Short-Term Use of Serotonin Reuptake Inhibitors and Risk of Upper Gastrointestinal Bleeding
Abstract
Objective
The association between selective serotonin receptor inhibitors (SSRIs) and risk of upper gastrointestinal bleeding remains controversial. Previous studies have generally evaluated the issue for approximately 3 months, even though the SSRI-mediated inhibition of platelet serotonin concentrations occurs within 7–14 days. The authors explored the risk of upper gastrointestinal bleeding after short-term SSRI exposure by a case-crossover design.
Method
The records of psychiatric inpatients with upper gastrointestinal bleeding were retrieved from the Taiwan National Health Insurance Database (1998−2009). Rates of antidepressant use were compared for case and control periods with time windows of 7, 14, and 28 days. The adjusted self-matched odds ratios from a conditional logistic regression model were used to determine the association between SSRI use and upper gastrointestinal bleeding.
Results
A total of 5,377 patients with upper gastrointestinal bleeding were enrolled. The adjusted odds ratio for the risk of upper gastrointestinal bleeding after SSRI exposure was 1.67 (95% CI=1.23–2.26) for the 7-day window, 1.84 (95% CI=1.42–2.40) for the 14-day window, and 1.67 (95% CI=1.34–2.08) for the 28-day window. SSRIs with high and intermediate, but not low, affinity for serotonin transporter were associated with upper gastrointestinal bleeding. An elevated risk of upper gastrointestinal bleeding after SSRI exposure was seen in male but not female patients.
Conclusions
Short-term SSRI use (7–28 days) is significantly associated with upper gastrointestinal bleeding. Gender differences may exist in the relationship between SSRI use and upper gastrointestinal bleeding. Physicians should carefully monitor signs of upper gastrointestinal bleeding even after short-term exposure to SSRIs, as is done with nonsteroidal anti-inflammatory drugs and aspirin.
Selective serotonin reuptake inhibitors (SSRIs) have become the most widely prescribed antidepressant class since fluoxetine first gained approval in the United States in 1987. SSRIs have increased in popularity in many countries in the past 20 years because they are thought to be relatively safe and well tolerated compared with earlier antidepressants, such as tricyclic antidepressants and monoamine oxidase (MAO) inhibitors (1). SSRIs are recommended as the first-line pharmacotherapy in treating depression, and they have been approved for the treatment of panic disorders, anxiety disorders, and posttraumatic stress disorder (2).
SSRIs have been found to be associated with upper gastrointestinal bleeding (3). The risk of upper gastrointestinal bleeding can be further increased when SSRIs are combined with nonsteroidal anti-inflammatory drugs (NSAIDs) (4), antiplatelet agents (5), or warfarin (6). However, controversy remains regarding whether there is actually an elevated risk of upper gastrointestinal bleeding with SSRI use (7, 8). The reason for the conflicting results is unclear. The absence of consideration of comorbidities and insufficient sample sizes of SSRI users could result in a failure to observe an elevated risk for upper gastrointestinal bleeding after SSRI exposure. Furthermore, a 3-month window is usually adopted in studies that evaluate the risk of upper gastrointestinal bleeding in SSRI users. To date, the risk of upper gastrointestinal bleeding after short-term SSRI exposure remains unclear. Within 7–14 days after starting SSRI, the intraplatelet serotonin concentration decreases by more than 80%, resulting in impairment of platelet aggregation (9–11). Within hours, SSRI use increases gastric acid secretion in rodents, which may potentiate the bleeding risk in the gastrointestinal tract (12). There has been a case report of upper gastrointestinal bleeding after only 7 days of SSRI exposure (3). Such observations prompted us to test the hypothesis that short-term SSRI use increases the risk of upper gastrointestinal bleeding. To overcome the potential biases stemming from inadequate control for comorbidities and underpowered sample sizes, we applied a case-crossover design in patients with psychiatric diagnoses from Taiwan’s nationwide population-based claims database.
Method
Case-Crossover Design
The case-crossover design is a research method that was proposed by Maclure in 1991 to survey the transient effects of acute events (13). In the case-crossover design, each patient serves as his or her own control. In previous observational studies investigating the risk of upper gastrointestinal bleeding after SSRI exposure, many possible confounding factors were not considered, such as lifestyle, smoking and alcohol use, gastrointestinal symptoms, stress symptoms, functional status, and other prescribed medications (14). Knowledge of these potentially confounding variables is not generally available in population studies. With the application of the case-crossover design, the same individual would serve as his or her own control (Figure 1), and therefore stable confounders that could not be measured, were poorly measured, or were unknown canceled each other out (15). This design has been used to demonstrate an elevated risk of cerebrovascular events after short-term exposure to antidepressants as well as lower gastrointestinal bleeding after short-term NSAID use (16, 17).

a Index date=date of upper gastrointestinal bleeding.
Data Source and Study Population
Our data set was derived from the Taiwan National Health Insurance Research Database (NHIRD) (http://www.nhri.org.tw/nhird/en/index.htm). The Taiwan government started a mandatory single-payer social health insurance system in 1995, covering over 23 million residents by 2010, which represents approximately 99% of Taiwan’s population. The database includes the entire registry and claims data from this health insurance system, ranging from demographic data to detailed orders from ambulatory and inpatient care. All data were de-identified by encrypting the identification codes of patients and medical facilities so that no individual could be identified during the investigation or processing of the database. Personal information, such as body weight, height, laboratory findings, lifestyle, and smoking and alcohol habits, was not available in the NHIRD. The accuracy of diagnoses of major diseases in the NHIRD, such as diabetes mellitus, hypertension, stroke, and gastrointestinal diseases, is high (18–20). The database has been extensively used in epidemiologic studies in Taiwan (1, 16, 17, 18).
In this study, we focused on patients with psychiatric diagnoses. The Psychiatric Inpatient Medical Claim (PIMC) data set contains the claims data for patients with a history of hospital admission to psychiatric wards, as well as those admitted to medical and surgical wards but with a concomitant psychiatric diagnosis, from 1998 to 2009. Adult patients (≥20 years old) with newly diagnosed upper gastrointestinal bleeding and required hospitalization were included for analysis. Upper gastrointestinal bleeding was defined by ICD-9-CM codes (21, 22). Patients who had a concomitant diagnosis of trauma (codes 800–804, 850–854, or V57) or had esophageal variceal bleeding (code 456.0 or 456.20) were excluded from the study. Patients found to be positive for gastrointestinal bleeding solely in a stool guaiac test were not included in the study.
Data Processing and Statistical Analysis
We assessed patients’ comorbidities, including history of upper gastrointestinal tract disease (defined as previous treatment with a proton pump inhibitor), depression, schizophrenia, anxiety, alcohol-related disease, cerebrovascular disease, myocardial infarction, heart failure, chronic pulmonary disease, diabetes mellitus, hypertension, dyslipidemia, arrhythmia, chronic renal disease, chronic liver disease, and malignancy according to the ICD-9-CM codes (see Table S1 in the data supplement that accompanies the online edition of this article). The Charlson comorbidity index was used to assess the severity of comorbidities (23). Health care utilization was measured by the frequency of ambulatory care visits and hospitalization in the year before the date of upper gastrointestinal bleeding (index date).
Information regarding prescribed drugs (according to the WHO Anatomical Therapeutic Chemical classification system), drug dosage, date of dispensation, supply days, and numbers of dispensed drug pills was extracted from the NHIRD/PIMC database. Antidepressants were further classified into groups according to their mechanism of action: SSRIs, tricyclic antidepressants, MAO inhibitors, serotonin-norepinephrine reuptake inhibitors (SNRIs), and other antidepressants. Medications that are potentially related to upper gastrointestinal bleeding were categorized as follows: proton pump inhibitors, histamine-2 receptor blockers, systemic corticosteroids, vitamin K antagonists, aspirin, clopidogrel, and NSAIDs. (A detailed list of the medications is presented in Table S2 in the online data supplement.)
The odds ratio for the risk of upper gastrointestinal bleeding after exposure to a specific drug was estimated by the ratio of patients who were exposed to that drug during the 14-day case period (1–14 days before the index date) to patients who were exposed only during the 14-day control period (15–28 days before the index date). The odds ratios of two other time windows, set at 7 days (1–7 days and 8–14 days before the index date for the case and control periods, respectively) and 28 days (1–28 days and 29–56 days before the index date for the case and control periods, respectively), were chosen for a sensitivity analysis (Figure 1). A conditional logistic regression model was used to calculate crude odds ratios by comparing the current use of SSRIs, SNRIs, tricyclic antidepressants, MAO inhibitors, and other antidepressants, with nonusers as the reference. In the multivariate analysis, we calculated an adjusted odds ratio that simultaneously controlled for the use of other individual NSAIDs and for potential time-varying confounding variables (proton pump inhibitors, histamine-2 receptor blockers, NSAIDs, vitamin K antagonists, clopidogrel, systemic corticosteroids, and aspirin) whose use might not be balanced between the case and control periods. Among the three time windows, the 14-day window showed the higher value in upper gastrointestinal bleeding risk after SSRI exposure, and it was therefore chosen for subsequent analyses. To evaluate the dose-response relationship between SSRIs and upper gastrointestinal bleeding, the average daily dose of SSRI was categorized into four ranges in relation to the defined daily dose (24): <0.5, 0.5–1, 1–2, and ≥2 defined daily doses. To evaluate the relationship between an SSRI’s affinity to serotonin receptor and risk of upper gastrointestinal bleeding, we divided the antidepressants into high (<1 nmol/L), intermediate (1–10 nmol/L), and low affinity (>10 nmol/L) according to the dissociation constant for the serotonin transporter (Kd) with the serotonin receptor.
We performed subgroup analyses by stratifying the characteristics of the included patients. The case subjects were separated according to gender, age (≥55 and <55 years), diabetes mellitus, history of myocardial infarction, chronic pulmonary disease, chronic kidney disease, hypertension, depression, schizophrenia, alcohol-related disease, history of previous upper gastrointestinal tract disease, Charlson comorbidity index score, and previous SSRI use. The interactions among SSRIs, NSAIDs, and aspirin regarding the risk of upper gastrointestinal bleeding were also analyzed. For quality assurance, we measured the risk of two other drugs (benzodiazepines and nonbenzodiazepine hypnotics) without known association with upper gastrointestinal bleeding as negative controls.
In a previous nationwide survey, 22% of psychiatric patients in Taiwan were estimated to have received prescriptions for at least one SSRI (1). Under the assumption of 0.8 as the correlation coefficient for SSRI use between the case and control periods and the assumption of 1.6 as the odds ratio for the upper gastrointestinal bleeding risk after SSRI exposure, 2,729 patients were required to achieve 90% power at 5% significance.
The study protocol was approved by the institutional review board of Taipei Veterans General Hospital. All statistical analyses were conducted using Stata, version 11.0 (StataCorp, College Station, Tex.).
Results
In a total of 187,117 patients who were included in the NHIRD-PIMC database from 1998 to 2009, 5,500 patients who were older than 20 years of age had new-onset episodes of upper gastrointestinal bleeding. We excluded 123 patients with concomitant trauma or esophageal variceal bleeding, leaving 5,377 patients for the analysis. The patients’ demographic and clinical characteristics are summarized in Table 1.
| Characteristic | ||
|---|---|---|
| Mean | SD | |
| Age (years) | 57.6 | 19.9 |
| Charlson comorbidity index scoreb | 3.64 | 2.76 |
| % | N | |
| Male | 74.6 | 4,011 |
| Previous upper gastrointestinal tract disease | 19.6 | 1,055 |
| Depression | 54.0 | 2,904 |
| Schizophrenia | 24.7 | 1,327 |
| Anxiety | 47.3 | 2,544 |
| Alcohol-related disease | 54.1 | 2,907 |
| Previous SSRI use | 42.6 | 2,290 |
| Cerebrovascular disease | 41.9 | 2,254 |
| Myocardial infarction | 2.8 | 149 |
| Heart failure | 8.9 | 476 |
| Chronic pulmonary disease | 34.4 | 1,849 |
| Diabetes mellitus | 8.9 | 477 |
| Hypertension | 41.1 | 2,212 |
| Dyslipidemia | 19.9 | 1,072 |
| Arrhythmia | 20.5 | 1,103 |
| Chronic renal disease | 11.0 | 592 |
| Chronic liver disease | 29.0 | 1,557 |
| Malignancy | 7.4 | 396 |
| Median | IQR | |
| Frequency of ambulatory visits | 29 | 16–42 |
| Frequency of hospitalization | 2 | 0–3 |
SSRIs were the only antidepressants associated with an elevated risk of upper gastrointestinal bleeding after we adjusted for the time-varying confounders (Table 2). An elevated risk of upper gastrointestinal bleeding was identified for all three time windows for SSRI users, with a higher value in the 14-day window. An analysis of fluoxetine (adjusted odds ratio=1.68, 95% confidence interval [CI]=1.10–2.57) and sertraline (adjusted odds ratio=1.87, 95% CI=1.16–3.02) showed an elevated risk of upper gastrointestinal bleeding, while for other SSRIs the elevation in risk fell short of statistical significance (Table 3). In the 14-day time window, the demographic data and distribution of comorbidities were similar between the control group (patients taking drugs in the control period but not in the case period) and the case group (patients taking drugs in the case period but not in the control period). Charlson comorbidity index score also did not differ significantly between the two groups (3.7 [SD=2.9] and 3.8 [SD=2.9], respectively). The frequencies of health care utilization before the index date were similar between SSRI users and users of other antidepressants (a median frequency of 32 for both groups for ambulatory care, and a median frequency of 2 for hospitalization for both groups).
| Time Window and Antidepressant Class | Case Subjects (N=5,377) | Control Subjects (N=5,377) | Crude Odds Ratio | 95% CI | p | Adjusted Odds Ratiob | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| 7-day window | ||||||||
| SSRIs | 660 | 606 | 1.81 | 1.34–2.43 | <0.001 | 1.67 | 1.23–2.26 | 0.001 |
| Tricyclic antidepressants | 152 | 152 | 1.00 | 0.58–1.72 | 1.000 | 0.87 | 0.50–1.53 | 0.64 |
| SNRIs | 142 | 141 | 1.06 | 0.55–2.05 | 0.89 | 0.92 | 0.46–1.82 | 0.80 |
| MAO inhibitors | 24 | 22 | 1.67 | 0.40–6.97 | 0.48 | 1.37 | 0.32–5.92 | 0.67 |
| Other antidepressants | 598 | 584 | 1.24 | 0.88–1.74 | 0.25 | 1.10 | 0.77–1.57 | 0.60 |
| 14-day window | ||||||||
| SSRIs | 727 | 640 | 2.00 | 1.55–2.59 | <0.001 | 1.84 | 1.42–2.40 | <0.001 |
| Tricyclic antidepressants | 178 | 163 | 1.50 | 0.95–2.38 | 0.09 | 1.31 | 0.81–2.11 | 0.28 |
| SNRIs | 159 | 144 | 1.94 | 1.06–3.54 | 0.03 | 1.90 | 1.00–3.62 | 0.05 |
| MAO inhibitors | 27 | 33 | 0.46 | 0.16–1.31 | 0.14 | 0.43 | 0.15–1.24 | 0.12 |
| Other antidepressants | 657 | 622 | 1.51 | 1.11–2.04 | 0.008 | 1.31 | 0.95–1.79 | 0.09 |
| 28-day window | ||||||||
| SSRIs | 814 | 718 | 1.72 | 1.39–2.124 | <0.001 | 1.67 | 1.34–2.08 | <0.001 |
| Tricyclic antidepressants | 208 | 192 | 1.33 | 0.92–1.92 | 0.14 | 1.19 | 0.81–1.75 | 0.39 |
| SNRIs | 175 | 164 | 1.36 | 0.85–2.16 | 0.20 | 1.27 | 0.79–2.05 | 0.33 |
| MAO inhibitors | 38 | 30 | 2.33 | 0.90–6.07 | 0.08 | 2.41 | 0.90–6.45 | 0.08 |
| Other antidepressants | 726 | 691 | 1.28 | 1.01–1.62 | 0.04 | 1.19 | 0.93–1.51 | 0.17 |
| Antidepressant Class and Agent | Case Subjects (N=5,377) | Control Subjects (N=5,377) | Crude Odds Ratio | 95% CI | p | Adjusted Odds Ratiob | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| SSRIs | 727 | 640 | 2.00 | 1.55–2.59 | <0.001 | 1.84 | 1.42–2.40 | <0.001 |
| Fluoxetine | 263 | 235 | 1.80 | 1.19–2.72 | <0.01 | 1.68 | 1.10–2.57 | 0.02 |
| Citalopram | 92 | 79 | 2.63 | 1.16–5.93 | 0.02 | 2.15 | 0.94–4.93 | 0.07 |
| Paroxetine | 127 | 111 | 1.94 | 1.08–3.49 | 0.03 | 1.76 | 0.97–3.23 | 0.07 |
| Sertraline | 194 | 168 | 2.00 | 1.25–3.20 | <0.01 | 1.87 | 1.16–3.02 | 0.01 |
| Fluvoxamine | 53 | 52 | 1.11 | 0.45–2.73 | 0.82 | 0.92 | 0.36–2.33 | 0.86 |
| Escitalopram | 26 | 19 | 2.17 | 0.82–5.70 | 0.12 | 2.35 | 0.86–6.43 | 0.10 |
| Tricyclic antidepressants | 178 | 163 | 1.50 | 0.95–2.38 | 0.09 | 1.31 | 0.81–2.11 | 0.28 |
| Imipramine | 101 | 94 | 1.30 | 0.76–2.25 | 0.34 | 1.12 | 0.64–1.99 | 0.70 |
| Clomipraminec | 6 | 5 | — | — | ||||
| Amitriptyline | 34 | 32 | 1.40 | 0.44–4.41 | 0.57 | 1.27 | 0.40–4.07 | 0.69 |
| Doxepin | 23 | 22 | 1.50 | 0.25–8.98 | 0.66 | 1.41 | 0.21–9.47 | 0.72 |
| Dothiepinc | 3 | 2 | — | — | ||||
| Maprotiline | 14 | 14 | 1.00 | 0.20–4.96 | 1.00 | 0.74 | 0.13–4.04 | 0.72 |
| SNRIs | 159 | 144 | 1.94 | 1.06–3.54 | 0.03 | 1.90 | 1.00–3.62 | 0.05 |
| Venlafaxine | 135 | 121 | 1.93 | 1.04–3.61 | 0.04 | 1.90 | 0.97–3.69 | 0.06 |
| Milnacipranc | 11 | 10 | — | — | ||||
| Duloxetine | 15 | 13 | 3.00 | 0.31–28.83 | 0.34 | 1.78 | 0.17–18.94 | 0.63 |
| MAO inhibitors | 27 | 33 | 0.46 | 0.16–1.31 | 0.14 | 0.43 | 0.15–1.24 | 0.12 |
| Moclobemide | 27 | 33 | 0.46 | 0.16–1.31 | 0.14 | 0.43 | 0.15–1.24 | 0.12 |
| Other antidepressants | 758 | 718 | 1.49 | 1.13–1.98 | <0.01 | 1.33 | 0.99–1.78 | 0.06 |
| Trazodone | 565 | 531 | 1.55 | 1.13–2.13 | 0.007 | 1.37 | 0.98–1.90 | 0.06 |
| Mirtazapine | 95 | 95 | 1.00 | 0.48–2.10 | 1.00 | 1.02 | 0.46–2.25 | 0.97 |
| Bupropion | 28 | 30 | 0.50 | 0.09–2.73 | 0.42 | 0.53 | 0.09–3.15 | 0.48 |
Antidepressants with high (adjusted odds ratio=1.75, 95% CI=1.31–2.34) and intermediate (adjusted odds ratio=1.74, 95% CI=1.23–2.45) serotonin transporter affinity were associated with an elevated risk of upper gastrointestinal bleeding, but not antidepressants with low affinity (adjusted odds ratio=1.27, 95% CI=0.94–1.72) (see Figure S1 in the online data supplement). SSRIs showed no dose-response relationship for risk of upper gastrointestinal bleeding (the adjusted odds ratios were 2.23 [p=0.08], 2.47 [p<0.001], 1.59 [p<0.010], and 1.75 [p=0.002] for <0.5, 0.5–1.0, 1.0–2.0, and ≥2.0 defined daily doses/day, respectively).
The effects of the interactions of SSRIs with NSAIDs and aspirin on the risk of upper gastrointestinal bleeding are summarized in Table 4. An elevated risk of upper gastrointestinal bleeding was observed in patients who were taking NSAIDs, SSRIs, or aspirin alone, with adjusted odds ratios ranging from 1.77 to 1.97. For patients taking SSRIs with aspirin, the risk of upper gastrointestinal bleeding increased slightly, to an odds ratio of 2.07. The highest upper gastrointestinal bleeding risk was observed with the combination of SSRIs and NSAIDs (adjusted odds ratio=3.44). The combination of all three drug types (SSRIs, NSAIDs, and aspirin) did not further increase the risk of upper gastrointestinal bleeding (adjusted odds ratio=3.27). Neither benzodiazepines (adjusted odds ratio=1.18, 95% CI=0.34–1.66) nor nonbenzodiazepine hypnotics (adjusted odds ratio=1.16, 95% CI=0.79–1.69) were associated with an elevated risk of upper gastrointestinal bleeding.
| Medications | Case Subjects (N=5,377) | Control Subjects (N=5,377) | Crude Odds Ratio | 95% CI | p | Adjusted Odds Ratiob | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| SSRIs alone | 504 | 464 | 1.79 | 1.39–2.29 | <0.001 | 1.77 | 1.38–2.28 | <0.001 |
| NSAIDs alone | 706 | 533 | 2.05 | 1.72–2.44 | <0.001 | 1.97 | 1.65–2.36 | <0.001 |
| Aspirin alone | 345 | 310 | 1.99 | 1.48–2.67 | <0.001 | 1.92 | 1.42–2.59 | <0.001 |
| SSRIs and aspirin | 71 | 69 | 2.25 | 1.13–4.49 | 0.02 | 2.07 | 1.02–4.21 | 0.04 |
| SSRIs and NSAIDs | 125 | 86 | 4.01 | 2.64–6.07 | <0.001 | 3.44 | 2.25–5.27 | <0.001 |
| NSAIDs and aspirin | 88 | 63 | 3.29 | 2.10–5.15 | <0.001 | 3.07 | 1.93–4.88 | <0.001 |
| SSRIs, NSAIDs, and aspirin | 27 | 21 | 3.78 | 1.48–9.61 | <0.01 | 3.27 | 1.25–8.60 | 0.02 |
In subgroup analyses, the risk of upper gastrointestinal bleeding was significantly elevated in males (adjusted odds ratio=2.43, 95% CI=1.75–3.38) but not in females (adjusted odds ratio=1.01, 95% CI=0.63–1.61). The risk of upper gastrointestinal bleeding was more easily observed in patients who were under age 55 (adjusted odds ratio=2.13, 95% CI=1.45–3.12), those who had a history of upper gastrointestinal tract diseases (adjusted odds ratio=3.17, 95% CI=1.70–6.00), and those who had no history of previous SSRI use (odds ratio=2.64, 95% CI=1.55–4.24). The bleeding risk was not associated with the patients’ comorbidities.
Discussion
In this case-crossover study, we provide new evidence that short-term exposure (7–28 days) to SSRIs is associated with a significant risk of upper gastrointestinal bleeding, a phenomenon similar to the short-term use of NSAIDs and antiplatelet agents.
Most previous studies exploring the effect of long-term (3 months) SSRI use on the risk of upper gastrointestinal bleeding were cohort, case-control, or nested case-control studies, which presented conflicting results (7, 25–27). The inconsistent conclusions may be due to the differences in enrollment criteria and matching strategies. Many of the previous studies did not take into consideration confounders (such as lifestyle factors and comorbidities) in the SSRI users or the patients with upper gastrointestinal bleeding (25, 27). Lifestyle factors such as alcoholism, smoking, and mental status are associated with peptic ulcer disease and even with a higher risk of upper gastrointestinal bleeding with or without SSRI exposure (28). Knowledge of these factors was typically unavailable or was not recorded in previous retrospective population studies. Some studies did not match with comorbidities (4, 26), while others did report comorbidities but with uneven distribution between case and control groups, which may adversely affect the final results (7, 8). To overcome this potential bias, we used a case-crossover design to assess the risk of upper gastrointestinal bleeding during a short interval (7–28 days) after SSRI exposure (13). Because lifestyle and the presence of chronic comorbid illnesses were unlikely to change during such a short period, the only differences between the case and control periods were the time-varying factors (medications). With this measurement, most of the unmeasured confounding factors that could cause selection bias would be eliminated.
We found that SSRIs were associated with an elevated risk of upper gastrointestinal bleeding in all three time windows. The risk after 14 days of exposure was higher than that for the 7- and 28-day windows, although the adjusted odd ratios overlapped (Figure 2). Multiple mechanisms may be involved in this association. Within 14 days of use, SSRIs can significantly reduce intraplatelet serotonin concentration (83% decrement) and impair platelet plug formation (9–11). SSRIs can also directly induce mucosal damage in the gastrointestinal tract and stimulate gastric acid secretion in rats within hours, which may potentiate the risk for upper gastrointestinal bleeding (29, 30). Our finding that the patients with preexisting upper gastrointestinal tract disease were predisposed to bleeding after short-term SSRI exposure further supports this observation.
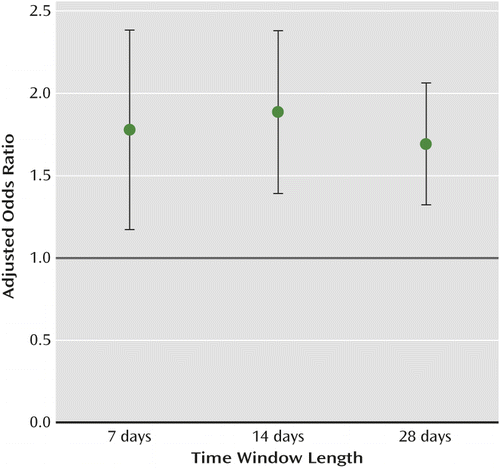
a Odds ratios were calculated for each of the three time windows and adjusted according to the use of proton pump inhibitors, histamine-2 receptor blockers, nonsteroidal anti-inflammatory drugs, clopidogrel, systemic corticosteroids, vitamin K antagonists, and aspirin. Results were significant at p<0.001 for all three time windows. Error bars indicate 95% confidence intervals.
In this study, we focused on patients with psychiatric diagnoses from a national population-based claims database. We believed that this specific group of patients served as an ideal group to test our hypothesis. First, in previous studies addressing the effects of SSRIs on the risk of upper gastrointestinal bleeding, the conclusions were almost always based on general-population data. These patients were usually highly exposed to other drugs related to bleeding (such as aspirin and NSAIDs), but a low percentage were SSRIs users (approximately 3% in the studied population) (6, 25). The role of SSRIs in the risk of upper gastrointestinal bleeding might be underestimated in patients with these conditions. Second, patients with psychiatric diagnoses constitute the main subpopulation that uses SSRIs, and their treatment course would be more regular and longer and would involve higher dosages compared with the general population. Thus, with the data set we used in this study, our results may better reflect the real effect of SSRIs on the risk of upper gastrointestinal bleeding.
Among the various SSRIs, fluoxetine and sertraline were both associated with an elevated risk of upper gastrointestinal bleeding. Although other SSRIs (citalopram, paroxetine, and escitalopram) were also associated with an elevated bleeding risk, the relationships fell short of statistical significance. We believe this may be due to the modest risk of upper gastrointestinal bleeding after SSRI exposure and the underpowered sample size for each individual SSRI, even though the number of individual drugs in this study outnumbered those in previous reports (25, 26). However, this assumption cannot be warranted until confirmed by studies with large enough subsamples of patients taking individual SSRIs.
Short-term exposure to tricyclic antidepressants, SNRIs, MAO inhibitors, and other antidepressants was not associated with an elevated risk of upper gastrointestinal bleeding. This finding suggests that these drugs might serve as alternatives to SSRIs in psychiatric patients with a history of gastrointestinal bleeding or peptic ulcer disease. Before the era of proton pump inhibitors, tricyclic antidepressants were used to treat peptic ulcer disease because of their anticholinergic and antihistamine effects (31). Mirtazapine also has antiulcer effects in rats and has fewer gastrointestinal side effects owing to its blocking effect on 5-HT2 and 5-HT3 receptors (32).
We found that male, but not female, SSRI users had an elevated risk for upper gastrointestinal bleeding. Previous studies have found that females have a lower but still significant risk of upper gastrointestinal bleeding compared with males after SSRI exposure (7, 26). Our results are in accord with a recent large-scale study showing that only male patients had a heightened risk of upper gastrointestinal bleeding following acute myocardial infarction after exposure to an SSRI and antiplatelet agents (5). There are several possible explanations for this finding. First, serotonin blood levels are higher in females than in males, because estradiol can stimulate serotonin uptake by platelets (33). Second, estrogen can stimulate platelet aggregation, resulting in higher platelet aggregation activity in females compared with males (34). Additionally, male gender is a risk factor for peptic ulcer disease, and the gastric microenvironment is more acidic in males than in females (35), which may also contribute to the gender differences in the risk of upper gastrointestinal bleeding after SSRI exposure. However, we should be careful in the interpretation of this result, because the power to detect a difference between case and control subjects in the female subgroup was inadequate in this study.
Although SSRIs are widely used in the treatment of depression and anxiety disorders, SSRIs can still be associated with significant side effects. For example, suicidality, birth defects, neonatal withdrawal syndrome, sexual disturbance, hyponatremia, weight gain, insomnia, and gastrointestinal bleeding have been associated with long-term SSRI use (36). Traditionally, short-term side effects of SSRI, such as nausea, diarrhea, headache, and agitation, are usually considered mild and subside after 2–3 weeks. However, this study demonstrated that short-term SSRI exposure (as little as 7 days) increased the risk of upper gastrointestinal bleeding. The extensive use of SSRIs has been criticized by some who question whether it is worthwhile to trade questionable symptom relief for these potentially serious and bothersome side effects, particularly when evidence-proven alternative interventions are available (37). Given the relatively high incidence of upper gastrointestinal bleeding in the general population (36–172 per 100,000) and the associated high mortality rate (2.4%−10%) (38), we suggest that physicians make treatment decisions on an individualized basis by balancing the potential side effects and treatment responses when prescribing SSRIs. Close monitoring of signs of gastrointestinal bleeding may be warranted soon after beginning SSRI treatment.
This study has several limitations. First, the exact reasons for SSRI prescriptions were not known. Prescription of an SSRI could be due to stressful life events, neuroticism, and anxiety. These psychological events are linked to the pathogenesis of peptic ulcer disease and might predispose the individual to upper gastrointestinal bleeding. Thus, these psychological factors may also contribute to upper gastrointestinal bleeding. Second, because patients’ identities were hidden for privacy protection, no information was available in the NHIRD about their adherence to the prescriptions, which is an inherent limitation of all studies using claims data. Nevertheless, previous information from official databases has shown high concordance between actual prescription adherence and self-reported medication use (39). In any case, nonadherence would eventually lead to underestimation of the actual risk. Finally, this study mainly focused on inpatients with psychiatric diagnoses whose illnesses are likely to be on the severe end of the spectrum. Our findings may or may not extend to patients with nonpsychiatric diagnoses.
1 : Utilization of antidepressants in Taiwan: a nationwide population-based survey from 2000 to 2009. Pharmacoepidemiol Drug Saf 2012; 21:980–988Crossref, Medline, Google Scholar
2 : Generic entry, reformulations, and promotion of SSRIs in the US. Pharmacoeconomics 2008; 26:603–616Crossref, Medline, Google Scholar
3 : Upper gastrointestinal bleeding in a patient with depression receiving selective serotonin reuptake inhibitor therapy. Indian J Pharmacol 2009; 41:51–53Crossref, Medline, Google Scholar
4 : Risk of serious upper gastrointestinal events with concurrent use of NSAIDs and SSRIs: a case-control study in the general population. Eur J Clin Pharmacol 2007; 63:403–408Crossref, Medline, Google Scholar
5 : Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ 2011; 183:1835–1843Crossref, Medline, Google Scholar
6 : Increased bleeding risk with concurrent use of selective serotonin reuptake inhibitors and coumarins. Arch Intern Med 2008; 168:180–185Crossref, Medline, Google Scholar
7 ;
8 : Selective serotonin reuptake inhibitors and gastrointestinal bleeding: a case-control study. PLoS ONE 2011; 6:e19819Crossref, Medline, Google Scholar
9 : Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther 2000; 68:435–442Crossref, Medline, Google Scholar
10 : Early effects of paroxetine on serotonin storage, plasma levels, and urinary excretion: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 2004; 24:536–539Crossref, Medline, Google Scholar
11 : Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes, and implications for use in elderly patients. Drugs Aging 2011; 28:345–367Crossref, Medline, Google Scholar
12 : The coadministration of paroxetine and low-dose aspirin synergistically enhances gastric ulcerogenic risk in rats. Biol Pharm Bull 2008; 31:1371–1375Crossref, Medline, Google Scholar
13 : The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133:144–153Crossref, Medline, Google Scholar
14 : Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology 2006; 17:268–275Crossref, Medline, Google Scholar
15 : The case-crossover study design in pharmacoepidemiology. Stat Methods Med Res 2009; 18:53–65Crossref, Medline, Google Scholar
16 : Non-steroidal anti-inflammatory drugs and risk of lower gastrointestinal adverse events: a nationwide study in Taiwan. Gut 2011; 60:1372–1378Crossref, Medline, Google Scholar
17 : Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry 2011; 168:511–521Link, Google Scholar
18 : A Study of Validation on Cormobidity Derived From Claims Data. Taiwan, National Yang-Ming University, 2004Google Scholar
19 : Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242Crossref, Medline, Google Scholar
20 : Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005; 104:157–163Medline, Google Scholar
21 : The accuracy of diagnosis and procedural codes for patients with upper GI hemorrhage. Gastrointest Endosc 2000; 51:423–426Crossref, Medline, Google Scholar
22 : Positive predictive value of ICD-9 codes in the identification of cases of complicated peptic ulcer disease in the Saskatchewan Hospital automated database. Epidemiology 1996; 7:101–104Crossref, Medline, Google Scholar
23 : Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619Crossref, Medline, Google Scholar
24 World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology: Guidelines for ATC Classification and DDD Assignment, 2012. Oslo, WHO, 2011Google Scholar
25 : Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ 1999; 319:1106–1109Crossref, Medline, Google Scholar
26 : An association between selective serotonin reuptake inhibitor use and serious upper gastrointestinal bleeding. Clin Gastroenterol Hepatol 2009; 7:1314–1321Crossref, Medline, Google Scholar
27 : Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 2003; 163:59–64Crossref, Medline, Google Scholar
28 : Gastro-intestinal haemorrhage risks of selective serotonin receptor antagonist therapy: a new look. Br J Clin Pharmacol 2008; 66:76–81Crossref, Medline, Google Scholar
29 : Fluoxetine and sertraline stimulate gastric acid secretion via a vagal pathway in anaesthetised rats. Pharmacol Res 2004; 50:309–316Crossref, Medline, Google Scholar
30 : High prevalence of gastroduodenal mucosal injury in patients taking selective serotonin reuptake inhibitors. Dig Endosc 2010; 22:77Crossref, Medline, Google Scholar
31 : Tricyclic antidepressant therapy for peptic ulcer disease. Arch Intern Med 1984; 144:566–569Crossref, Medline, Google Scholar
32 : Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Dig Dis Sci 2009; 54:1868–1875Crossref, Medline, Google Scholar
33 : Effects of sex steroids on serotonin uptake in blood platelets. Acta Endocrinol (Copenh) 1976; 83:420–428Crossref, Medline, Google Scholar
34 : The effects of estrone, estradiol, and estriol on platelet aggregation induced by adrenaline and adenosine diphosphate. Platelets 2006; 17:441–447Crossref, Medline, Google Scholar
35 : Age does not affect basal gastric acid secretion in normal subjects or in patients with acid-peptic disease. Am J Gastroenterol 1994; 89:712–716Medline, Google Scholar
36 : Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J Psychopharmacol 2009; 23:967–974Crossref, Medline, Google Scholar
37 : Statistical alchemy for drug treatment of generalized anxiety disorder: a commentary on the meta-analysis by Baldwin et al [BMJ 2011;342:d1199]. Psychother Psychosom 2011; 80:261–263Crossref, Medline, Google Scholar
38 : Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008; 22:209–224Crossref, Medline, Google Scholar
39 : High concordance between self-reported medication and official prescription database information. Eur J Clin Pharmacol 2007; 63:1069–1074Crossref, Medline, Google Scholar



