Bipolar Disorder and a History of Suicide Attempts With a Duplication in 5HTR1A
To the Editor: Serotonergic function has long been thought to be important in mood regulation, and first-line treatments for mood disorders involve the use of selective serotonin reuptake inhibitors. There are numerous receptors for serotonin in the brain, and most have been investigated in candidate gene studies or gene expression studies using blood or brain samples from mood disorder patients or those who have died by suicide. One particular brain receptor for serotonin, 5HTR1A, has been studied thoroughly with conflicting results: some studies suggest increased expression or binding in the frontal cortex and rostral raphe of suicide completers (1, 2), while others show decreased expression or binding in the brains of depressed subjects (3). Nevertheless, all studies suggest that altered expression of 5HTR1A may have a role in suicidal behavior and mood. We identified one individual recruited in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) cohort (4) with a 617,395 Kb duplication on chromosome 5 (Hg18: 63166781–63784175) that included a complete duplication of the 5HTR1A gene (Figure 1A). We validated this duplication with quantitative real-time polymerase chain reaction using three independent probes across the copy number variant (CNV) comparing the patient’s DNA to seven control samples without a CNV at this locus (Figure 1B). There were no other rare CNVs in this individual within the detection resolution of this technology.
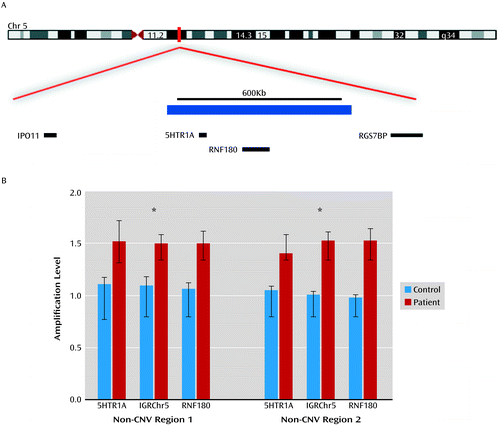
a Panel A shows duplication from a single patient (blue bar) on chromosome 5q12.2 involving 5HTR1A, with genes at and near the locus noted. Panel B shows real-time polymerase chain reaction using probes across the CNV region (introns of 5HTR1A, RNF180, and an intergenic region on chromosome 5, IGRChr5) and normalized to two independent genomic regions with no dosage change (non-CNV regions 1 and 2), in the patient and seven control samples known not to carry the 5HTR1A-related CNV.
* p<0.05 or equivalent.
The patient was a 49-year-old woman with bipolar I disorder. She had no history of psychosis or alcohol and drug abuse or dependence, but she did have a history of multiple suicide attempts. She was divorced, was living on disability, and had no history of family or partner violence. In the year prior to enrollment in the study, her illness had a rapid cycling course (70% of days depressed, 90% irritable, 80% anxious, and 10% elevated mood). She entered the study euthymic, but she experienced a major depressive episode that was treated with lamotrigine; she exited the study before recovery. She reported the onset of her illness at age 16, with no history of antidepressant-associated switching from depression to mania. She characterized depressive and manic episodes in her life as “too numerous to count.”
The STEP-BD data set comprises 945 individuals (433 of them male) with bipolar disorder, 383 of whom reported a previous suicide attempt. All participants were screened on the Affymetrix 6.0 array, and CNVs were called using Birdsuite and processed in PLINK. In line with the serotonergic hypothesis of mood disorders, we further screened all STEP-BD participants for CNVs in any serotonergic genes but found none. Only one participant of unknown psychiatric history in the Database of Genomic Variants (subject 36425)—a database of over 100,000 CNVs consisting of nonpsychiatrically screened control subjects from multiple studies—also had a large duplication that included 5HTR1A, suggesting that CNVs at this locus are not common. While it is impossible to determine whether expression of 5HTR1A was increased in the brain of this individual as a result of having three copies of the gene instead of two, these data support the hypothesis that increased expression of 5HTR1A contributes to mood dysregulation and suicidal behavior and that potential alterations lead to psychopathology rather than altered mood leading to increased expression of 5HTR1A. No statistically significant causation can be shown from our analysis; that is, all individuals have private variation nonspecific to disease and thus variants cannot be conclusively linked to disease in a single case report.
1 : Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol 2011; 15:435–447Crossref, Medline, Google Scholar
2 : Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res 2008; 42:433–442Crossref, Medline, Google Scholar
3 : Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000; 57:174–180Crossref, Medline, Google Scholar
4 : Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003; 53:1028–1042Crossref, Medline, Google Scholar



