Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders
Abstract
Impaired emotion regulation contributes to the development and severity of substance use disorders (substance disorders). This review summarizes the literature on alterations in emotion regulation neural circuitry in substance disorders, particularly in relation to disorders of negative affect (without substance disorder), and it presents promising areas of future research. Emotion regulation paradigms during functional magnetic resonance imaging are conceptualized into four dimensions: affect intensity and reactivity, affective modulation, cognitive modulation, and behavioral control. The neural circuitry associated with impaired emotion regulation is compared in individuals with and without substance disorders, with a focus on amygdala, insula, and prefrontal cortex activation and their functional and structural connectivity. Hypoactivation of the rostral anterior cingulate cortex/ventromedial prefrontal cortex (rACC/vmPFC) is the most consistent finding across studies, dimensions, and clinical populations (individuals with and without substance disorders). The same pattern is evident for regions in the cognitive control network (anterior cingulate and dorsal and ventrolateral prefrontal cortices) during cognitive modulation and behavioral control. These congruent findings are possibly related to attenuated functional and/or structural connectivity between the amygdala and insula and between the rACC/vmPFC and cognitive control network. Although increased amygdala and insula activation is associated with impaired emotion regulation in individuals without substance disorders, it is not consistently observed in substance disorders. Emotion regulation disturbances in substance disorders may therefore stem from impairments in prefrontal functioning, rather than excessive reactivity to emotional stimuli. Treatments for emotion regulation in individuals without substance disorders that normalize prefrontal functioning may offer greater efficacy for substance disorders than treatments that dampen reactivity.
The ability to monitor and control affect, or “emotion regulation,” refers to the processes by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions (1). Impairments in emotion regulation contribute to substance use disorder (substance disorder) development, persistence, and severity. In adolescence, difficulties in emotion regulation may increase the likelihood of initiating, or perpetuating, substance use (2, 3), and adults with substance disorders have more emotion regulation difficulties than comparison subjects (see review in reference 4). Individuals who use substances to relieve negative affect develop addictive patterns of drug use more quickly (2, 5), and emotion regulation difficulties are associated with greater substance use severity in individuals in whom a substance disorder has already developed (6, 7). As impaired emotion regulation would render an individual with a substance disorder more vulnerable to cue-induced cravings or impulsive responding (1), it is not surprising that impaired emotion regulation predicts poor response to treatment (8, 9) and accentuates the risk of relapse during negative affect (10).
Although several well-established pharmacologic treatments for anxiety disorders, depressive disorders, and other disorders associated with impaired emotion regulation have been tested in substance disorders (11), most show little or no effect on substance use. Identifying the neural circuitry underlying impaired emotion regulation, and how it differs from the neural circuitry in those with emotion regulation difficulties without substance disorders, may help identify important treatment targets for substance disorders. Once identified, normalization of the neural underpinnings of impaired emotion regulation in individuals with substance disorders could serve as a proximal marker of the substance disorder’s treatment response.
To provide a framework for identifying these alterations in neural circuitry, this review will first present different components of emotion regulation, the imaging tasks used to assess each component, and their associated neural circuitry. We will focus on studies that used task-based functional magnetic resonance imaging (fMRI) to examine functional connectivity (particularly resting state functional connectivity) and structural connectivity. The neural circuitry associated with impaired emotion regulation in individuals with dysregulated emotion without substance disorders (particularly anxiety, depressive, and borderline personality disorders) will be compared with the circuitry in people with substance disorders, with a focus on the amygdala, insula, and prefrontal cortex and associated networks. The review concludes with treatment implications and targets, limitations of the studies to date, and suggested future directions of research.
Four Dimensions Underlying Emotion Regulation
A number of conceptual approaches have been posited for emotion regulation (see reviews in references 1, 12–15). Although an in-depth discussion of these approaches is outside the scope of this review, we posit four dimensions of emotion regulation that are consistent with previous conceptual approaches. These dimensions—affect intensity/reactivity, affective modulation, cognitive modulation, and behavioral control—will provide an organizational schema to categorize the broad array of fMRI paradigms described (see reference 4). Commonly used self-report scales measuring emotion regulation can also be categorized into these four dimensions (see Table S1 in the data supplement accompanying the online version of this article), and impairments in all four of these dimensions are observed in substance disorders (4).
Affect Intensity/Reactivity
The initial affective response may occur outside of conscious awareness and prior to the engagement of most top-down modulatory processes (15, 16). Individuals with higher intensity (magnitude) and reactivity (degree of changeability) of affect may be more likely to suffer from emotional instability, especially if modulatory processes (described below) are not intact. Affect intensity/reactivity is tested by the rapid presentation (i.e., less than 2 seconds) of stressful, disturbing, or emotional cues. The short stimulus presentation timing induces intense affect but does not allow for a prominent regulatory response (17–22).
Affective Modulation and Cognitive Modulation
Two modulatory processes are involved in emotion regulation, each testable by distinct functional approaches and thus considered separately. These two strategies (affective modulation and cognitive modulation) roughly correspond to hot and cold executive functioning (23), implicit and explicit emotion regulation (14), and automatic and voluntary cognitive/behavioral emotion control (24), respectively. Affective modulation automatically engages processes that evaluate reward salience, assess environmental cues for potential threats, help with social and emotional functioning, and produce motivational biases in emotionally significant contexts. Cognitive modulation voluntarily engages processes involved in problem solving, strategic planning, and the conscious efforts to modulate internal affective states (13, 15, 16, 23).
Affective modulation tasks utilize paradigms exposing participants to prolonged (up to 2 minutes) exposure to negative cues, allowing for engagement of regulatory responses. Such tasks include reading a personalized stress script (25, 26) or a social stress task (Montreal Imaging Stress Task) (27), multiple negative stimuli presented over blocks of time (28), and cue conditioning, in which a neutral conditioned stimulus is paired with an unpleasant unconditioned stimulus, evoking a negative emotional response (29). Cognitive modulation tasks, which include “cognitive reappraisal” (16) and “reinterpretation” (30), require participants to use cognitive reframing techniques (reappraisal) to alter their emotional response to a stimulus (16, 30–35). For example, individuals may be shown negative images and asked to lessen the intensity of their emotional response (31).
Behavioral Control
Impulsivity refers to difficulties in regulating behavior. The behavioral control dimension is a subfacet of impulsivity related to engaging in a (maladaptive) behavior in the context of or in response to an intense emotion. Individuals with poor behavioral control are more likely to have a strong emotion “take over” their actions, corresponding to “negative urgency” (36) or “regulation” (16).
These tasks assess the effects of distracting affective stimuli. Examples include emotional go/no-go tasks (inhibition of prepotent responses are tested in the presence of emotionally distracting cues) (37), emotional oddball tasks (the ability to respond to an infrequent target is assessed in the setting of a disturbing cue) (38), emotional distractor tasks (threat-related distractors are presented during performance of simple cognitive tasks) (20, 39), conflict tasks (categorizing facial affect while ignoring overlaid affect label words) (40), and tasks of expressive suppression (participants are asked to “keep their face still” while watching negative images) (30, 32).
Neural Circuitry of Emotion Regulation
Selected Regions of Focus
In the following sections, the neural circuitry of emotion regulation in psychiatric populations with and without substance disorders is reviewed. We focus on five brain regions or groups of regions based on their essential roles in evaluating threatening stimuli, emotional processing, emotion regulation, and behavioral control (15, 16, 41–44). These regions roughly fall within two functional categories—emotion-generating or emotion-processing regions, i.e., amygdala and insula—and three emotion regulatory regions—1) the dorsomedial PFC (dmPFC), including the dorsal anterior cingulate cortex (dACC) and the presupplementary and supplementary motor area; 2) the lateral PFC (lPFC), including the lateral orbitofrontal cortex (lOFC) and ventrolateral PFC (vlPFC); and 3) the rostral ACC/ventromedial PFC (rACC/vmPFC), including the perigenual ACC, subgenual ACC, and medial OFC (mOFC)] (Table 1, Figure 1A) (15, 16, 41–44). Two general characteristics of intact emotion regulation processes are that emotion-generating/processing regions are activated by negative emotional stimuli and that this neural response is dampened by emotion regulatory regions, automatically by the rACC/vmPFC and voluntarily by the dmPFC and lPFC (15, 16, 24, 42).
| Region of Focus and Region With Functional Connections | Structurally Connected via |
|---|---|
| Emotion-generating and emotion-processing regions | |
| Amygdalab: assigns value to positive and negative emotional cues, is involved in fear conditioning, activates during stress and exposure to negative stimuli, generates fear response (21, 44) | |
| Insula | Ventrolateral branch of UF; ventral amygdalofugal pathway |
| dmPFC | Anteromedial branch of the UF; external capsule; cingulum |
| rACC/vmPFC | UF; cingulum; anterior corona radiata via the internal capsule; ventral amygdalofugal pathway; inferior thalamic peduncle/radiation |
| lPFC | Ventrolateral branch of UF; external capsule; cingulum |
| Amygdala | AC (interhemispheric connections) |
| Insula (SN)c: activates in response to salient stimuli (positive and negative cues), processes interoceptive information (ascending visceral inputs to insula) (42), activates during stress and exposure to negative stimuli, is involved in fear conditioning (21, 44) | |
| Amygdala | Ventrolateral branch of UF; ventral amygdalofugal pathway |
| dmPFC | Superior fronto-occipital fasciculus |
| rACC/vmPFC | Unnamed tracts (structural connectivity demonstrated through seed-based studies) |
| lPFC | Extreme capsule; short association fibers (fronto-insular tracts) |
| Insula | Midbody of the CC (interhemispheric connections) |
| Regulatory regions | |
| dmPFCd (includes dACC, preSMA, SMA) (CCN/ECN): activates in response to salient stimuli (positive and negative cues), is involved in performance monitoring/error monitoring, conflict processing, integrating emotional response during goal selection, response conflict, response execution (preSMA/SMA) (24, 41, 42, 44) | |
| lPFC | Short association fibers (frontal aslant tract) (intrahemispheric connections) |
| dmPFC | Regions of the CC (genu, rostrum, rostral body, anterior midbody, midbody) (interhemispheric connections) |
| rACC/vmPFC | Short association fibers (interhemispheric connections) |
| lPFCe (includes dlPFC, vlPFC, lOFC) (CCN/ECN): involved in planning, selection of goals, sequencing, holding information online (dlPFC), response inhibition (especially vlPFC/lOFC), conscious/voluntary regulation of amygdala and insula activation (24, 41, 42, 44) | |
| dmPFC | Short association fibers (frontal aslant tract) (intrahemispheric connections) |
| lPFC | Regions of the CC (genu, rostrum, rostral body, anterior midbody) (interhemispheric connections) |
| rACC/vmPFC | Short association fibers (interhemispheric connections) |
| rACC/vmPFCf (includes rACC, vmPFC, mOFC, pgACC, sgACC) (DMN): is involved in the subjective valuation of cues (assigns motivational salience and encodes outcome expectancies during emotional decision making, determines motivational priorities), is involved in self-referential introspection (tags information as personally relevant), processes emotional conflict (15, 48), mediates extinction (16), provides automatic/unconscious regulation of amygdala and insula activation (15, 24) | |
| dmPFC | Regions of the CC (genu, rostrum, rostral body) (interhemispheric connections) |
| rACC | Frontal forceps (interhemispheric connections) |
| lPFC | Short association fibers (frontal orbitopolar tract) (intrahemispheric connections) |
TABLE 1. Brain Regions of Focus, Functional Role in Emotion Regulation, and Related Connections (White Matter Tracts) in Substance Use Disordersa
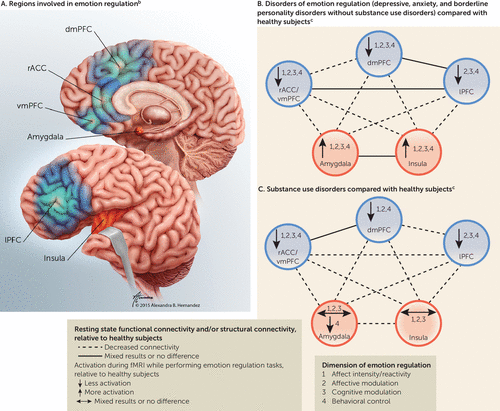
FIGURE 1. Brain Regions Involved in Emotion Regulation and Alterations in Depressive, Anxiety, and Borderline Personality Disorders and in Substance Use Disordersa
a The dorsomedial prefrontal cortex (dmPFC) includes the dorsal anterior cingulate cortex (dACC), presupplementary motor area (preSMA), and supplementary motor area (SMA). The lateral prefrontal cortex (lPFC) includes the dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), and lateral orbitofrontal cortex (lOFC). Other abbreviations: rostral anterior cingulate cortex (rACC), ventromedial prefrontal cortex (vmPFC).
b The illustration in part A is by Alexandra B. Hernandez of Gory Details (used by permission). Regions shaded in red are categorized as emotion-generating or emotion-processing regions; regions depicted in blue are categorized as regulatory regions. Further details about the roles of these regions in emotion regulation are specified in Table 1.
c Emotion regulation tasks were performed during functional magnetic resonance imaging.
These brain areas are also components of neural “networks,” defined by the strength of the temporal correlation of low-frequency fMRI blood oxygen labeled dependent (BOLD) fluctuations between discrete anatomical regions (45). This review focuses on the described regions rather than networks because some regions in the network overlap and some regions may be activated in isolation of a network (Table 1). The dmPFC and lPFC are considered part of the cognitive (or executive) control network (35, 41), which plays a critical role in “the internal representation, maintenance, and updating of context information in the service of exerting control over thoughts and behavior” (42, 46). The dmPFC and insula are components of the “salience network” (42, 43), which activates in response to and integrates information concerning salient stimuli during cognitive control (41). (The dmPFC is considered part of both the cognitive control and salience networks.) The rACC/vmPFC is an integral component of the “default mode network,” which is actively engaged during rest, mind wandering, and self-referential introspective states and is deactivated when executive control is engaged (45, 47–49).
Connections between regions and/or networks can be assessed with functional or structural connectivity; the former assesses temporal coherence between regions, and the latter uses diffusion tensor imaging to assess white matter connection. Although measures of functional and structural connectivity are frequently correlated, the strength of correlation varies with the network examined (50). Connectivity between regions may occur via indirect pathways, and consequently, functional connectivity may be observed in the absence of structural connectivity. Functional, relative to structural, connectivity also varies more across time (50). Higher levels of fractional anisotropy and lower levels of mean diffusivity are both markers of greater white matter integrity.
Neural Circuitry of Emotion Regulation During fMRI Tasks
The rACC/vmPFC and dmPFC (dACC) are activated during tasks of all four dimensions of emotion regulation, consistent with rACC/vmPFC involvement in automatic regulation and inhibition of intense affect (15, 16, 24, 51) and the dmPFC dual role of responding to salient stimuli (dACC, in particular) and mediating cognitive control (41, 42). Activation of the lPFC is most notable during tasks of the latter three dimensions (27, 29, 31–35, 37, 39, 52). Emotion-generating/processing regions (amygdala and insula) are activated during tasks of affect intensity or reactivity (18–22), but inconsistently or rarely during affective or cognitive modulation tasks (27, 29, 31–35, 52). The absence of consistent amygdala/insula activation during affective/cognitive modulation may result from down-regulation by regulatory regions (15, 16, 24, 35) or habituation to the repeated presentation of emotional stimuli (21, 53, 54). In contrast, the amygdala is often activated during behavioral control (32, 37, 40), presumably due to the effort required to control behavior, diminishing the availability of neural resources to attenuate emotional reactivity (32).
Neural Circuitry in Disorders of Negative Affect
fMRI Tasks of Emotion Regulation
Affect intensity/reactivity.
Anxiety and borderline personality disorders are associated with insula/amygdala hyperactivation during tasks of affect intensity/reactivity, and amygdala/insula activation is positively associated with self-reported negative valence of task stimuli (18, 21, 44, 53, 55, 56). In contrast, hypoactivation in the rACC/vmPFC and dmPFC is described in these disorders, with possibly greater hypoactivation in some diagnoses (generalized anxiety disorder, posttraumatic stress disorder [PTSD]) relative to others (panic and social phobia) (18, 21, 53, 55).
Affective modulation.
Anxiety and depressive disorders are associated with greater amygdala and insula activation during tasks of affective modulation (21, 44, 51, 57). When participants recall unresolved life events, amygdala/insula hyperactivation is also observed in individuals with borderline personality (58). With respect to regulatory regions, PTSD is associated with lower activation in all regulatory regions in most (21, 57, 59), but not all (44), studies during these tasks. Depression, by contrast, is associated with hyperactivation in regulatory regions (59).
Cognitive modulation.
Cognitive modulation tasks consistently reveal that individuals with higher anxiety levels or with anxiety disorders (31, 34, 60) or borderline personality disorder (56) experience increased activation in emotion-generating/processing regions and lower activation in all regulatory regions. Depression is associated with decreased activity in the lPFC (51). Hyperactivation in emotion-generating/processing regions, coupled with hypoactivation in regulatory areas during these tasks, may lead to difficulties in down-regulating intense emotion. Treatment of social anxiety disorder is associated with greater inverse dmPFC–amygdala connectivity and greater connectivity within regulatory regions (dmPFC–lPFC and dmPFC–rACC/vmPFC) during a cognitive modulation task (60), and improvement of depressive symptoms during treatment is also associated with greater activity in the lPFC (33).
Behavioral control.
During tasks of behavioral control, higher subjective anxiety levels and anxiety disorder diagnoses are associated with heightened activation in emotion-generating/processing regions (40, 61) and attenuated activation in all regulatory regions (39, 40, 61). Impairment in amygdala–rACC/vmPFC anticorrelation (40) and lower connectivity between the insula and dmPFC are observed in individuals with anxiety disorders during behavioral control (62).
In summary (Figure 1B), greater problems with emotion regulation in individuals with disorders associated with negative affect (without substance disorders) are associated with hyperactivation in the amygdala/insula and hypoactivation in the rACC/vmPFC and dmPFC (specifically dACC) during tasks of affect intensity/reactivity and hyperactivation of the amygdala/insula and hypoactivation in regulatory regions (rACC/vmPFC, dmPFC, and lPFC) during tasks of affective modulation, cognitive modulation, and behavioral control.
Resting State Functional Connectivity and Structural Connectivity
Decreased resting state connectivity between emotion-generating/processing and regulatory regions is associated with disorders of emotion dysregulation. Resting state connectivity between the amygdala/insula and all three regulatory regions is lower in individuals with anxiety and depressive disorders relative to controls in most studies and improves with treatment (44, 63, 64). Structural connectivity is also impaired in disorders of emotion regulation. Trait anxiety, anxiety disorder, major depressive disorder, and borderline personality are associated with decreased fractional anisotropy or increased mean diffusivity in the uncinate fasciculus and cingulum (tracts connecting the amygdala/insula to regulatory regions and the amygdala to the insula) (20, 65–67).
Decreased within-regulatory region functional and structural connectivity are also associated with emotion dysregulation. Decreased resting state connectivity is observed between the rACC/vmPFC and dmPFC in veterans with PTSD relative to healthy combat veterans (68). Impaired interhemispheric connections among individuals with major depressive disorder also occur, evidenced by decreased fractional anisotropy within the genu of the corpus callosum (69). Studies in individuals with anxiety disorders, however, demonstrated mixed results in the genu (65).
In summary, individuals with disorders of negative affect generally exhibit decreased resting state functional and structural connectivity between emotion-generating/processing regions and regulatory regions and, to some degree, within regulatory regions as well.
Neural Circuitry of Emotion Regulation in Substance Use Disorders
The emerging literature exploring the neural underpinnings of emotion regulation in substance disorders points to intriguing similarities—and differences—relative to individuals with disturbed emotion regulation but without substance disorders. While we will consider potential confounds, our goal is to highlight congruent findings that are shared among the various addictions. Moreover, we will report on differences that are associated with relapse risk (26, 70) and craving intensity (71). fMRI activation studies are summarized in Figure 1C, Table 2, and Table S2 in the online data supplement, and resting state functional and structural connectivity studies are summarized in Figure 1C, Tables 3 and 4, and online Table S3.
| Study | Task | Stress Versus Baseline Contrast | Stress Versus Neutral Contrast | Overall Effect: Neutral Plus Stressc | Neutral Trials Only | Regions |
|---|---|---|---|---|---|---|
| Affect intensity/reactivity tasks | ||||||
| Gilman et al. 2008 (17) | Negative or neutral pictures paired with alcohol or neutral beverages | AUD > controls | R amygdala | |||
| O’Daly et al. 2012d (73) | Fearful faces | AUD < controls | R amygdala | |||
| AUD < controls | B insula (AUD with history of multiple detoxifications < AUD with history of single detoxification < controls); B middle/lOFC (AUD with history of multiple detoxifications < AUD with history of single detoxification and controls) | |||||
| Salloum et al. 2007 (72) | Negative emotional faces | AUD < controls | Fear: L vmPFC/middle OFC, L rACC, L insula, R sgACC; disgust: L dlPFC, L dACC, L rACC, R amygdala, R insula | |||
| AUD > controls | Disgust: L dlPFC; anger: L insula | |||||
| Affective modulation tasks | ||||||
| Potenza et al. 2012 (75) | Personalized script | Women only: cocaine use disorder > controls | B insula, dACC, L dlPFC, B amygdala, B posterolateral OFC (BA 47) | |||
| Women only: cocaine use disorder > controls | L vlPFC/B posterolateral OFC (BA 47), vmPFC, B dlPFC, rACC | |||||
| Men only: cocaine use disorder > controls | L insula, dACC | |||||
| Men only: cocaine use disorder > controls | sgACC, rACC, B vmPFC, dACC, B insula, B amygdala, B vlPFC (BA 47), B dlPFC (lateral BA 10) | |||||
| Seo et al. 2013 (26) | Personalized script | AUD < controls | L insula, dmPFC (BA 8, 9), L lOFC, B dlPFC (BA 6, 8), vmPFC, sgACC | |||
| AUD > controls | vmPFC, sgACC | |||||
| AUD < controls | L lOFC, L insula, L dlPFC (BA 6, 8) | |||||
| Worse-outcome AUD < better-outcome AUD | rACC, vmPFC, R vlPFC | |||||
| Worse-outcome AUD > better-outcome AUD | sgACC, vmPFC, rACC | |||||
| Sinha et al. 2005 (25) | Personalized script | Cocaine use disorder < controls | dACC | |||
| Sinha et al. 2007 (118) | Personalized script | Worse-outcome cocaine use disorder > better-outcome cocaine use disorder | dmPFC/frontal pole (BA 9, 10) | |||
| Wang et al. 2010 (28) | Short negative stimuli (IAPS) in block design | Opioid use disorder < controls | R amygdala | |||
| Xu et al. 2013 (119) | Personalized script | Cocaine use disorder with higher-relapse-risk genotype (CG) > cocaine use disorder with lower-relapse-risk genotype (CC) | R amygdala | |||
| Yang et al. 2013 (74) | Conditioned paradigm | AUD < controls (AUD deactivated to high threat, controls did not) | rACC | |||
| AUD < controls (AUD deactivated more than controls to high threat) | rACC | |||||
| AUD > controls (controls deactivated more than AUD to low threat) | rACC | |||||
| Cognitive modulation tasks | ||||||
| Albein-Urios et al. 2014 (76) | Reappraisal | Cocaine use disorder < controls | L dlPFC, L insula | |||
| Behavioral control tasks | ||||||
| Smoski et al. 2011 (38) | Emotional oddball task | Opioid use disorder < controls | B amygdala, dACC, R dlPFC, L sgACC | |||
| Opioid use disorder < controls | B amygdala, dACC | |||||
| Opioid use disorder > controls | dACC | |||||
| Opioid use disorder > controls | L IFG/vlPFC (BA 45) | |||||
TABLE 2. Differences Between Individuals With Substance Use Disorders and Control Subjects in Activation in Regions of Focus (Amygdala, Insula, lPFC, dmPFC, rACC/vmPFC) During Tasks of Emotion Regulationa,b
| Study | Seeds | Connectivity | Regions |
|---|---|---|---|
| Camchong et al. 2013 (80) | sgACC | Worse-outcome AUD < better-outcome AUD | sgACC–L dlPFC, sgACC–B insula |
| Gu et al. 2010 (77) | B amygdala, B rACC | Cocaine use disorder < controls | B amygdala–rACC, B rACC–R insula, B rACC–B amygdala |
| McHugh et al. 2014 (70) | L and R BL amygdala, L and R CM amygdala | Worse-outcome cocaine use disorder < better-outcome cocaine use disorder and controls | L CM amygdala–vmPFC/rACC |
| Müller-Oehering et al. 2014 (81) | dACC, B dlPFC | AUD > controls | dACC–B vmPFC |
| AUD < controls | B dlPFC–R dACC/insula | ||
| O’Daly et al. 2012 (73) | R and L insula, R and L amygdala | AUD < controls | L insula–L rACC (AUD with history of multiple detoxifications versus controls), L insula–L vmPFC (AUD with history of multiple detoxifications versus controls), L insula–R vlPFC (AUD with history of multiple detoxifications versus controls) |
| AUD > controls | L insula–R vlPFC (AUD with history of single detoxification versus controls), L insula–L vmPFC (AUD with history of single detoxification versus controls) | ||
| Positive correlation with number of past detoxifications (more detoxifications > fewer detoxifications) | L amygdala–L dlPFC | ||
| Negative correlation with number of past detoxifications (more detoxifications < fewer detoxifications) | L insula–L vlPFC | ||
| Pujol et al. 2014 (79) | R and L insula, but authors report only R because L was similar | Cannabis use disorder < controls | R insula–dACC, R insula–rACC/vmPFC (more anticorrelated in cannabis use disorder than in controls) |
| Sutherland et al. 2013 (71) | R and L insulac | Nicotine use disorder with greater alexithymia and craving in withdrawal < nicotine use disorder with lower alexithymia and craving in withdrawal | R insula–sgACC/rACC |
| Upadhyay et al. 2010 (78) | B insulad, B BL amygdala, B CM amygdala | Opioid use disorder < controls | B insula–B lOFC, B insula–vmPFC, B insula–B BL amygdala, B insula–dACC, B CM amygdala–rACC, B BL amygdala–B lOFC, B BL amygdala–dmPFC |
TABLE 3. Differences Between Individuals With Substance Use Disorder and Control Subjects in Resting State Functional Connectivity in Regions of Focus (Amygdala, Insula, lPFC, dmPFC, rACC/vmPFC)a,b
| Study | Tracts/Volumes of Interest (and Diffusion Tensor Imaging Measures) | Structural Connectivity | Substance Use Disorder Group | Control Group | Exclusion Criteria |
|---|---|---|---|---|---|
| Alhassoon et al. 2012 (88) | Body of CC, genu of CC (fractional anisotropy, radial diffusivity) | AUD < controls | 15 abstinent AUD (15 male; mean age=51.4, SD=6); minimum 2 weeks abstinent; 11 smokers; 0 with other SUD | 15 controls (15 male; mean age=51.8, SD=7.4); 3 smokers; 0 with SUD | Any axis I disorder except major depressive disorder, other SUD except nicotine or cannabis, not having “urine and blood assured sobriety for 2 weeks” |
| Durkee et al. 2013 (120) | AC (fractional anisotropy) | AUD/AUD+PTSD < controls | 19 AUD (14 male; mean age=32.6, SD=7; 10 smokers, 1 cocaine use disorder, 1 cannabis use disorder, 2 major depressive disorder; 16 mean days abstinent before scan); 17 AUD+PTSD (9 male; mean age=37, SD=8.6; 11 smokers, 2 cocaine use disorder, 1 cannabis use disorder, 3 major depressive disorder; 24 mean days abstinent before scan) | 19 controls (10 male; mean age=26, SD=4; 0 smokers) | Positive urine screen for drugs or positive breath alcohol reading at time of study; other axis I disorders not an exclusion |
| Harris et al. 2008 (91) | R lOFC, R cingulum in R dACC (fractional anisotropy) | AUD < controls | 15 AUD (15 male; mean age=48, SD=13; 3 with history of tobacco dependence; abstinent minimum 4 weeks before MRI) | 15 controls (15 male; mean age=56, SD=9); similar to AUD group on depression and anxiety measures | History of depression, schizophrenia, or other SUD |
| Hudkins et al. 2012 (86) | B cingulum, genu of CC, L internal capsule (fractional anisotropy) | Nicotine use disorder > controls | 18 nicotine use disorder (10 male; mean age=33.7, SD=7.9) | 18 controls (9 male; mean age=33, SD=10) | Any axis I disorder, dependence on alcohol or drug of abuse, positive drug test on day of scan |
| L internal capsule, R cingulum (fractional anisotropy) | Negative correlation with level of dependencec (higher dependence < lower dependence) | ||||
| R ACR, R cingulum (fractional anisotropy) | Negative correlation with cigarettes/day (more cigarettes < fewer cigarettes) | ||||
| Lane et al. 2010 (83) | B ACR (fractional anisotropy) | Cocaine use disorder < controls | 15 cocaine use disorder (10 male; mean age=38.47, SD=2; 13 smokers, 9 other SUD [sedative 1, opiate 2, cannabis 5, hallucinogens 1, PCP 1, stimulant 2, alcohol 4]) | 18 controls (9 male; mean age=35, SD=2.6; 4 smokers, 0 other SUD) | Comorbid alcohol dependence, positive urine drug test on day of scan, DSM IV axis I disorders other than SUD |
| Anterior body of CC (fractional anisotropy, mean λ2 λ3) | Cocaine use disorder < controls | ||||
| Lin et al. 2013 (87) | L genu of CC, L rostral body of CC (fractional anisotropy, axial diffusivity, radial diffusivity) | Heavy smokers < controls; positive correlation between years of regular smoking and diffusivity (radial diffusivity and mean diffusivity, not fractional anisotropy) (more smoking < less smoking) | 34 heavy smokers (27 male; mean age=47, SD=7) | 34 nonsmokers (28 male; mean age=47, SD=9) | Drug abuse or dependence, psychiatric disease (Mini International Neuropsychiatric Interview); no subjects were daily drinkers, had had social consequences of drinking, or had difficulty stopping drinking |
| Pfefferbaum et al. 2006 (90) | Genu of CC, body of CC (mean diffusivity) | AUD < controls | 57 AUD (age stratified by gender: 40 male [mean age=53, SD=10], 17 female [mean age=50, SD=10]); recruited from rehab centers; mean days abstinent 92; 8 anxiety disorder, 15 mood disorder, 17 depressive disorders, 26 smokers | 74 controls (age stratified by gender: 32 male [mean age=52, SD=14], 42 female [mean age=55, SD=12]; 2 smokers) | DSM IV axis I diagnosis of bipolar disorder or schizophrenia, history of non-alcohol substance dependence |
| Qiu et al. 2013 (89) | R OFC, CC, B thalamic radiation (fractional anisotropy) | Opioid use disorder < controls | 18 short-duration opioid use disorder (18 male; mean age=35, SD=8; heroin use duration <10 years); 18 long-duration opioid use disorder (18 male; mean age=38, SD=4; heroin use duration 10–20 years); all regular smokers | 16 controls (16 male; mean age=38, SD=4; all regular smokers) | Schizophrenia, bipolar disorder, ever used any other types of drugs |
| B UF (radial diffusivity) | Opioid use disorder < controls | ||||
| R OFC (fractional anisotropy) | Negative correlation with length of drug dependence (more years < fewer years) | ||||
| Sorg et al. 2012 (82) | Frontal forceps, genu of CC, anterior body of CC, B UF, L anterior internal capsule (fractional anisotropy, axial diffusivity, radial diffusivity) | AUD who relapsed < AUD who remained abstinent | 29 AUD, abstinent at 6-month follow up (minimum 2 weeks sobriety) (28 male; mean age=48, SD=7; inpatients; 21 smokers, 8 past SUD; mean BDI score=8, SD=7); 16 AUD who returned to heavy use (minimum 2 weeks sobriety) (16 male; mean age=48, SD=10; inpatients; 15 smokers, 2 past SUD; mean BDI score=11, SD=8) | 30 controls (29 male; mean age=49, SD=10; 3 smokers; mean BDI score=3, SD=2; no history of axis I disorder) | DSM-IV diagnosis of non-alcohol SUD, hospitalization for a psychiatric condition that preceded AUD |
| Upadhyay et al. 2010 (78) | Ventral amygdalofugal pathway, B UF, B internal capsule, B external capsule, genu of CC, anterior midbody of CC, B anterior thalamic radiation (fractional anisotropy) | Opioid use disorder < controls | 10 opioid use disorder (prescription opioid dependence; 7 male; mean age=29, SD=9; 0 smokers) | 10 controls (7 male; mean age=30, SD=8; 0 smokers) | Positive urine screen at time of scan, chronic pain, dependence on other drugs including heroin and alcohol, other psychiatric disorders |
| Viswanath et al. 2015 (84) | CC, B internal capsule, B corona radiata (fractional anisotropy) | SUD < psychiatric controls | 99 SUD (alcohol, tobacco, cannabis most prevalent; 64 male; mean age=30, SD=1; inpatients; mean average length of stay=45 days, SD=1; 40% depression, 53% anxiety, 14% bipolar, 37% personality disorder) | 52 psychiatric controls (21 male; mean age=33, SD=2; 38% depression, 50% anxiety, 17% bipolar, 41% personality disorder) | |
| Yeh et al. 2009 (85) | R ACR, genu of CC, B UF, B internal capsule, B cingulum, R extreme capsule (fractional anisotropy) | AUD < controls | 10 AUD (10 male; mean age=47, SD=7.6; mean days of abstinence=6, SD=3;10 chronic smokers, 2 with alcohol-induced mood disorder with depressive features, 1 with alcohol-induced psychotic disorder) | 10 controls (10 male; mean age=42.7, SD=9.4; light drinkers, 2 smokers) | General psychiatric conditions, SUD other than nicotine |
| Genu of CC (mean diffusivity) | Negative correlation with increased drinking (more drinks < fewer drinks) |
TABLE 4. Differences Between Individuals With Substance Use Disorder and Control Subjects in Structural Connectivity in White Matter Tracts Connecting Regions of Focus (Amygdala, Insula, lPFC, dmPFC, rACC/vmPFC)a,b
fMRI Tasks of Emotional Regulation
Affect intensity/reactivity.
Amygdala hyperactivation is consistently observed in individuals with disorders of negative affect during tasks of affect intensity/reactivity (preceding section). In contrast, individuals with substance disorders show evidence of no activation, hypoactivation (72, 73), or hyperactivation (17, 72) of the amygdala during exposure to negative images in the International Affective Picture System (IAPS) (17) or facial expressions (72, 73). Insula activation is equally mixed, with no or hypoactivation (17, 72, 73) or hyperactivation (72) observed in substance disorders compared with controls. These disparate findings (even within the same cohort [72]) are evident even though all cited studies were conducted in individuals with alcohol use disorders (alcohol disorders) and the subjects had been abstinent for at least 2 weeks (17, 72, 73). Notably, two of these studies (17, 72) were small (11 subjects per group).
Similar to groups with disorders of negative affect without substance disorders, individuals with substance disorders show a dampening of the rACC/vmPFC and dmPFC. Decreased activation in the rACC/vmPFC during fear and disgust (72, 73) and in the dmPFC (dACC) during disgust (72) is observed in alcohol disorders. Therefore, diminished regulatory activity but not heightened activation in emotion-generating/processing regions is observed in substance disorders during tasks of affect intensity/reactivity.
Affective modulation.
Affective modulation tasks in substance disorders are also not associated with the heightened activation of emotion-generating/processing regions observed in individuals with disorders of negative affect without substance disorders. In individuals (primarily male) with alcohol disorders (26, 74), opioid use disorders (opioid disorders) (28), and cocaine use disorders (cocaine disorders) (25, 75), tasks of affective modulation showed no change or dampened amygdala (25, 26, 28, 74, 75) and insula (25, 26, 28, 74) activation relative to controls. In contrast to a cohort of matched male participants with cocaine disorders who showed no amygdala and limited insula activation during a personalized stressful narrative, however, female participants demonstrated a marked response (75).
Attenuated activity in emotion regulatory regions during affective modulation, on the other hand, is again generally consistent with the observation of attenuated activation in individuals with disorders of negative affect without substance disorders. rACC/vmPFC activation was significantly lower in individuals with alcohol disorders than in control subjects (26, 74) and in individuals who relapsed earlier (26). Similar findings in other regulatory regions have been observed in most comparisons of people with substance disorders versus control subjects: hypoactivation was demonstrated in the dmPFC (dACC) in individuals with cocaine disorder (25) and in the lPFC in alcohol disorder (26), and lower activation in the lPFC predicted relapse in alcohol disorder (26). The exceptions to these findings include increased activation (lPFC) in women-only participants with cocaine disorder (75) and increased dACC activation in both women and men with cocaine disorder (75).
Cognitive modulation.
The only published study, to our knowledge, that assessed cognitive modulation in individuals with substance disorders asked participants to suppress a negative affective response during exposure to negative IAPS stimuli (76). These epochs were compared with periods when participants were asked to maintain their affective response. In individuals with cocaine disorders, suppression of affect resulted in reduced activation in both emotion-generating/processing regions (insula) and emotion-regulating regions (lPFC).
Behavioral control.
When exposed to neutral distractor images while performing an emotional oddball task (requiring attendance to a target stimulus), individuals with borderline personality plus opioid disorder demonstrated less activation in both emotion-generating/processing regions (amygdala) and all three emotion-regulating regions, relative to individuals without either disorder (38). Findings in the amygdala are in contrast to the increase in activation that would be expected in individuals with borderline personality only.
Summary
The most consistent finding distinguishing people with substance disorders from healthy subjects, and often predictive of relapse, is hypoactivation in regulatory regions, particularly the rACC/vmPFC, during tasks of emotion regulation (with the exception of a single study in cocaine disorder [75]). These findings persist across dimensions and substance-disordered populations and mirror studies of individuals with anxiety, depressive disorders, and borderline personality disorder. Unlike the observed increase in amygdala/insula activation in individuals with impaired emotion regulation without substance disorders, however, activation in emotion-processing/generating regions is not reliably observed during emotion regulation in substance disorders. The critical caveat to these observations is an apparent gender effect. The only study assessing women separately (75) found increased activation of emotion-generating/processing regions in women but not men.
Resting State Functional and Structural Connectivity
The strength of resting state connectivity between the amygdala (77, 78) or insula (73, 77–80) and the rACC/vmPFC is weaker in individuals with substance disorder compared with controls, in individuals with a greater risk of relapse (70), and in individuals with heightened craving during withdrawal relative to those with less craving (71). Similarly, lower strength of resting state connectivity between the amygdala and the lPFC and dmPFC is observed in opioid disorders (78), between the insula and lPFC in alcohol and opioid disorders (73, 78, 81), and between the insula and dmPFC (78, 79) in opioid and cannabis disorders. Finally, lower insula–amygdala connectivity strength is observed in substance disorders (78).
Likewise, alterations in structural connectivity between emotion-generating/processing regions and regulatory regions and between the insula and amygdala are observed in substance disorders. Fractional anisotropy in the uncinate fasciculus (78, 82) and ventral amygdalofugal pathway (78) (amygdala–regulatory regions, amygdala–insula), anterior corona radiata (amygdala–rACC) (83), internal capsule (amygdala–lPFC) (82, 84), and external capsule (amygdala–lPFC, amygdala–dmPFC) (78) is reduced in substance disorders compared with controls. Reduced fractional anisotropy of the extreme capsule (lPFC–insula) is also observed in substance disorders (85). One reported exception was in smokers; these individual showed increased fractional anisotropy in the internal capsule and cingulum (amygdala–regulatory regions). This was posited as related to a trajectory wherein there is increased fractional anisotropy at earlier ages, which decreases with more years of smoking and greater dependence (86).
Measures of resting state connectivity within regulatory regions are less consistent than results between emotion-generating/processing and regulatory regions. In alcohol disorder, connectivity is increased between the rACC/vmPFC and dACC but decreased between the dlPFC and dACC (81). Connectivity between the rACC/vmPFC and the lPFC is also attenuated in individuals who later relapse, relative to those who abstain (80).
In contrast, structural connectivity within and between regulatory regions is more consistently reduced in substance disorders relative to controls and in more severe substance disorders relative to those that are less severe. Except for some conflicting results in smokers (in the genu) (86, 87), fractional anisotropy within the frontal forceps (82), within the genu of the corpus callosum (78, 82, 88), and within the rostral body (87) is reduced in substance disorders. Decreased fractional anisotropy in the genu is also associated with a longer duration of substance disorder (89), and reduced fractional anisotropy in the genu and frontal forceps predicts relapse in alcohol disorder (82). Further, mean diffusivity of the genu of the corpus callosum is increased (90) in substance disorders. A number of studies assessing white matter integrity utilizing a region-of-interest approach have also demonstrated within-PFC reductions in white matter integrity in individuals with substance disorders, including reduced fractional anisotropy in lOFC (91) and in a region encompassing the cingulum and the right dACC (91). Duration of drug dependence negatively correlated with fractional anisotropy within the right mOFC (89).
In summary, individuals with substance disorders reliably demonstrate weakened strength of resting state connectivity between the amygdala/insula and regulatory regions, consistent with observations in individuals with disorders associated with negative affect. Decreased resting state functional connectivity is also generally observed between and within regulatory regions in substance disorders relative to controls. This weakening of functional connectivity strength may be caused by impairment in the integrity of white matter tracts.
Potential Treatment Targets
Routes of Dysfunction
Impaired functioning in rACC/vmPFC, dACC, lPFC.
Unlike the augmented amygdala/insula reactivity observed in individuals with emotion regulation difficulties without substance disorders, individuals with substance disorders rarely exhibit hyperactivation in emotion-generating/processing regions during emotional provocation. This is consistent with reduced sensitivity to nondrug emotional stimuli in substance disorders (92), whereas emotion-generating/processing regions are highly reactive to drug cues (26, 75, 92). Similar to what is commonly reported in individuals with emotion regulation difficulties without substance disorders, hypoactivation in PFC regulatory regions is reliably observed in substance disorders (rACC/vmPFC in all tasks of emotion regulation and the dmPFC and lPFC during affective modulation and cognitive modulation). Therefore, emotion regulation disturbances in substance disorders may stem primarily from impairments in PFC activation, as a direct result of disrupted neural functioning, rather than from excessive reactivity to negatively charged affective stimuli.
Decreased resting state functional and structural connectivity between amygdala/insula and PFC.
Impairments in resting state connectivity and white matter tract integrity in individuals with substance disorders, in particular in connections between emotion-generating/processing regions and regulatory regions (rACC/vmPFC, lPFC, dmPFC) and between the insula and amygdala, may contribute to impaired down-regulation of emotion-generating/processing regions. However, disruptions in connectivity could also be the genesis of hypoactivation in regulatory regions in substance disorders. Proper engagement of PFC modulatory responses may depend on receiving information from the amygdala and/or insula; the primary deficit in emotion dysregulation may be delayed or weak communication from emotion-generating/processing regions to the PFC. In fact, individuals with anxiety disorders show delayed dlPFC and dmPFC activation during cognitive modulation tasks (60).
Hyperactivation in default mode network during rest/baseline.
In substance disorders, task-induced rACC/vmPFC hypoactivation may reflect a relatively heightened fMRI signal during baseline or neutral periods (26, 74, 75). rACC/vmPFC (a primary locus of the default mode network) is active during rumination and self-monitoring but deactivated during outwardly focused cognitive tasks (47–49). Rumination is associated with unhappiness (47) and may be a form of emotionality itself; hyperactivation in this network could contribute to difficulties in emotion regulation in substance and other disorders. Heightened intraregional rACC/vmPFC connectivity has been observed in depression (93), and heightened within-network connectivity has been observed in alcohol disorders (94). Although our theory is more concerned with basal default mode network activity, it is still notable that difficulty “shutting down” the network has been observed in PTSD (57), ADHD (48), and cocaine disorders (95).
Treatment Implications
It is not yet known which, if any, of the described alterations in substance disorders 1) reliably contributes to relapse risk and 2) will respond to treatment, particularly with respect to emotion regulation. Proposed avenues of future study are herein discussed.
Augment PFC activation during emotion regulation tasks.
Treatments for emotion regulation in populations without substance disorders that normalize PFC function (increase task-related activation) may have greater efficacy for substance disorders than dampening reactivity in emotion-generating/processing regions, as the latter is generally not observed in substance disorders. Selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines dampen amygdala, insula (96–98), and lOFC (97, 99) activity and are useful in the treatment of disorders of negative affect without substance disorders but not particularly in the treatment of substance disorders (11). A caveat is that late-onset alcohol disorder—associated with heightened anxiety and higher rates of comorbid anxiety/depression—tends to respond better to SSRIs than does early-onset disorder (100), possibly suggesting a typology-specific difference in the reactivity of emotion-generating/processing regions. In contrast to SSRIs, norepinephrine reuptake inhibitors (e.g., reboxetine) increase activation in the dlPFC and dmPFC in response to negative stimuli (97). Norepinephrine reuptake inhibitors, as well as other medications that increase noradrenergic function (e.g., bupropion, tricyclic antidepressants, SNRIs such as venlafaxine) may, therefore, deserve further study for the treatment of substance disorders. Bupropion is already a mainstay of treatment for nicotine dependence, and although it has not proven reliably effective in the treatment of stimulant disorders (11), targeting individuals with PFC hypoactivation may improve its effectiveness. Similarly, modafinil, which is most beneficial in alcohol disorders with comorbid impairments in cognitive control (101), was reported to attenuate behavioral disinhibition in alcohol disorder while increasing dmPFC activation (102). Targeted studies focused on substance-disordered individuals with attenuated PFC activation during emotion regulation tasks may improve the effectiveness of both pharmacological and behavioral interventions.
Improve white matter tract integrity or resting state connectivity strength.
Oxytocin enhances resting state connectivity between the amygdala and rACC/vmPFC (64) and is being investigated (preclinically) as a treatment for substance disorders (103). Cognitive-behavioral therapy for anxiety disorders increases connectivity within regulatory regions (dmPFC–rACC/vmPFC, dmPFC–lPFC) and increases anticorrelation between the amygdala and dmPFC during cognitive modulation tasks (60). Identifying either medications or psychosocial therapies that increase the strength of resting state functional connectivity or the integrity of white matter tracts between emotion-generating/processing and regulatory regions may, therefore, prove particularly useful in substance disorders.
Decrease default mode network activation at rest or increase deactivation during tasks.
Finally, identifying treatments that ameliorate heightened basal activation in this network may also prove useful. Performing a complex task is known to deactivate the default mode network, and boredom is a well-accepted relapse trigger. Simply encouraging patients to “stay busy” may work, in part, by deactivating this network. Mindfulness is under investigation for the treatment of a variety of substance disorders (104) and may be working by means of this mechanism, as meditation decreases network activation (49, 105).
Limitations and Future Work
This review has some notable limitations. First, the relatively comprehensive literature cited assessing the neural circuitry associated with negative affect in individuals without substance disorders is not matched by a commensurate literature in substance disorders. Second, more task-based studies in substance disorders are needed to further explore activation patterns within each dimension (especially in affect intensity/reactivity, cognitive modulation, and behavioral control). Third, most studies of emotion regulation in substance disorders have male majorities; those that included sufficient numbers of women to explore gender differences observed stark gender contrasts (75). Consequently, further studies into the effects of gender on brain activation are required. Fourth, the quandary of whether the neural differences in individuals with substance disorders are a consequence of pre-existing vulnerabilities or of persistent substance use was not considered in this review. Nevertheless, almost all of the studies assessing neural activity evaluated individuals with substance disorders following at least two weeks of abstinence. Thus, even substance-induced alterations appear to persist beyond the initial withdrawal period and may therefore impact relapse risk and, subsequently, require treatment. Fifth, we focused our work on the insula, amygdala, and certain regions within the PFC and their interactions partially because they fell within relevant networks of interest. However, other regions also play key roles in emotion regulation, including the hippocampus, dorsal and ventral striatum, and posterior cingulate. Although not a focus of this review, these and other regions may be of equal importance in accounting for altered emotion regulation in substance disorder. Sixth, it will be important in future work to identify differences between different substances of abuse and at different stages of abstinence as well as premorbid alterations versus those that are substance-induced. Seventh, because substance disorder subtype may influence treatment response, future work could explore the relationships between subtype and alterations in emotion regulation circuitry, which could help in treatment matching efforts (11).
Finally, the extant literature did not allow us to assess the potential impact of comorbid psychiatric disorders associated with negative affect (e.g., depressive, borderline personality, posttraumatic stress, and other anxiety disorders) on disruptions in neural circuitry. Comorbid diagnoses could account for many of the similarities described between substance disorders and disorders of negative affect without substance disorders, as well as much of the variability reported in the substance disorder groups. Other comorbid disorders commonly observed in substance disorders and events associated with emotion dysregulation (e.g., attention deficit, bipolar, and conduct/antisocial disorders as well as childhood and adult trauma) could also play an important role in the alterations described. For example, similar to persons with substance use disorders, individuals with conduct disorder and callous-unemotional traits demonstrate a blunted amygdala response to emotional stimuli (106), individuals with ADHD histories show decreased activation in the amygdala, rACC/vmPFC, and lOFC during behavioral control (107), and those with trauma histories alone evidence decreased PFC activation during cognitive modulation relative to controls (108). The majority of the articles did not provide extensive details on the rates of these disorders in their samples (see Tables S2 and S3 in the online data supplement); these questions remain open for future exploration.
Identifying the root neural causes contributing to emotion regulation disturbances in substance disorders, the relationship of these disturbances to relapse, and approaches for normalizing these processes is imperative. Knowing the neural underpinnings will help us in efforts to match treatments, which may lead to improved treatment efficacy.
1 : Emotion regulation: a theme in search of definition. Monogr Soc Res Child Dev 1994; 59:25–52Crossref, Medline, Google Scholar
2 : Alcohol abuse and dependence symptoms: a multidimensional model of common and specific etiology. Psychol Addict Behav 2009; 23:415–427Crossref, Medline, Google Scholar
3 : Self-regulation as a protective factor against risky drinking and sexual behavior. Psychol Addict Behav 2010; 24:376–385Crossref, Medline, Google Scholar
4 : Using neuroimaging to improve emotion regulation treatments for substance use disorders, in Neuroimaging and Psychosocial Addiction Treatment. Edited by Feldstein Ewing SW, Witkiewitz K, Filbey FM. London, UK, Palgrave, 2015Crossref, Google Scholar
5 : Emotion regulation and the anxiety disorders: an integrative review. J Psychopathol Behav Assess 2010; 32:68–82Crossref, Medline, Google Scholar
6 : The relative and unique contributions of emotion dysregulation and impulsivity to posttraumatic stress disorder among substance dependent inpatients. Drug Alcohol Depend 2013; 128:45–51Crossref, Medline, Google Scholar
7 : The role of anxiety sensitivity and difficulties in emotion regulation in posttraumatic stress disorder among crack/cocaine dependent patients in residential substance abuse treatment. J Anxiety Disord 2009; 23:591–599Crossref, Medline, Google Scholar
8 : Early recovery from alcohol dependence: factors that promote or impede abstinence. J Subst Abuse Treat 2010; 38:42–50Crossref, Medline, Google Scholar
9 : How does stress lead to risk of alcohol relapse? Alcohol Res 2012; 34:432–440Medline, Google Scholar
10 : Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol 2011; 79:307–318Crossref, Medline, Google Scholar
11 : Psychopharmacology for addictions, in Addictions: A Comprehensive Guidebook, 2nd ed. Edited by McCrady BS, Epstein EE. New York, Oxford University Press, 2013Google Scholar
12 : Emotion regulation and psychopathology. Annu Rev Clin Psychol 2015; 11:379–405Crossref, Medline, Google Scholar
13 : The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Crossref, Medline, Google Scholar
14 : Explicit and implicit emotion regulation: a dual-process framework. Cogn Emotion 2011; 25:400–412Crossref, Medline, Google Scholar
15 : Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Front Psychiatry 2014; 5:123Crossref, Medline, Google Scholar
16 : Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15:85–93Crossref, Medline, Google Scholar
17 : Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol 2008; 13:423–434Crossref, Medline, Google Scholar
18 : Regional cerebral changes and functional connectivity during the observation of negative emotional stimuli in subjects with post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci 2013; 263:575–583Crossref, Medline, Google Scholar
19 : Volitional control of anterior insula activity modulates the response to aversive stimuli: a real-time functional magnetic resonance imaging study. Biol Psychiatry 2010; 68:425–432Crossref, Medline, Google Scholar
20 : The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 2009; 29:11614–11618Crossref, Medline, Google Scholar
21 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Link, Google Scholar
22 : A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 2005; 62:146–152Crossref, Medline, Google Scholar
23 : Identifying component-processes of executive functioning that serve as risk factors for the alcohol-aggression relation. Psychol Addict Behav 2012; 26:201–211Crossref, Medline, Google Scholar
24 : A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13:829, 833–857Crossref, Medline, Google Scholar
25 : Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005; 183:171–180Crossref, Medline, Google Scholar
26 : Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 2013; 70:727–739Crossref, Medline, Google Scholar
27 : Neural correlates of processing stressful information: an event-related fMRI study. Brain Res 2009; 1293:49–60Crossref, Medline, Google Scholar
28 : Alterations in the processing of non-drug-related affective stimuli in abstinent heroin addicts. Neuroimage 2010; 49:971–976Crossref, Medline, Google Scholar
29 : Striatal-limbic activation is associated with intensity of anticipatory anxiety. Psychiatry Res 2012; 204:123–131Crossref, Medline, Google Scholar
30 : Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: a comparative fMRI investigation. Neuroimage 2014; 101:298–309Crossref, Medline, Google Scholar
31 : Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage 2012; 62:1575–1581Crossref, Medline, Google Scholar
32 : The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 2008; 63:577–586Crossref, Medline, Google Scholar
33 : Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry 2013; 70:1181–1189Crossref, Medline, Google Scholar
34 : Negative emotion regulation in patients with posttraumatic stress disorder. PLoS One 2013; 8:e81957Crossref, Medline, Google Scholar
35 : Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 2014; 45:202–211Crossref, Medline, Google Scholar
36 : The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif 2001; 30:669–689Crossref, Google Scholar
37 : Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage 2007; 36:1026–1040Crossref, Medline, Google Scholar
38 : Functional imaging of emotion reactivity in opiate-dependent borderline personality disorder. Pers Disord 2011; 2:230–241Crossref, Medline, Google Scholar
39 : Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 2004; 7:184–188Crossref, Medline, Google Scholar
40 : Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 2010; 167:545–554Link, Google Scholar
41 : Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 2004; 56:129–140Crossref, Medline, Google Scholar
42 : Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015; 16:55–61Crossref, Medline, Google Scholar
43 : Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72:305–315Crossref, Medline, Google Scholar
44 : Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag 2015; 11:115–126Medline, Google Scholar
45 : Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711Crossref, Medline, Google Scholar
46 : A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev 2002; 26:809–817Crossref, Medline, Google Scholar
47 : A wandering mind is an unhappy mind. Science 2010; 330:932Crossref, Medline, Google Scholar
48 : Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 2009; 33:279–296Crossref, Medline, Google Scholar
49 : Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012; 59:750–760Crossref, Medline, Google Scholar
50 : Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct 2009; 213:525–533Crossref, Medline, Google Scholar
51 : Neural correlates of dysfunctional emotion regulation in major depressive disorder: a systematic review of neuroimaging studies. Neurosci Biobehav Rev 2013; 37:2529–2553Crossref, Medline, Google Scholar
52 : Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 2011; 32:1998–2013Crossref, Medline, Google Scholar
53 : The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am J Psychiatry 2014; 171:82–90Link, Google Scholar
54 : Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci 1999; 19:10869–10876Crossref, Medline, Google Scholar
55 : A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62:273–281Crossref, Medline, Google Scholar
56 : Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry 2011; 69:564–573Crossref, Medline, Google Scholar
57 : Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry 2012; 69:360–371Crossref, Medline, Google Scholar
58 : Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychol Med 2006; 36:845–856Crossref, Medline, Google Scholar
59 : Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res 2007; 155:45–56Crossref, Medline, Google Scholar
60 : Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry 2013; 70:1048–1056Crossref, Medline, Google Scholar
61 : From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 2009; 66:649–655Crossref, Medline, Google Scholar
62 : Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol 2012; 89:273–276Crossref, Medline, Google Scholar
63 : A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord 2014; 172C:8–17Medline, Google Scholar
64 : Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 2014; 39:2061–2069Crossref, Medline, Google Scholar
65 : Diffusion tensor imaging in anxiety disorders. Curr Psychiatry Rep 2012; 14:197–202Crossref, Medline, Google Scholar
66 : Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology 2012; 37:959–967Crossref, Medline, Google Scholar
67 : White matter integrity and its association with affective and interpersonal symptoms in borderline personality disorder. Neuroimage Clin 2015; 7:476–481Crossref, Medline, Google Scholar
68 : Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp 2015; 36:99–109Crossref, Medline, Google Scholar
69 : Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci 2013; 38:49–56Crossref, Medline, Google Scholar
70 : Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry 2014; 5:16Crossref, Medline, Google Scholar
71 : Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl) 2013; 228:143–155Crossref, Medline, Google Scholar
72 : Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res 2007; 31:1490–1504Crossref, Medline, Google Scholar
73 : Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 2012; 37:2267–2276Crossref, Medline, Google Scholar
74 : Altered neural processing of threat in alcohol-dependent men. Alcohol Clin Exp Res 2013; 37:2029–2038Crossref, Medline, Google Scholar
75 : Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 2012; 169:406–414Link, Google Scholar
76 : Re-appraisal of negative emotions in cocaine dependence: dysfunctional corticolimbic activation and connectivity. Addict Biol 2014; 19:415–426Crossref, Medline, Google Scholar
77 : Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 2010; 53:593–601Crossref, Medline, Google Scholar
78 : Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010; 133:2098–2114Crossref, Medline, Google Scholar
79 : Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res 2014; 51:68–78Crossref, Medline, Google Scholar
80 : Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res 2013; 37:75–85Crossref, Medline, Google Scholar
81 : The resting brain of alcoholics. Cereb Cortex 2014; bhu134Google Scholar
82 : Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry 2012; 71:262–268Crossref, Medline, Google Scholar
83 : Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 2010; 5:e11591Crossref, Medline, Google Scholar
84 : Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology 2015; 92:63–68Crossref, Medline, Google Scholar
85 : Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res 2009; 173:22–30Crossref, Medline, Google Scholar
86 : Cigarette smoking and white matter microstructure. Psychopharmacology (Berl) 2012; 221:285–295Crossref, Medline, Google Scholar
87 : Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend 2013; 129:82–87Crossref, Medline, Google Scholar
88 : Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res 2012; 36:1922–1931Crossref, Medline, Google Scholar
89 : Progressive white matter microstructure damage in male chronic heroin dependent individuals: a DTI and TBSS study. PLoS One 2013; 8:e63212Crossref, Medline, Google Scholar
90 : Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging 2006; 27:994–1009Crossref, Medline, Google Scholar
91 : Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res 2008; 32:1001–1013Crossref, Medline, Google Scholar
92 : Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157:1789–1798Link, Google Scholar
93 : Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76:517–526Crossref, Medline, Google Scholar
94 : Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol (Epub ahead of print, June 3, 2015)Google Scholar
95 : Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug Alcohol Depend 2013; 133:235–241Crossref, Medline, Google Scholar
96 : Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 2005; 62:282–288Crossref, Medline, Google Scholar
97 : Acute neural effects of selective serotonin reuptake inhibitors versus noradrenaline reuptake inhibitors on emotion processing: implications for differential treatment efficacy. Neurosci Biobehav Rev 2013; 37:1786–1800Crossref, Medline, Google Scholar
98 : Functional effects of chronic paroxetine versus placebo on the fear, stress and anxiety brain circuit in social anxiety disorder: initial validation of an imaging protocol for drug discovery. Eur Neuropsychopharmacol 2014; 24:105–116Crossref, Medline, Google Scholar
99 : The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology 2005; 30:1724–1734Crossref, Medline, Google Scholar
100 : Comparison of alcoholism subtypes as moderators of the response to sertraline treatment. Alcohol Clin Exp Res 2012; 36:509–516Crossref, Medline, Google Scholar
101 : Effect of modafinil on impulsivity and relapse in alcohol dependent patients: a randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2013; 23:948–955Crossref, Medline, Google Scholar
102 : Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry 2013; 73:211–218Crossref, Medline, Google Scholar
103 : Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. Int J Neuropsychopharmacol 2015; 18:pyu009Crossref, Google Scholar
104 : Craving to quit: psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychol Addict Behav 2013; 27:366–379Crossref, Medline, Google Scholar
105 : The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Ann N Y Acad Sci 2014; 1307:19–27Crossref, Medline, Google Scholar
106 : The neurobiology of psychopathic traits in youths. Nat Rev Neurosci 2013; 14:786–799Crossref, Medline, Google Scholar
107 : Emotional bias of cognitive control in adults with childhood attention-deficit/hyperactivity disorder. Neuroimage Clin 2014; 5:1–9Crossref, Medline, Google Scholar
108 : A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry 2009; 66:656–664Crossref, Medline, Google Scholar
109 : Short frontal lobe connections of the human brain. Cortex 2012; 48:273–291Crossref, Medline, Google Scholar
110 : Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res 2009; 174:217–222Crossref, Medline, Google Scholar
111 : Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J Neurosci 2015; 35:6020–6027Crossref, Medline, Google Scholar
112 : Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res 2013; 214:260–268Crossref, Medline, Google Scholar
113 : Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007; 130:630–653Crossref, Medline, Google Scholar
114 : Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 2009; 30:3127–3141Crossref, Medline, Google Scholar
115 : How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res 2011; 223:211–221Crossref, Medline, Google Scholar
116 : Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp 2009; 30:3172–3187Crossref, Medline, Google Scholar
117 : Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 2011; 54:49–59Crossref, Medline, Google Scholar
118 : Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 2007; 26:25–31Crossref, Medline, Google Scholar
119 : A variant on the kappa opioid receptor gene (OPRK1) is associated with stress response and related drug craving, limbic brain activation and cocaine relapse risk. Transl Psychiatry 2013; 3:e292Crossref, Medline, Google Scholar
120 : White matter microstructure alterations: a study of alcoholics with and without post-traumatic stress disorder. PLoS One 2013; 8:e80952Crossref, Medline, Google Scholar



