A Diffusion Tensor Imaging Study in Children With ADHD, Autism Spectrum Disorder, OCD, and Matched Controls: Distinct and Non-Distinct White Matter Disruption and Dimensional Brain-Behavior Relationships
Abstract
Objective:
Neurodevelopmental disorders (NDDs) (attention deficit hyperactivity disorder [ADHD], autism spectrum disorder [ASD], and obsessive-compulsive disorder [OCD]) share genetic vulnerability and symptom domains. The authors present direct comparison of structural brain circuitry in children and adolescents with NDDs and control subjects and examine brain circuit-behavior relationships across NDDs using dimensional measures related to each disorder.
Method:
Diffusion imaging and behavioral measures were acquired in 200 children and adolescents (ADHD: N=31; OCD: N=36; ASD: N=71; controls: N=62; mean age range: 10.3–12.6 years). Following Tract-Based Spatial Statistics, multigroup comparison of white matter indices was conducted, followed by pairwise comparisons. Relationships of fractional anisotropy with dimensional measures of inattention, social deficits, obsessive-compulsive symptoms, and general adaptive functioning were conducted across the NDD sample.
Results:
Lower fractional anisotropy within the splenium of the corpus callosum was found in each NDD group, compared with the control group. Lower fractional anisotropy in additional white matter tracts was found in the ASD and ADHD groups, compared with the control group, but not in the OCD group. Fractional anisotropy was lower in the ASD and ADHD groups compared with the OCD group but was not different in ADHD participants compared with ASD participants. A positive relation between fractional anisotropy (across much of the brain) and general adaptive functioning across NDDs was shown.
Conclusions:
This study identified disruption in interhemispheric circuitry (i.e., fractional anisotropy alterations in the corpus callosum) as a shared feature of ASD, ADHD, and OCD. However, fractional anisotropy alterations may be more widespread and severe in ASD and ADHD than in OCD. Higher fractional anisotropy throughout the brain appears to be related to better adaptive function across NDDs.
Attention deficit hyperactivity disorder (ADHD), pediatric obsessive-compulsive disorder (OCD), and autism spectrum disorder (ASD) are relatively common childhood neurodevelopmental disorders (NDDs). In line with evidence that the higher-order tasks that are impaired in NDDs (e.g., social communication in ASD, regulation of attention and behavior in ADHD and OCD) rely on tight control of activation across neural networks (1), neuroimaging studies have recently been focused on understanding network connectivity in NDDs. Although published studies consistently implicate differences in structural and functional connectivity measures in a single NDD group, compared with controls (2–4), we have yet to uncover disease-specific neurobiological features for any NDD. Consistent with neuroimaging findings that are not specific to any one NDD, considerable phenotypic (5, 6) and genetic overlap has been found between NDDs (7). For example, some of the same genes that are involved in regulating neural migration and synaptic development (e.g., ASTN2, contactin-associated proteins) have been implicated across cases of OCD, ASD, and ADHD (7, 8). Nonspecific and overlapping findings across these disorders suggest that early developmental disruption of neural connections may be a common etiopathogenic risk factor across different NDDs.
Diffusion tensor imaging (DTI) is the only MRI-based neuroimaging method that can infer properties of structural brain connectivity in vivo. Fractional anisotropy, the most widely reported DTI index in NDDs (2, 4, 9, 10), provides a measure of directionally dependent water molecule diffusion that correlates with coherence and integrity of neural fibers (11). Voxel-wise analyses applied to DTI data enable exploration of white matter microstructure across the brain (large-scale structural connectivity). In NDDs, voxel-wise DTI studies largely point to decreased fractional anisotropy affecting widespread regions housing white matter connections in ASD (2), increased and decreased fractional anisotropy most commonly localized to the anterior corona radiata, internal capsule, corpus callosum, and cerebellar white matter in ADHD (4), and fractional anisotropy values that are either no different or higher for voxels along the cingulum bundle and corpus callosum in OCD (3), compared with controls. However, small sample sizes, and conflicting results, continue to limit our understanding of how neural connectivity differs in NDDs.
To date, no voxel-wise DTI study, to our knowledge, has directly compared different NDDs with each other (12, 13). Direct comparison of different NDDs may provide new understanding of biological mechanisms that are either shared between disorders or unique to any one disorder. These types of insight may be vital for treatment innovation and development of biologically informed classification systems. In addition, some symptoms and general functional impairment are often shared across NDDs (e.g., attention problems in ADHD and ASD [6], repetitive behaviors in ASD and OCD [5], general functional impairment across ADHD, ASD, and OCD [14, 15]). Exploration of the relations between neural circuitry and dimensional behavioral impairment may uncover brain-behavior relationships in a manner not possible using a categorical, disease-based approach (16).
In the present study, Tract-Based Spatial Statistics (17), a voxel-wise approach optimized for examination of diffusion properties of white matter across the brain, was applied in a relatively large sample including children and adolescents diagnosed with ADHD, ASD, or OCD and controls. Our primary aims were to 1) compare DTI indices across NDD groups and controls to see whether the direction and extent of white matter alterations were distinct for different NDDs and 2) when “lumping” NDD participants together, to assess differences with controls and relations with clinical symptom dimensions that cut across NDDs. Based on previous case-control DTI studies, we hypothesized that 1) fractional anisotropy would be lower in corpus callosum and fronto-striatal (corona radiata and internal capsule) white matter in ASD and ADHD but not OCD (2–4), when compared with controls, respectively, and 2) fractional anisotropy would correlate with disease burden for clinical symptoms that cut across NDDs.
Method
Study Participants
A total of 234 participants (NDDs, N=170; controls, N=64) in this study were recruited through the Province of Ontario Neurodevelopmental Disorders Network (POND) and from the Hospital for Sick Children and Holland Bloorview Kids Rehabilitation Hospital (Toronto). Clinical participants were included if they had a primary clinical diagnosis of ADHD, OCD, or ASD, sufficient English comprehension to complete required testing, and no contraindications for MRI. Previous clinical diagnoses were confirmed using the Parent Interview for Child Symptoms (18) for ADHD, the Children’s Yale-Brown Obsessive Compulsive Scale (19) for OCD, and the Autism Diagnostic Observation Schedule-2 (20) and Autism Diagnostic Interview-Revised (21) for ASD. Control participants were recruited through flyers posted at the Hospital for Sick Children and in the community, as well as through word-of-mouth, from 2011 to 2014. Exclusion criteria for controls included history of premature birth (<35 weeks) or presence of a neurodevelopmental, psychiatric, or neurologic diagnosis on interview. Full-scale IQ was estimated in the current sample using age-appropriate Wechsler or Stanford Binet scales. Each participating institution received approval for this study from their respective research ethics boards. Written, informed consent/assent from primary caregivers/study participants was obtained after complete description of the study was given.
Continuous Behavioral Measures Used for Brain-Behavior Analyses
Associations between white matter structure and behavior across children with NDDs focused on measures of attention problems, social deficits, and obsessive-compulsive symptoms (i.e., continuous symptom scores that are both characteristic of one NDD and expressed in varying degrees across other NDDs) and a general measure of adaptive functioning, capturing cross-disorder functional impairment that is not characteristic of any one disorder (22). The attention problem subscale from the Child Behavior Checklist (23) for 6–18 year-olds, an age-appropriate standardized parent-report questionnaire, was used to measure attention problems. The Toronto Obsessive-Compulsive Scale (24), a 21-item parent or youth self-report scale, provided a quantitative measure of obsessive-compulsive features. The Social Communication Questionnaire, a 40-item scale adapted from the Autism Diagnostic Interview-Revised, was used to measure social communication deficits (25). The General Adaptive Composite from the Adaptive Behavior Assessment System-II (26) was used to provide a general measure of adaptive functioning. For additional information on continuous behavioral measures, see the data supplement accompanying the online version of this article.
Participants’ Demographic Characteristics
After quality control of our imaging data, the analyzed sample totaled 200 (ADHD, N=31; OCD, N=36; ASD, N=71; control, N=62) (the demographic characteristics of the sample are summarized in Table 1; also see Tables S1 and S2 in the online data supplement detailing psychotropic medication use and additional symptom data). In the 34 (of 234) participants who were excluded due to failure to pass imaging quality control, NDD status was as follows: ADHD, 16/47 (34%); ASD, 13/84 (15%); OCD, 3/39 (8%); control, 2/64 (3%). After exclusion of subjects with missing behavioral scores, brain-behavior analyses were carried out across 106 of the 138 children with NDDs (ADHD, N=27; ASD, N=60; OCD, N=19). Because age ranges differed between the OCD group and both the ADHD group and the control group, participants were matched for age prior to carrying out OCD versus control and ADHD versus OCD pairwise comparisons. No prior published study, to our knowledge, has reported on the imaging data presented for participants included in the present study.
| Characteristic | Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ASD (N=71) | 2. ADHD (N=31) | 3. OCD (N=36) | 4. Control (N=62) | Analysis | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | Post Hoc | |
| Age (years) | 11.4 | 3.4 | 10.3 | 1.8 | 12.6 | 2.6 | 10.8 | 2.8 | 4.3 | 3, 196 | 0.006 | 3>4, 2 |
| N | % | N | % | N | % | N | % | χ2 | df | p | Post Hoc | |
| Gender (male) | 56 | 78.9 | 25 | 80.6 | 22 | 61.1 | 37 | 59.7 | 8 | 3 | 0.047 | Male:female ratio=1, 2>4 |
| Handedness (right) | 70 | 98.9 | 30 | 96.8 | 32 | 88.9 | 61 | 98.4 | 7.8 | 3 | 0.05 | |
| Taking psychiatric medications | 29 | 40.8 | 13 | 41.9 | 13 | 36.1 | 0 | 0 | 0.3 | 2 | 0.86 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | Post Hoc | |
| Full-scale IQ | 95.0 | 19.7 | 103.4 | 12.6 | 112.5 | 17.1 | 112.5 | 17.1 | 10.8 | 3, 147 | <0.001 | 4, 3>1 |
| N | % | N | % | N | % | N | % | |||||
| Documented comorbidity (yes) | 3 | 4.2 | 23 | 74.2 | 13 | 36.1 | ||||||
| Secondary neurodevelopmental disorder | ||||||||||||
| ADHD | 2 | 2.8 | 6 | 16.7 | ||||||||
| ASD | 2 | 6.5 | ||||||||||
| OCD | 1 | 3.2 | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | Post Hoc | |||
| Toronto Obsessive-Compulsive Disorder Scale score | –5.5 | 20.2 | –17.4 | 22.4 | 16.1 | 19.1 | 2.7 | 2, 120 | <0.001 | 3>1>2 | ||
| Social Communication Questionnaire score | 19.3 | 7.5 | 7.7 | 5.3 | 4.7 | 4.6 | 66 | 2, 120 | <0.001 | 1>2, 3 | ||
| Child Behavior Checklist | ||||||||||||
| Attention problem T score | 67.3 | 9.6 | 72.9 | 7.0 | 59.3 | 6.8 | 19 | 2, 113 | <0.001 | 2>1>3 | ||
| Adaptive Behavior Assessment System-II | ||||||||||||
| General adaptive composite scaled score | 68.2 | 14.1 | 74.0 | 12.8 | 95.9 | 20.0 | 23.7 | 2, 101 | <0.001 | 3>1, 2 | ||
| Functional academics scaled score | 6.2 | 3.0 | 6.7 | 2.8 | 10.8 | 3.9 | 22 | 2, 107 | <0.001 | 3>1, 2 | ||
TABLE 1. Demographic Characteristics of Children and Adolescents With Autism Spectrum Disorder (ASD), Attention Deficit Hyperactivity Disorder (ADHD), and Obsessive-Compulsive Disorder (OCD) and Control Subjects
MRI
All brain imaging for the present study was performed on a 3T MRI (Siemens, Tim Trio, Malvern, Pa.) system at the Hospital for Sick Children, using a 12-channel head coil. Anatomical scans were acquired using a three-dimensional T1-weighted MPRAGE sequence (field of view=192×240×256 mm, 1 mm cubic voxels, time to repeat/echo time/TI=2,300 ms/2.96 ms/900 ms, fractional anisotropy=9°, GRAPPA=2).
Diffusion imaging.
Diffusion scans were acquired using a two-dimensional diffusion-weighted echoplanar imaging sequence (axial, field of view=244×244 mm, 70 interleaved 2-mm thick slices, 2×2 mm2 in-plane resolution, time to repeat/echo time=8,800 ms/87 ms, GRAPPA=2, b=1,000 seconds/mm2, 60 directions) (for further information regarding diffusion imaging acquisition, see the online data supplement).
Image Analysis
Data were analyzed offline using a combination of FSL [FMRIB Software Library] Diffusion Toolbox (27) and locally developed software. Diffusion-weighted scans were registered to the nondiffusion-weighted image by affine transformations to minimize distortions due to eddy currents and head motion.
Quality control.
To remove the influence of slice-wise artifacts per encoding direction in the raw diffusion images, we calculated (for each participant) each slice’s mean within-brain image intensity per encoding direction and the slice’s standard deviation across all encoding directions, excluding b=0 images. Participants who had more than five direction means that exceeded the standard deviation of that slice were removed from further analysis by a rater blind to diagnosis.
Following removal of nonbrain tissue, the diffusion tensor was fitted at each voxel using FSL Diffusion Toolbox software (27), producing diffusion maps for λ1, λ2, λ3, fractional anisotropy, and mean diffusivity. For image analyses using Tract-Based Spatial Statistics (17), each study participant's fractional anisotropy map was registered to fractional anisotropy maps for all other participants to identify the most representative map from the current data set (scan requiring the least average warping to align with other scans). All fractional anisotropy maps were then registered to this data set-derived pediatric template. Following visual inspection to ensure quality of registration, a mean of all aligned fractional anisotropy maps was used to create a skeletonized image representing the center of white matter tracts throughout the brain that were common across subjects. The white matter skeleton was thresholded to include voxels with fractional anisotropy values >0.2, to suppress areas of low fractional anisotropy and/or high intersubject variability. Each participant’s aligned fractional anisotropy map was then projected onto the mean skeleton, and fractional anisotropy values were taken from the nearest relevant tract center (i.e., local maxima) for voxel-wise comparisons (17). In the present study, we chose to focus our analyses on fractional anisotropy (reported below). See additional details of analyses of mean, radial, and axial diffusivity measures in the online data supplement.
Statistical Analyses
Voxel-wise analyses were performed nonparametrically with permutation-based analysis using Randomize (FSL) (28) and the threshold-free cluster enhancement method (29). Statistical maps were then thresholded at a p value <0.05, fully corrected for multiple comparisons across space using family-wise error. The most probable anatomic localization of each significant cluster was determined using gray matter, white matter, and the John Hopkins University white matter tractography atlas tools in FSL (30).
Analysis 1.
To examine structural properties of white matter that were distinct or nondistinct across different NDDs, a univariate test comparing fractional anisotropy across the ASD, ADHD, OCD, and control groups at the same time was conducted and followed by pairwise (case-control and case-case) voxel-wise comparisons between groups.
Analysis 2.
To examine how structural connectivity might differ between all individuals with an NDD and controls, we conducted a pairwise comparison between an NDD group versus controls, matching for age and sex and excluding participants with below-average IQ. We then examined the relation between structural connectivity in all NDD participants and our four continuous behavioral measures.
To control for potentially confounding variables, all analyses were run while controlling for: age, age2, sex, and medication status. Because medication status involved a number of different medication classes in different combinations (see Table S1 in the data supplement), for the present study we coded medication status for participants as medicated (coded 1) versus unmedicated (coded 0).
Results
Analysis 1
Multigroup analysis showed that fractional anisotropy differences among all four groups were localized to voxels within the splenium of the corpus callosum (F=8.8, df=3, 196, p<0.001; significance remained when IQ was added into the model as a covariate). Post hoc pairwise comparisons indicated that splenium fractional anisotropy values that differed on multigroup analysis were lower in each NDD group, compared with controls, but were not different between NDDs (Figure 1). Splenium fractional anisotropy findings remained significant when multigroup analysis was rerun including only males (see the online data supplement).
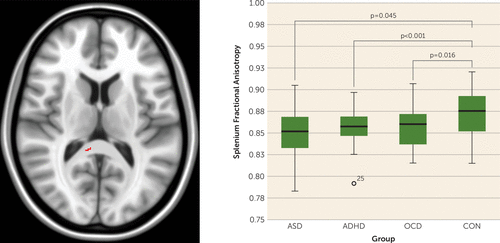
FIGURE 1. Differences in Splenium Fractional Anisotropy Found on Multigroup Comparison of Autism Spectrum Disorder (ASD), Attention Deficit Hyperactivity Disorder (ADHD), Obsessive-Compulsive Disorder (OCD), and Control (CON) Groupsa
a Voxels within the splenium of the corpus callosum where white matter fractional anisotropy differed between groups on multigroup comparison of ASD, ADHD, OCD, and controls are presented in the left image (F=8.8, df=3, 196, p<0.001). All voxels displayed in red (left image) are significant at a p value <0.05, fully corrected for multiple comparisons across space using family-wise error. The boxplot on the right shows results of post hoc pairwise comparisons indicating that splenium fractional anisotropy values that differed on multigroup analysis were lower in each neurodevelopmental disorder group, compared with controls, but were not different between neurodevelopmental disorders.
On voxel-wise case-case and case-control pairwise comparisons, we found lower fractional anisotropy in the ASD group compared with the control group and in the ADHD group compared with the control group. Affected voxels were located along all cortico-cortical, interhemispheric, and cortico-striatal fibers. Significant fractional anisotropy differences were not found in the OCD group, compared with controls. Lower fractional anisotropy was found in the ASD group compared with the OCD group for voxels located along all cortico-cortical, interhemispheric, and cortico-striatal fibers and in the ADHD group compared with the OCD group for voxels along the anterior thalamic radiation, genu of the corpus callosum, cortico-spinal tract, arcuate, and inferior-fronto-occipital fasciculi (see the data supplement for results of post hoc analyses examining relations between clinical symptoms and findings on pairwise comparison of the ADHD and OCD groups). No significant fractional anisotropy differences were found in the ASD group compared with the ADHD group (see Figure 2 for results of pairwise comparisons).
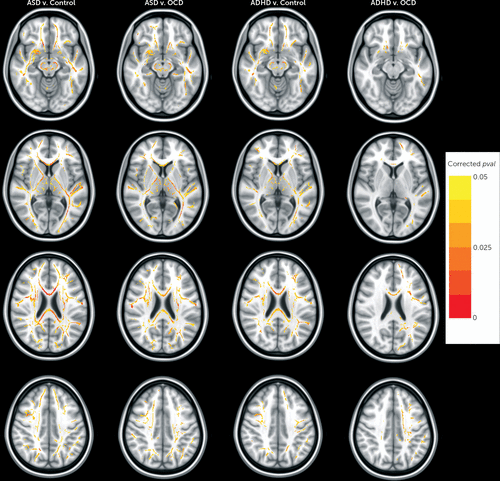
FIGURE 2. Significant White Matter Anisotropy Differences Found on Pairwise Case-Case and Case-Control Comparisonsa
a Each column represents results from voxel-wise pairwise comparison where a significant difference in fractional anisotropy was found (columns left to right: autism spectrum disorder [ASD] versus control, ASD versus obsessive-compulsive disorder [OCD], attention deficit hyperactivity disorder [ADHD] versus control, ADHD versus OCD). All voxels displayed in red or yellow are significant at a p value <0.05, fully corrected for multiple comparisons across space using family-wise error.
On multigroup comparison of additional diffusivity measures, axial diffusivity was found to be significantly different among our four groups within voxels corresponding to the left thalamus and extending into the internal capsule (F=18.9, df=3, 196, p<0.001). No significant differences were found for mean or radial diffusivity (see the data supplement for further details).
Analysis 2
NDD versus control comparison showed lower fractional anisotropy in the NDD group for voxels along the genu and splenium of the corpus callosum, cortico-spinal tract, inferior longitudinal, arcuate, and inferior-fronto-occipital fasciculi (see Figure S2 in the data supplement). Across all NDD participants, significant positive correlations were found between adaptive functioning scores and fractional anisotropy in voxels along the genu and splenium of the corpus callosum, cortico-spinal tract, inferior longitudinal, arcuate, and inferior-fronto-occiptal fasciculi (F=15.99, df=1, 102, p=0.0001 [Figure 3]). Of note, voxels that were significantly associated with adaptive functioning across NDDs overlapped to a large degree with voxels exhibiting lower fractional anisotropy in the NDD group compared with the control group. No significant brain-behavior correlations were found on examination of additional continuous behavioral measures.
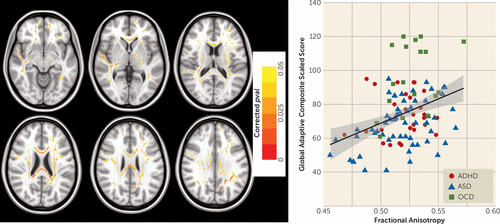
FIGURE 3. Relation Between White Matter Anisotropy and General Adaptive Functioning Across Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), and Obsessive-Compulsive Disorder (OCD)a
a The voxels where fractional anisotropy was positively correlated with General Adaptive Composite scores from the Adaptive Behavioral Assessment System-II across ASD, ADHD, and OCD participants are shown. All voxels displayed in red or yellow (left images) are significant at a p value <0.05, fully corrected for multiple comparisons across space using family-wise error. The scatterplot (right) shows linear regression illustrating a significant positive association between average white matter fractional anisotropy (for colored voxels depicted in the left images) and adaptive functioning score for each participant (F=15.99, df=1, 102, p=0.0001). (For visualization, a scaled range of fractional anisotropy values including all mean values found for our sample was used; the full range of potential fractional anisotropy scores for the present study was >0.2–1.)
Discussion
In the present study, we examined distinct and nondistinct features of structural brain connectivity in a large sample that included children and adolescents with and without NDDs. First, we found lower fractional anisotropy in voxels along the splenium of the corpus callosum in all three NDD groups, compared with the control group. Pairwise comparisons revealed that both the ASD and ADHD groups had reductions in fractional anisotropy in a number of additional white matter tracts, compared with controls, that were not found in the OCD group. No significant fractional anisotropy differences were found between the ASD and ADHD groups; however, reduced fractional anisotropy was present in both the ASD and ADHD groups compared with the OCD group. Examination of NDD participants together, compared with controls, revealed lower fractional anisotropy in NDD individuals in voxels along the genu and splenium of the corpus callosum, cortico-spinal tract, fronto-occipital, fronto-temporal, and occipito-temporal white matter connections; fractional anisotropy within these same white matter tracts was also associated with adaptive functioning across all NDD participants.
Our finding of reduced fractional anisotropy in the splenium that was not different between NDD groups is consistent with prior pediatric case-control studies finding lower splenium fractional anisotropy in either ADHD or ASD samples, compared with controls (2, 4). Splenium fractional anisotropy differences have also been shown in OCD samples compared with controls, although the direction of findings varies (3). Lower fractional anisotropy in brain white matter is reflective of decreased directional organization of neural fibers and may be due to changes in axon diameter, density, axonal packing properties, or myelin integrity (11). The corpus callosum facilitates interhemispheric connectivity/inhibition across the cortex (31). Corpus callosum structure in infancy predicts executive function in childhood and has been shown to correlate with tasks related to cognition in preschool-age children (32). Corpus callosum axons undergo extensive refinement in the postnatal period, and splenium fractional anisotropy increases rapidly over the first 10 years of life (33), a period that overlaps with the onset of NDDs. Given the role of the corpus callosum in interhemispheric connectivity across the cortex, early developmental disruption within this tract may influence structural development in related long-range white matter tracts that undergo more protracted maturation. In addition to fractional anisotropy findings, we found lower axial (but no difference in radial or mean) diffusivity in NDDs compared with controls in the present study (see the online data supplement). Although previous studies have interpreted axial and radial diffusivity differences in NDDs as a specific indicator of axonal or myelin disruption, respectively (2–4), evidence has indicated that this measure is sensitive to a number of additional factors in humans and may not be an appropriate indicator of specific tissue pathology (34).
In line with several previous studies, case-control comparisons showed lower fractional anisotropy that was neuroanatomically widespread in ADHD or ASD, compared with controls (4). However, no fractional anisotropy differences were found in the ADHD group compared with the ASD group, and fractional anisotropy differences did not extend beyond the splenium in the OCD group compared with the control group. Our results in ADHD and ASD are consistent with two recent imaging studies that directly compared connectivity patterns in children and adolescents with ASD or ADHD and controls (35, 36). These studies indicated that both ASD and ADHD are disorders involving large-scale connectivity impairments based on the diffuse nature of structural and functional connectivity differences found across the brain in each NDD compared with controls. The presence of a similar scale of disrupted connectivity in ASD and ADHD is consistent with the early timing of onset for each disorder, which corresponds to a period of dynamic maturational change for long-range white matter tracts across the brain (33). It is also consistent with evidence of enrichment for rare genetic variants that encode cell-adhesion and neuronal synapse scaffolding in both conditions (7). Although we did not find disease-specific fractional anisotropy alterations in ASD or ADHD in the present sample, two previous studies found differences between these disorders in terms of network topology (35, 36).
In contrast to the absence of significant differences between ADHD and ASD, we found differences in white matter structure in both the ASD group and the ADHD group, compared with the OCD group. Lower fractional anisotropy in ASD compared with OCD was widespread, while fractional anisotropy reductions in ADHD compared with OCD included voxels along the cortico-spinal tract, genu of the corpus callosum, and inferior-fronto-occipital fasciculus, frontal white matter connections that have been implicated in both ADHD and OCD previously (13, 37). It may be that the later timing of disease onset in pediatric OCD (middle-childhood as opposed to early childhood in ASD and ADHD) explains the more substantial and widespread fractional anisotropy reductions found in children and adolescents with ASD and ADHD compared with OCD in our sample. Post hoc analyses indicated that fractional anisotropy differences in fronto-cortical white matter tracts in the ADHD group compared with the OCD group were also negatively correlated with attention problems (characteristic ADHD symptoms) and positively correlated with obsessive-compulsive symptoms in participants with either ADHD or OCD (see the data supplement). Positive correlations between fronto-cortical fractional anisotropy and OCD symptoms (3) and negative associations between fronto-cortical fractional anisotropy and ADHD symptoms (4) have been found in separate case-control studies examining OCD and ADHD groups, compared with controls, respectively. These converging results may shed light on how divergent structural properties within the same fronto-cortical network may influence expression of seemingly opposing clinical symptoms (inattentive/impulsive versus obsessional/compulsive symptoms). However, these results must be considered preliminary, given the sample size of our pairwise ADHD-OCD comparison and the presence of diagnostic overlap in a small number of included participants.
Finally, we found lower fractional anisotropy in individuals with an NDD compared with controls in voxels along a number of long-range white matter connections with particularly prominent effects on the genu and splenium of the corpus callosum. Our brain-behavior analyses across all NDDs showed that general adaptive functioning was positively correlated with fractional anisotropy in voxels along major interhemispheric and cortico-cortical connections. The tissue properties of long-range white matter connections are thought to facilitate control over the speed and timing of activation across neural networks and are of critical importance for efficient performance in higher-order tasks (1) that support adaptive functioning. Impaired adaptive functioning is a part of the diagnostic criteria for each of the NDDs sampled in the present study (22). Unlike the clinical symptom measures used in the study, our measure of general adaptive functioning was not designed to support categorical diagnosis of any specific NDD and may therefore be more suitable for identifying neurobiological features that cut across different NDDs.
Adaptive functioning is most often measured in studies of ASD, wherein the level of adaptive impairment is not necessarily consistent with IQ or symptom severity among affected individuals (38). Despite a prior call for imaging research in ASD that considers dimensional quantifiers, such as adaptive functioning, to better understand brain-behavior relationships in this disorder (38), we are not aware of any imaging study that has examined the association of adaptive behavior with imaging measures in any NDD, nor of any studies that control for this variable. Recent work speaks to the importance of general adaptive functioning as a predictor of outcome in childhood NDDs (39). Therefore, heterogeneous functioning in NDDs may serve as an important confounding factor to be considered in future studies. As found in a recent longitudinal study of outcome in ASD, improvement in adaptive functioning is not necessarily associated with improvement in clinical severity (39). Novel interventions are needed to improve long-term outcome across NDDs. Our data suggest that intervention studies aimed at improving adaptive functioning may also investigate white matter fractional anisotropy as a mediating mechanism.
Although the present study is, to our knowledge, the largest pediatric imaging comparison of different neuropsychiatric disorders, our OCD and ADHD groups could still be considered moderately sized following rigorous quality control procedures, as necessary for pediatric neuroimaging studies (40). Therefore, sample size and potential effects of unequal sample sizes may have limited the overall power of our study to detect other white matter alterations and brain-behavior relationships in NDDs. Furthermore, just under 30% of NDD participants in the current sample were medicated, and the pattern of medication use (with different neurochemical effects) varied in NDD groups. Although we controlled for medication status in the present study, analysis of larger samples enabling the examination of the specific effects of various medications and medication-by-diagnosis interaction effects on white matter are still needed. In addition, our results in NDDs may not generalize to age ranges outside of those that we studied. Future efforts to collect large longitudinal NDD data sets and larger numbers of female participants are needed to further resolve discrepant findings in the literature and extend understanding of developmental trajectories, the influence of IQ, and sex-specific findings in these disorders. It is noteworthy that in the present sample, multigroup comparison findings were maintained when a male-only analysis was conducted.
Conclusions
We identified disruption in interhemispheric circuitry across ASD, ADHD, and OCD children and adolescents. However, white matter disruption, as indexed by lower fractional anisotropy values, was more widespread in ASD and ADHD participants, compared with OCD participants and controls. Finally, white matter disruption across NDD participants was related to impairment in adaptive functioning. Additional research examining the association between adaptive functioning and brain structure and function is warranted in NDDs.
1 : White matter in learning, cognition and psychiatric disorders. Trends Neurosci 2008; 31:361–370Crossref, Medline, Google Scholar
2 : Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex 2015; 62:158–181Crossref, Medline, Google Scholar
3 : Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. J Psychiatr Res 2014; 54:26–35Crossref, Medline, Google Scholar
4 : Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev 2012; 36:1093–1106Crossref, Medline, Google Scholar
5 : Autism and ADHD symptoms in patients with OCD: are they associated with specific OC symptom dimensions or OC symptom severity? J Autism Dev Disord 2010; 40:580–589Crossref, Medline, Google Scholar
6 Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry 2012; 51:1160–1172Crossref, Medline, Google Scholar
7 : Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med 2011; 3:95ra75Crossref, Medline, Google Scholar
8 : Disruption of the ASTN2/TRIM32 locus at 9q33.1 is a risk factor in males for autism spectrum disorders, ADHD and other neurodevelopmental phenotypes. Hum Mol Genet 2014; 23:2752–2768Crossref, Medline, Google Scholar
9 : White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology 2012; 37:2730–2739Crossref, Medline, Google Scholar
10 : Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry 2011; 70:1083–1090Crossref, Medline, Google Scholar
11 : The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002; 15:435–455Crossref, Medline, Google Scholar
12 : Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry 2009; 65:63–74Crossref, Medline, Google Scholar
13 : Imaging the ADHD brain: disorder-specificity, medication effects and clinical translation. Expert Rev Neurother 2014; 14:519–538Crossref, Medline, Google Scholar
14 : Brief report: Adaptive functioning in children with ASD, ADHD and ASD+ADHD. J Autism Dev Disord 2015; 45:2235–2242Crossref, Medline, Google Scholar
15 : Depression in youth with obsessive-compulsive disorder: clinical phenomenology and correlates. Psychiatry Res 2012; 196:83–89Crossref, Medline, Google Scholar
16 : Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 2012; 14:29–37Medline, Google Scholar
17 : Tract-Based Spatial Statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505Crossref, Medline, Google Scholar
18 : The Parent Interview for Child Symptoms: a situation-specific clinical research interview for attention-deficit hyperactivity and related disorders. Can J Psychiatry 2006; 51:325–328Crossref, Medline, Google Scholar
19 : Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852Crossref, Medline, Google Scholar
20 : The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000; 30:205–223Crossref, Medline, Google Scholar
21 : Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
22 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association Publishing, 2013Crossref, Google Scholar
23 : Child Behavior Checklist (CBCL 6-18). Burlington, Vt, University Associates in Psychiatry, 2001Google Scholar
24 : The Toronto Obsessive-Compulsive Scale: psychometrics of a dimensional measure of obsessive-compulsive traits. J Am Acad Child Adolesc Psychiatry 2016; 55:310–318Crossref, Medline, Google Scholar
25 : Autism Screening Questionnaire: diagnostic validity. Br J Psychiatry 1999; 175:444–451Crossref, Medline, Google Scholar
26 : Assessing Psychological Functioning in Metabolic Disorders: Validation of the Adaptive Behavior Assessment System, Second Edition (ABAS-II), and the Behavior Rating Inventory of Executive Function (BRIEF) for Identification of Individuals at Risk. JIMD Rep 2015; 21:35–43Crossref, Medline, Google Scholar
27 : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(suppl 1):S208–S219Crossref, Medline, Google Scholar
28 : Permutation inference for the general linear model. Neuroimage 2014; 92:381–397Crossref, Medline, Google Scholar
29 : Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44:83–98Crossref, Medline, Google Scholar
30 : Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230:77–87Crossref, Medline, Google Scholar
31 : Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002; 17:77–94Crossref, Medline, Google Scholar
32 : White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry 2013; 170:899–908Link, Google Scholar
33 : Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008; 40:1044–1055Crossref, Medline, Google Scholar
34 : About “axial” and “radial” diffusivities. Magn Reson Med 2009; 61:1255–1260Crossref, Medline, Google Scholar
35 : Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry 2013; 74:623–632Crossref, Medline, Google Scholar
36 : Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: a rich club-organization study. Hum Brain Mapp 2014; 35:6032–6048Crossref, Medline, Google Scholar
37 : Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br J Psychiatry 2008; 192:25–31Crossref, Medline, Google Scholar
38 : Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. J Autism Dev Disord 2007; 37:748–759Crossref, Medline, Google Scholar
39 : Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry 2015; 72:276–283Crossref, Medline, Google Scholar
40 : Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 2012; 60:623–632Crossref, Medline, Google Scholar



