. These factors contributed to his mood and behavior changes, in the context of a progressive neurodegenerative disease. In our opinion, these psychiatric symptoms cannot be directly attributed to DBS. The preponderance of the evidence suggests that the patient’s progressive neurocognitive disorder, his alcohol use, and his diagnosis of adjustment disorder with disturbance of emotions and conduct contributed to his behavioral changes. This case highlights the crucial role of the psychiatrist in the assessment and treatment of patients with Parkinson’s disease undergoing DBS.
1 : Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21(suppl 14):S247–S258Crossref, Medline, Google Scholar
2 : Atlas for Stereotaxy of the Human Brain, 2nd ed. Edited by Walker EA. Stuttgart, Thieme Medical Publishers, 1977Google Scholar
3 : Deep Brain Stimulation Programming: Principles and Practice. New York, Oxford University Press, 2010Google Scholar
4 : Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 2006; 21(suppl 14):S290–S304Crossref, Medline, Google Scholar
5 : Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PLoS One 2012; 7:e29153Crossref, Medline, Google Scholar
6 : The subthalamic nucleus in the context of movement disorders. Brain 2004; 127:4–20Crossref, Medline, Google Scholar
7 : Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord 2006; 12:265–272Crossref, Medline, Google Scholar
8 : Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson’s disease. Eur J Neurol 2012; 19:121–127Crossref, Medline, Google Scholar
9 : Unmasking psychiatric symptoms after STN DBS in Parkinson’s disease. Acta Neurol Scand Suppl 2008; 188:41–45Crossref, Medline, Google Scholar
10 : Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a review of the literature. J Neuropsychiatry Clin Neurosci 2012; 24:102–110Crossref, Medline, Google Scholar
11 : Contact dependent reproducible hypomania induced by deep brain stimulation in Parkinson’s disease: clinical, anatomical, and functional imaging study. J Neurol Neurosurg Psychiatry 2011; 82:607–614Crossref, Medline, Google Scholar
12 : Manic behaviour induced by deep-brain stimulation in Parkinson’s disease: evidence of substantia nigra implication? J Neurol Neurosurg Psychiatry 2006; 77:1363–1366Crossref, Medline, Google Scholar
13 : Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 1999; 340:1476–1480Crossref, Medline, Google Scholar
14 : Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. J Neurol Sci 2009; 287:159–171Crossref, Medline, Google Scholar
15 : Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord 2008; 23:416–421Crossref, Medline, Google Scholar
16 : Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013; 12:37–44Crossref, Medline, Google Scholar
17 : Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol 2009; 65:586–595Crossref, Medline, Google Scholar
18 : Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg 2014; 121:709–718Crossref, Medline, Google Scholar
19 : Impulsive behavior and associated clinical variables in Parkinson’s disease. Psychosomatics 2011; 52:41–47Crossref, Medline, Google Scholar
20 : Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007; 318:1309–1312Crossref, Medline, Google Scholar
21 : Impulse control disorders following deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: clinical aspects. Parkinsons Dis 2011; 2011:658415 (http://dx.doi.org/10.4061/2011/658415)Medline, Google Scholar
22 : Do patients get angrier following STN, GPi, and thalamic deep brain stimulation. Neuroimage 2011; 54(suppl 1):S227–S232Crossref, Medline, Google Scholar
23 : Pearls in patient selection for deep brain stimulation. Neurologist 2007; 13:253–260Crossref, Medline, Google Scholar
24 : Deep brain stimulation for Parkinson’s disease: patient selection and evaluation. Mov Disord 2002; 17(suppl 3):S94–S101Crossref, Medline, Google Scholar
25 : Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 2011; 68:165–171Crossref, Medline, Google Scholar
26 : Neuropsychological assessment for management of patients with deep brain stimulation. Mov Disord 2002; 17(supp 3):S116–S122Crossref, Medline, Google Scholar
27 : Voltage-dependent mania after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a case report. Biol Psychiatry 2011; 70:e5–e7Crossref, Medline, Google Scholar
28 : Calculating total electrical energy delivered by deep brain stimulation systems. Ann Neurol 2005; 58:168–169Crossref, Medline, Google Scholar



