Risk for Addiction-Related Disorders Following Mild Traumatic Brain Injury in a Large Cohort of Active-Duty U.S. Airmen
Abstract
Objective
Military personnel are at increased risk for traumatic brain injury (TBI) from combat and noncombat exposures. The sequelae of moderate to severe TBI are well described, but little is known regarding long-term performance decrements associated with mild TBI. Furthermore, while alcohol and drug use are well known to increase risk for TBI, little is known regarding the reverse pattern. The authors sought to assess possible associations between mild TBI and addiction-related disorders in active-duty U.S. military personnel.
Method
A historical prospective study was conducted using electronically recorded demographic, medical, and military data for more than a half million active-duty U.S. Air Force service members. Cases were identified by ICD-9-CM codes considered by an expert panel to be indicative of mild TBI. Outcomes included ICD-9-CM diagnoses of selected addiction-related disorders. Cox proportional hazards modeling was used to calculate hazard ratios while controlling for varying lengths of follow-up and potential confounding variables.
Results
Airmen with mild TBI were at increased risk for certain addiction-related disorders compared with a similarly injured non-mild TBI comparison group. Hazards for alcohol dependence, nicotine dependence, and nondependent abuse of drugs or alcohol were significantly elevated, with a consistent decrease over time.
Conclusions
A novel finding of this study was the initial increased risk for addiction-related disorders that decreased with time, thus eroding war fighter performance in a military population. Moreover, these results suggest that mild TBI is distinguished from moderate to severe TBI in terms of timing of the risk, indicating that there is a need for screening and prevention of addiction-related disorders in mild TBI. Screening may be warranted in military troops as well as civilians at both short- and long-term milestones following mild TBI.
Traumatic brain injury (TBI) is considered a signature injury in the wars in Iraq and Afghanistan (1). The prevalence of TBI among U.S. troops is estimated to be between 23% (2) and 30% (combat-exposed troops) (3). Advances in protective equipment and battlefield medicine have resulted in roughly 90% survival rates for wounded U.S. troops (4), leaving more of them to deal with the lingering effects of TBI than in previous wars. The associated projected health care costs over the next two decades are in the billions of dollars (5). The evolution and pervasive deployment of improvised explosive devices has often resulted in mild TBI. Mild TBI, commonly known as a concussion, occurs when trauma to the head is combined with one or more of the following attributable symptoms: a brief alteration of mental status, such as confusion or disorientation; loss of memory for events immediately before or after the injury; and/or loss of consciousness lasting less than 30 minutes. Trauma to the brain is known to cause long-term cytoarchitectural and functional damage that often leads to neurological disease (6, 7), psychiatric symptoms (4, 8–11), or disability (12, 13). Unfortunately, most TBI research involves moderate to severe forms of the injury, with little attention to sequelae (14).
Addiction-related disorders portend an increased risk for TBI (e.g., in motor vehicle accidents and in falls). However, little research has assessed the reverse pattern. This is an important issue, as demonstrated in a 5-year study of biopsychosocial outcomes following TBI, such as functional independence, sickness, medical consequences, community integration, disability assessment, life satisfaction, and other factors, whereby the only measure indicating deterioration was the proportion of TBI patients abusing drugs or alcohol (15). Proposed mechanisms of an increased risk for addiction-related disorders in mild TBI include substance use as a coping response to the psychosocial stressors of disability, substance use as a consequence of damage to brain circuits known to mediate the medical disease of addiction (disrupting incentive-motivation neurocircuitry or causing persistent executive cognitive deficits) (16), and substance use as self-medication for chronic pain or attentional/cognitive dysfunction. All three mechanisms are plausible in our study population.
Most studies of post-TBI addiction-related disorders focused primarily on alcohol use, did not include a comparison group, were weighted by moderate to severe TBI, and examined heterogeneous subjects. While the results of some studies support increased alcohol use or problems after TBI (15, 17–19), others have found decreases after TBI, with some finding a later, gradual return to near or below pre-TBI levels long-term (20–25). Some studies also have shown a reduction in drug use after TBI (24, 26, 27). A commonly held opinion is that “TBI is a minimal, short-term risk factor for substance abuse ... the prevalence of substance abuse after TBI appears to reflect enduring, pre-morbid abuse patterns and coping strategies. As such, TBI is more often a consequence than a cause of substance abuse” (28). Thus, both the literature on mild TBI and clinical practice recommendations are conflicted and incomplete.
According to a recent Capitol Hill briefing presented by the National Institute on Drug Abuse, there is “no information regarding [the effects of mild TBI] on substance use disorders” (29). However, given the above evidence, we hypothesized that mild TBI may increase the risk for addiction-related disorders. This hypothesis can be tested using existing Department of Defense electronic personnel and medical data. Therefore, our objective was to conduct a historical perspective cohort study of active-duty male and female U.S. Air Force enlisted and officer personnel (airmen) to assess possible associations between mild TBI and addiction-related disorders.
Method
Population and Data Sources
Electronic personnel data were obtained from the Defense Manpower Data Center, which maintains demographic and military electronic records for all U.S. service personnel for the duration of their military careers. Outpatient electronic medical record data were obtained from the Military Health System, which is maintained by TRICARE Management Activity. Study subjects’ demographic and military data were linked to electronic medical records using personal identifiers. Individual diagnoses of addiction-related disorders were compiled and are listed in Table 1. To better manage the number of data analyses while still assessing addiction-related disorders as a whole, we limited the number of drug classes to those that would possibly counter the cognitive dysfunction or pain issues stemming from mild TBI. However, cannabis and cocaine use were included in the categories of drug dependence and nondependent abuse of drugs.
| Addiction-Related Disorders | ICD-9-CM Code |
|---|---|
| Alcohol dependence | 303.90 |
| Drug dependence | 304.00–304.93 |
| Nondependent abuse of drugs (including alcohol) | 305.20–305.83 |
| Nicotine dependence | 305.10–305.13 |
| Opioid dependence or abuse | 304.00–304.03, 305.50–305.53 |
| Caffeine-related disorders | 305.90–305.93 |
| Amphetamine dependence or abuse | 304.40–304.43, 305.70–305.73 |
| Pathological gambling disorder | 312.31 |
Mild TBI was identified using ICD-9-CM codes listed by the Centers for Disease Control and Prevention (CDC) in their 2003 report to Congress on mild TBI in the United States (30). The CDC list comprises ICD-9-CM codes (31) that are considered by an expert panel to be indicative of mild TBI, meaning transient confusion or disorientation, memory loss, and/or brief loss of consciousness. ICD-9-CM diagnoses for mild TBI found in electronic outpatient health records were used to identify cases of mild TBI.
For this study, airmen who were on active duty for at least 180 days between October 1, 2001, and September 30, 2008, were selected. To increase the probability of including only incident cases (N=5,065), individuals with a history of mild TBI, other head injuries, or addiction-related disorders 2 years prior to entering the study were removed from consideration. The comparison group (N=44,733) consisted of airmen who were diagnosed as having sustained an outpatient injury to the torso, spinal cord, abdomen, pelvis, digestive tract, or genitourinary tract (ICD-9-CM 805–810, 860–870, 900–905, 922–923, 926–927, and 933–959). The comparison group was referred to as the “other-injured group” for the purposes of this study. The other-injured group was used to minimize medical surveillance bias that may occur as a result of increased medical observation after injury and to reduce the likelihood of confounding associated with having sustained any injury.
The study was conducted in accordance with all applicable federal regulations governing the protection of human subjects in research as approved by the Air Force Research Laboratory/Wright Site Institutional Review Board (protocol F-WR-2009-0066-H).
Statistical Analyses
Demographic and military data were analyzed using frequency distributions and Pearson’s chi-square tests to determine statistical significance, univariate differences, and possible confounding. Cox proportional hazards models were used in the multivariate analysis. Each ICD-9-CM category was investigated separately to calculate hazard ratios among individuals with a diagnosis in that category. For each individual, person-time began on the date at which the individual was diagnosed with a mild TBI or other bodily injury. If an individual suffered another mild TBI or other bodily injury, person-time ended the day before the subsequent event. All Cox proportional hazards models were adjusted for sex, marital status, race/ethnicity, birth year category, deployment status, education level, rank, career field, and most axis I disorders (as coded by ICD-9-CM codes listed in Table 2). The axis I diagnoses used in the adjustments are often comorbid with TBI, and in our study population these diagnoses were made after the TBI and were not present during the 2 years prior to study entry. No significant interactions or multicollinearity were detected among any independent variables in these models.
| Diagnosis | ICD-9-CM Code |
|---|---|
| Cognitive disorders | |
| Attention deficit disorder/attention deficit hyperactivity disorder | 314.00, 314.01 |
| Schizophrenia and other psychotic disorders | 293.81, 293.82, 295.10–295.45, 295.60–295.75, 295.90–295.95, 297.1, 297.3, 298.8, 298.9 |
| Mood disorders | |
| Unipolar depression | 296.30–296.36, 300.4, 311 |
| Unspecified/episodic mood disorders | 293.83, 296.90–296.99 |
| Bipolar and cyclothymic disorders | 296.00–296.06, 296.40–296.89, 301.13 |
| Anxiety disorders | |
| Generalized anxiety disorder or anxiety disorder not otherwise specified | 293.84, 300.00–300.09 |
| Panic/phobic disorders | 300.20–300.29 |
| Obsessive-compulsive disorder | 300.3, 301.4 |
| Acute stress disorder | 308.3 |
| Posttraumatic stress disorder | 309.81 |
| Adjustment reactions | 309.0, 309.1, 309.24, 309.28, 309.3, 309.4, 309.82–309.9 |
To examine the association between mild TBI and the outcomes of interest, postexposure time was divided into three periods: 1–30 days, 31–179 days, and ≥180 days. The first occurrence of each outcome was used in the analysis; therefore, individuals with an outcome diagnosis in a subsequent time interval were not previously diagnosed with that outcome in a preceding time interval. Thus, data for individuals used in either of the first two time periods were removed from the analyses for any succeeding time periods. Adjusted hazard ratios with 95% confidence intervals (CIs) were calculated to compare the risk of the specified outcomes between the mild TBI group and the other-injured group.
Additional analyses using propensity score matching were conducted by replacing potentially confounding covariates with a single score that measured the propensity of an individual in the other-injured group to be similar to an individual in the mild TBI group. For each mild TBI case subject, one closely matched (based on demographic variables) comparison subject was selected from the other-injured group. Adjusted Cox proportional hazards models were then run on the matched pairs. All statistical analyses were conducted using SAS, version 9.2 (SAS Institute, Cary, N.C.).
Results
Our study included 5,065 active-duty airmen who met the CDC administrative data criteria for a mild TBI. The other-injured (comparison) group consisted of 44,733 airmen. In the univariate analysis, individuals in the mild TBI group were more likely to be male, to be white, to be born after 1975, never to have been married, to have an education level of high school or less, to be enlisted, and to have worked in the logistics/maintenance career field (Table 3). In the univariate analysis, all demographic and military characteristic differences were statistically significant (p<0.001, using Pearson’s chi-square test). These univariate differences were adjusted for in the multivariate analysis because they were thought to be possible confounding variables in the comparison between the mild-TBI and other-injured groups with respect to addiction-related disorders.
| Characteristic | Mild Traumatic Brain Injury Group (N=5,065) | Other-Injured Comparison Group (N=44,733) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 4,158 | 82.09 | 33,674 | 75.28 |
| Female | 907 | 17.91 | 11,059 | 24.72 |
| Race/ethnicity | ||||
| White (non-Hispanic) | 3,802 | 75.06 | 32,772 | 73.26 |
| Black (non-Hispanic) | 588 | 11.61 | 6,162 | 13.78 |
| Asian or Pacific Islander | 126 | 2.49 | 1,269 | 2.84 |
| Hispanic | 329 | 6.50 | 2,604 | 5.82 |
| Native American | 35 | 0.69 | 368 | 0.82 |
| Other/unknown | 185 | 3.65 | 1,558 | 3.48 |
| Birth year | ||||
| Before 1965 | 340 | 6.71 | 6,259 | 13.99 |
| 1966–1975 | 795 | 15.70 | 10,020 | 22.40 |
| 1976 or later | 3,930 | 77.59 | 28,454 | 63.61 |
| Marital status | ||||
| Currently married | 1,481 | 29.24 | 18,588 | 41.55 |
| Never married | 3,418 | 67.48 | 24,228 | 54.16 |
| No longer married | 166 | 3.28 | 1,917 | 4.29 |
| Education | ||||
| High school diploma or less | 4,536 | 89.56 | 36,277 | 81.10 |
| Some college/bachelor’s degree | 364 | 7.19 | 5,614 | 12.55 |
| Advanced degree | 150 | 2.96 | 2,699 | 6.03 |
| Unknown | 15 | 0.30 | 143 | 0.32 |
| Rank | ||||
| Enlisted | 4,814 | 95.04 | 40,307 | 90.11 |
| Officer | 251 | 4.96 | 4,426 | 9.89 |
| Deployed | ||||
| Never | 2,526 | 49.87 | 22,163 | 49.55 |
| Once | 1,400 | 27.64 | 12,274 | 27.44 |
| Twice | 661 | 13.05 | 5,971 | 13.35 |
| More than twice | 478 | 9.44 | 4,325 | 9.67 |
| Career field | ||||
| Operations | 774 | 15.28 | 8,196 | 18.32 |
| Logistics/maintenance | 1,940 | 38.30 | 14,724 | 32.92 |
| Support | 1,466 | 28.94 | 12,596 | 28.16 |
| Medical | 381 | 7.52 | 4,116 | 9.20 |
| Professional/acquisitions/finance | 112 | 2.21 | 1,350 | 3.02 |
| Other/unknown | 392 | 7.74 | 3,751 | 8.39 |
Most striking in the Cox proportional hazards analyses was that the hazard for alcohol dependence was significantly elevated across all three periods of observation (1–30 days, 31–179 days, and ≥180 days after TBI) for the mild TBI group when compared with the other-injured group (Table 4, Figure 1). With regard to time trends, the highest hazard ratio for alcohol dependence occurred in the earliest period, between 1 and 30 days following a mild TBI diagnosis (hazard ratio=3.48; 95% CI=1.86–6.51), with a consistent decrease (31–179 days post-TBI: hazard ratio=2.66; 95% CI=1.86–3.81) over time (≥180 days post-TBI: hazard ratio=1.70; 95% CI=1.31–2.21). Nicotine dependence and nondependent abuse of drugs or alcohol demonstrated a similar pattern. The highest hazard ratios for nicotine dependence (hazard ratio=2.03; 95% CI=1.56–2.66) and nondependent abuse of drugs or alcohol (hazard ratio=2.11, 95% CI=1.65– 2.70) occurred in the first 30 days. Although the hazard ratios were also significantly elevated for drug dependence, opioid dependence or abuse, and caffeine-related disorders, the total number of cases in the mild TBI group was less than five, and the confidence intervals were wide, indicating uncertainty associated with these estimates.
| Category | Mild Traumatic Brain Injury (TBI) Group | Hazard Ratio | 95% CI | |
|---|---|---|---|---|
| N | % | |||
| 1–30 days post-TBI diagnosis | ||||
| Substance use disorder | ||||
| Alcohol dependence | 15 | 0.30 | 3.48b | 1.86–6.51 |
| Drug dependence | 4 | 0.08 | 7.71b | 1.87–31.78 |
| Nondependent abuse of drugs or alcohol | 82 | 1.62 | 2.11b | 1.65–2.70 |
| Nicotine dependence | 69 | 1.36 | 2.03b | 1.56–2.66 |
| Opioid dependence or abuse | 3 | 0.06 | 6.14b | 1.20–31.31 |
| Caffeine-related disorders | 4 | 0.08 | 3.78b | 1.09–13.08 |
| Amphetamine dependence or abuse | 1 | 0.02 | 4.82 | 0.30–77.70 |
| Pathological gambling disorder | 0 | 0.00 | —c | |
| 31–179 days post-TBI diagnosis | ||||
| Substance use disorder | ||||
| Alcohol dependence | 42 | 0.83 | 2.66b | 1.86–3.81 |
| Drug dependence | 5 | 0.10 | 1.09 | 0.42–2.80 |
| Nondependent abuse of drugs or alcohol | 152 | 3.00 | 1.14 | 0.96–1.35 |
| Nicotine dependence | 110 | 2.17 | 0.92 | 0.75–1.12 |
| Opioid dependence or abuse | 4 | 0.08 | 3.98b | 1.14–13.93 |
| Caffeine-related disorders | 7 | 0.14 | 1.58 | 0.69–3.63 |
| Amphetamine dependence or abuse | 0 | 0.00 | —c | |
| Pathological gambling disorder | 0 | 0.00 | —c | |
| 180 days post-TBI diagnosis | ||||
| Substance use disorder | ||||
| Alcohol dependence | 71 | 1.40 | 1.70b | 1.31–2.21 |
| Drug dependence | 2 | 0.45 | 1.35 | 0.86–2.11 |
| Nondependent abuse of drugs or alcohol | 534 | 10.54 | 1.01 | 0.92–1.11 |
| Nicotine dependence | 513 | 10.13 | 1.00 | 0.91–1.10 |
| Opioid dependence or abuse | 10 | 0.20 | 1.22 | 0.62–2.40 |
| Caffeine-related disorders | 22 | 0.43 | 1.30 | 0.82–2.05 |
| Amphetamine dependence or abuse | 1 | 0.02 | 0.83 | 0.10–6.75 |
| Pathological gambling disorder | 2 | 0.04 | 1.15 | 0.25–5.24 |
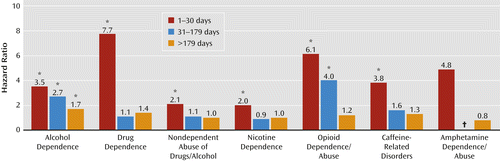
a Results were adjusted for sex, marital status, race/ethnicity, birth year, deployment, education, rank, and career field and for posttraumatic stress disorder, depression, and other mental disorders frequently comorbid with TBI.
* Differences are statistically significant at an alpha level of 0.05.
† The percentage of outcome in the comparison population was not sufficient to generate a hazard ratio with a 95% confidence interval.
Other than for alcohol dependence, the only other significant finding was an elevated hazard ratio for opioid dependence or abuse in both the 1–30 days (hazard ratio=6.14, 95% CI=1.20–31.31) and 31–179 days (hazard ratio=3.98, 95% CI=1.14–13.93) post-TBI categories. However, these hazard ratios were based on only three and four diagnoses, respectively, in the mild TBI group. The propensity score matching results were similar to our adjusted hazard ratios (Table 4) but with wider confidence intervals because of the reduced comparison population.
Discussion
To our knowledge, this is the first large, well-controlled, adjusted study of the association between mild TBI and subsequent addiction-related disorders. Mild TBI was associated with an increased risk for alcohol dependence, nondependent abuse of drugs or alcohol, and nicotine dependence in the first 30 days following mild TBI, with alcohol dependence being significant across all three independent time periods. Contrary to published observations of moderate to severe TBI, there was no period during which significant risk did not occur following mild TBI. Furthermore, whereas previous research indicated that mild TBI sequelae resolved quickly, our findings suggest that alcohol dependence may be a long-lasting adverse health outcome following mild TBI. Given the increasing emphasis and awareness of mild TBI in both military and civilian populations, these findings may have far-reaching clinical and military readiness implications.
These results should be interpreted within possible limitations, most importantly the use of multiple ICD-9-CM codes to identify mild TBI. We used a series of codes that were recommended in the CDC’s 2003 report to Congress (30). As part of an internal validation substudy, this project included a blinded medical record review with a board-certified neurologist. Data showed a moderate level of agreement between the CDC-recommended codes and evidence of mild TBI in the medical records that matched the date of diagnosis in the electronic data. A Cohen’s kappa value of 0.51 was statistically significant (95% CI=0.29–0.72).
Although there were specific ICD-9-CM codes for the addiction-related disorders studied, it is possible that that codes were not always assigned accurately, causing some misclassification of the outcomes of interest (14). The extent to which this occurred could not be accurately assessed, but it is most likely nondifferential with respect to TBI status and would most probably have biased our findings toward the null. It is also likely that ICD-9-CM codes underestimate the prevalence of addiction-related disorders, resulting in high specificity but low sensitivity (32); that is, they correctly identify those without the condition but are not as successful in identifying those with the condition. We believe that the strategy of using the other-injured comparison group, as well as examining the association for different time periods, mitigates these limitations.
Since this was a study of association and not causation, any cause-effect interpretations must be guarded. Although a causal mechanism seems biopsychosocially plausible, it is not clinically intuitive that hazard ratios would be elevated so soon after the incident mild TBI (within 30 days). That is, a delay might be expected for onset of addiction-related disorders after mild TBI, with a period of at least 12 months typically considered before an addiction-related disorders diagnosis is made (33). Perhaps biological stress from brain trauma or psychological stress relating to the mild TBI experience serves to hasten the expression of addiction-related disorders (34). It is known that TBI is associated with a variety of behavioral consequences (i.e., symptoms of depression, anxiety, aggression, and impulse control) and may diminish inhibitory control over certain behaviors while exacerbating clinical expression of psychiatric symptoms (34). TBI frequently results in injury to prefrontal cortical systems in the brain, which are central to impulse control and decision making, and TBI survivors are known to have blunted dopamine systems, which are significant to reward and salience attribution (35, 36). Alternatively, perhaps addiction-related disorders are diagnosed earlier in military populations (compared with the civilian population) as a result of more aggressive drug testing, widely implemented addiction-related disorders screening (required at annual medical, dental, deployment/readiness, and other assessments), access to addiction/mental health services at no cost, and use of commander-directed evaluations.
Our use of the other-injured group may have mediated the effect that injury stress and management of chronic pain may have on the development of addiction-related disorders. However, individuals coping with the cognitive deficits of head injury may have been referred to a substance-related specialist for assessment, resulting in an “incident” case of substance use disorder. For example, mood or behavioral symptoms may moderate the association between TBI and addiction-related disorders, as well as certain psychiatric disorders known to have a higher association with substance use disorders.
Moreover, not all individuals with mild TBI or mental disorders actually seek medical care (30, 37). Addiction-related disorders may lead to punitive actions in the military, and thus these disorders may be underreported in our study. Although individuals were excluded from the analyses if they had a preexisting (2 years prior to inclusion in the study) diagnosis of an addiction-related disorder, this would not necessarily exclude all alcohol- or substance-related behaviors. The effect of any underreporting on study findings is not clear and depends on whether or not underreporting is differential with respect to TBI status. Since we only used incident cases, we could not evaluate the consequence of multiple TBI events. Given the continuous exposure of military personnel to multiple sources of TBIs, further study of this issue is essential.
Our study has several strengths. Limitations of previous research on mild TBI and subsequent addiction-related disorders, reviewed above (16) support the quality of our analyses (which involved homogeneous subjects, exclusion of preexisting addiction-related disorders, assessing addiction-related disorders and TBI together, focus on mild severity only, and adjusting for axis I disorders). The use of Department of Defense electronic data eliminated the possibility of recall bias and resulted in a large sample size, which allowed us to examine a number of relatively uncommon addiction-related disorders. Using observations in three time periods enabled us to identify an earlier period of risk as well as a trend. Since this trend decreased over time, some researchers may have averaged over and minimized significant effects. Our results, especially as they pertain to alcohol dependence, suggest a decreasing hazard over time, which may be explained by 1) a gradual reduction in TBI-related psychosocial stress over time, 2) improved brain function over time as a result of tissue repair or neuroplasticity, 3) restoration of cognitive function over time, or 4) reduction in pain over time and subsequent reduced risk for self-medication. The use of the other-injured group as the comparison group is significant. Although our findings support those of perhaps the only other study (17) to assess mild TBI separately for the risk for addiction-related disorders, that study did not include an injured comparison group. By using the injured comparison group, our study isolated mild TBI as a significant contributing factor to addiction-related disorders. A focus extending beyond alcohol to all other drugs of abuse, legal drugs (nicotine, caffeine), and gambling is unique, as is the temporal approach to better assess for causality.
Conclusions
Our findings suggest an increased risk for incidence of alcohol dependence, nondependent abuse of drugs or alcohol, and nicotine dependence during the first 30 days following mild TBI and a risk thereafter for alcohol dependence for at least 6 months after injury. Any alcohol or drug use after TBI is concerning given the potential for reduction in spontaneous healing, risk of seizure or repeat TBI, and exacerbation of residual cognitive, emotional, and behavioral impairments (26). When considered against the existing literature, our results suggest that the Substance Abuse and Mental Health Services Administration approach to addiction-related disorders in TBI should be revised. Furthermore, screening for addiction-related disorders should be considered as part of routine care for mild TBI and might best capture the first 30 days post-mild TBI, with repeat alcohol screening thereafter for at least 6 months following the injury.
1 : An achievable vision: report of the Department of Defense Task Force on Mental Health, June 2007. Washington, DC, Department of Defense. http://www.health.mil/dhb/mhtf/mhtf-report-final.pdfGoogle Scholar
2 : Traumatic brain injury screening: preliminary findings in a US Army brigade combat team. J Head Trauma Rehabil 2009; 24:14–23Crossref, Medline, Google Scholar
3 : Invisible wounds of war: summary and recommendations for addressing psychological and cognitive injuries. Santa Monica, Calif, Rand Corp, 2008. http://rand.org/pubs/monographs/2008/RAND_MG720.1.pdfGoogle Scholar
4 : Neuroscience: shell shock revisited: solving the puzzle of blast trauma. Science 2008; 319:406–408Crossref, Medline, Google Scholar
5 : Casualties of war: military care for the wounded from Iraq and Afghanistan. N Engl J Med 2004; 351:2471–2475Crossref, Medline, Google Scholar
6 : Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry 2002; 59:17–22Crossref, Medline, Google Scholar
7 : Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991 Am J Public Health 1996; 86:1281–1288Crossref, Medline, Google Scholar
8 : Mental health encounters and diagnoses following deployment to Iraq and/or Afghanistan, US Armed Forces, 2001–2006. Med Surveill Monthly Report 2007; 14:2–8. http://www.afhsc.mil/viewMSMR?file=2007/v14_n04.pdf#Article1Google Scholar
9 : A prospective study of depression following combat deployment in support of the wars in Iraq and Afghanistan. Am J Public Health 2010; 100:90–99Crossref, Medline, Google Scholar
10 : Depression amongst outpatients with traumatic brain injury. Brain Inj 2001; 15:811–818Crossref, Medline, Google Scholar
11 : Psychosocial functioning during the year following severe traumatic brain injury. Brain Inj 2001; 15:683–696Crossref, Medline, Google Scholar
12 : Posttraumatic stress disorder symptoms during the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2006; 18:501–508Crossref, Medline, Google Scholar
13 : Re: Psychiatric diagnoses in historic and contemporary military cohorts: combat deployment and the healthy warrior effect. Am J Epidemiol 2008; 168:1095–1096, author reply 1096–1098Crossref, Medline, Google Scholar
14 : Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med 2008; 358:453–463Crossref, Medline, Google Scholar
15 : Outcomes in the first 5 years after traumatic brain injury. Arch Phys Med Rehabil 1998; 79:298–305Crossref, Medline, Google Scholar
16 : Alcohol and drug use following traumatic brain injury: a prospective study. Brain Inj 2007; 21:1385–1392Crossref, Medline, Google Scholar
17 : A prospective longitudinal multicenter analysis of alcohol use patterns among persons with traumatic brain injury. J Head Trauma Rehabil 1996; 11:58–69Crossref, Google Scholar
18 : Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry 2004; 61:53–61Crossref, Medline, Google Scholar
19 : Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil 1998; 13:24–39Crossref, Medline, Google Scholar
20 : Patterns of alcohol use 1 year after traumatic brain injury: a population-based, epidemiological study. J Int Neuropsychol Soc 2005; 11:322–330Crossref, Medline, Google Scholar
21 : Post-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj 2002; 16:583–592Crossref, Medline, Google Scholar
22 : Alcohol use before and after traumatic head injury. Ann Emerg Med 1995; 26:167–176Crossref, Medline, Google Scholar
23 : Alcohol use among persons with traumatic brain injury. J Head Trauma Rehabil 1990; 5:9–20Crossref, Google Scholar
24 : Alcohol and drug use among young persons with traumatic brain injury. J Learn Disabil 1996; 29:643–651Crossref, Medline, Google Scholar
25 : The natural history of drinking and alcohol-related problems after traumatic brain injury. Arch Phys Med Rehabil 2003; 84:185–191Crossref, Medline, Google Scholar
26 : The nature and extent of substance abuse problems among persons with traumatic brain injuries. J Head Trauma Rehabil 1995; 10:29–45Crossref, Google Scholar
27 : Treating Clients With Traumatic Brain Injury, vol 9. Rockville, Md, Substance Abuse and Mental Health Services Administration, 2010, pp 2Google Scholar
28 : Psychiatric comorbidity following traumatic brain injury. Brain Inj 2007; 21:1321–1333Crossref, Medline, Google Scholar
29 Compton WM: Substance abuse among military personnel, veterans, and their families, in Proceedings at the Congressional Briefing, Washington, DC, 2011, May 3. http://www.psych.org/MainMenu/AdvocacyGovernmentRelations/GovernmentRelations/Advocacy-Highlights.aspxGoogle Scholar
30 : Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, Centers for Disease Control and Prevention, 2003Google Scholar
31 : International Classification of Diseases, 9th Revision, Clinical Modification, 6th ed, vol 1–3. Washington, DC, US Department of Health and Human Services, 2006Google Scholar
32 : Accuracy of Veterans Affairs databases for diagnoses of chronic diseases. Prev Chronic Dis 2009; 6:1–11. http://www.cdc.gov/pcd/issues/2009/oct/pdf/08_0263.pdfGoogle Scholar
33 : Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
34 : Behavioral and psychiatric comorbidities of TBI, in Traumatic Brain Injury: A Clinician’s Guide to Diagnosis, Management, and Rehabilitation. Edited by Tsao JW. New York, Springer, 2012, pp 223–224Crossref, Google Scholar
35 : Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem 2005; 95:457–465Crossref, Medline, Google Scholar
36 : Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med 2000; 27:1410–1414Crossref, Medline, Google Scholar
37 : Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13–22Crossref, Medline, Google Scholar



