Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies
Abstract
Objective
The authors performed a comprehensive meta-analysis of task-based functional MRI studies of attention deficit hyperactivity disorder (ADHD).
Method
The authors searched PubMed, Ovid, EMBASE, Web of Science, ERIC, CINAHAL, and NeuroSynth for studies published through June 30, 2011. Significant differences in brain region activation between individuals with ADHD and comparison subjects were detected using activation likelihood estimation meta-analysis. Dysfunctional regions in ADHD were related to seven reference neuronal systems. The authors performed a set of meta-analyses focused on age groups (children and adults), clinical characteristics (history of stimulant treatment and presence of psychiatric comorbidities), and specific neuropsychological tasks (inhibition, working memory, and vigilance/attention).
Results
Fifty-five studies were included (39 for children and 16 for adults). In children, hypoactivation in ADHD relative to comparison subjects was observed mostly in systems involved in executive function (frontoparietal network) and attention (ventral attentional network). Significant hyperactivation in ADHD relative to comparison subjects was observed predominantly in the default, ventral attention, and somatomotor networks. In adults, ADHD-related hypoactivation was predominant in the frontoparietal system, while ADHD-related hyperactivation was present in the visual, dorsal attention, and default networks. Significant ADHD-related dysfunction largely reflected task features and was detected even in the absence of comorbid mental disorders or a history of stimulant treatment.
Conclusions
A growing literature provides evidence of ADHD-related dysfunction in multiple neuronal systems involved in higher-level cognitive functions but also in sensorimotor processes, including the visual system, and in the default network. This meta-analytic evidence extends early models of ADHD pathophysiology that were focused on prefrontal-striatal circuits.
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood-onset psychiatric conditions, with an estimated worldwide-pooled prevalence exceeding 5% in children (1). Impairing ADHD symptoms persist into adulthood in as many as 65% of cases (2). Despite a voluminous literature (3), ADHD pathophysiology remains incompletely understood. To gain insight into the neural correlates of ADHD, Dickstein et al. (4) conducted a quantitative meta-analysis of 16 functional MRI (fMRI) studies published before February 2006. They found evidence suggesting significant neuronal hypoactivation in individuals with ADHD relative to comparison subjects, mostly in the fronto-striatal and parietal regions. A substantial number of studies included in Dickstein et al. (4) assessed response inhibition as a potential contributor to the particular dysfunctional regions identified in ADHD, reflecting the influence of a neuropsychological theory positing inhibitory dysfunction as the core deficit in ADHD (5).
The ADHD fMRI literature has grown substantially since, and neuropsychological paradigms beyond response inhibition have been more frequently investigated (6). In addition, the field has shifted to reporting between-group contrasts (i.e., between individuals with ADHD and comparison subjects) instead of relying on qualitative comparisons of within-group results, as was common in the early literature. Finally, from a theoretical perspective, ADHD is increasingly thought to reflect altered connectivity within and among several neural networks rather than abnormalities of discrete, isolated brain regions (7, 8).
Accordingly, we present an updated meta-analytic review of the ADHD fMRI literature. We included pertinent task-based fMRI studies reporting between-group contrasts regardless of the type of task examined. We conducted a set of meta-analyses focusing on clinically relevant issues that can now be addressed with greater precision than was possible at the time of the Dickstein et al. study (4). In particular, the larger number of available studies allowed us to explore possible ADHD-related dysfunctions in relation to specific age groups (children and adolescents or adults), clinical characteristics (history of stimulant treatment and presence of comorbid psychiatric disorders), or neuropsychological paradigms (inhibitory control, working memory, and vigilance/attention).
Based on the perspective that ADHD is a disorder reflecting dysfunction of large-scale neuronal systems (7), we interpreted abnormally activated brain regions from our meta-analysis as dysfunctional nodes of large-scale networks described in the current neuroscience literature. We used a set of functional networks recently derived from a large data set of resting-state functional imaging as a reference (9). As proposed in a recent qualitative review (7), we hypothesized ADHD-related dysfunctions in networks that are involved not only in higher-level cognitive-behavioral functions, such as the frontoparietal, dorsal attention, and default networks, but also in sensorimotor processes, including somatomotor and visual networks. Consistent with qualitative reviews of fMRI studies in ADHD (10, 11), we expected ADHD-related dysfunctions 1) to differ in adults compared with children, 2) to be present regardless of comorbid psychiatric disorders or history of stimulant treatment, and 3) to differ according to the specific neuropsychological task examined.
Method
Search Strategy
We searched the following databases: PubMed, Ovid (including PsycINFO and Ovid MEDLINE), EMBASE, Web of Science (Science Citation Index Expanded, Social Sciences Citation Index, and Arts and Humanities Citation Index), ERIC, CINAHL, and NeuroSynth (www.neurosynth.org). Details of the search strategy are reported in section A1 of the data supplement that accompanies the online edition of this article.
Study Eligibility Criteria
Studies were included if they 1) used a diagnosis of ADHD according to DSM-IV, DSM-IV-TR, or ICD-10, 2) used a typically developing comparison group, 3) reported data as three-dimensional coordinates in stereotactic space, and 4) used between-group contrasts.
Studies were excluded if they 1) used a neuroimaging method other than fMRI; 2) included participants with ADHD symptoms but without a formal diagnosis of ADHD; 3) assessed the effect of medication without reporting fMRI data at baseline or after washout; 4) reported only within-group contrasts; 5) conducted a priori region-of-interest analyses (as these violate the assumption, under the null hypothesis, that the likelihood of locating activated foci is equal at every voxel); 6) reported only deactivations (this occurred in only one study [12], which was thus not comparable to the others); or 7) included adults with ADHD in partial remission, as it has not been established whether the neuronal correlates of individuals with ADHD in partial remission are similar to those with the full syndrome.
Data Extraction
Two authors (S.C. and C.C.) independently searched the literature, examined the retrieved articles, and extracted and cross-checked data. Initial disagreements on seven of 2,287 screened articles were resolved by consensus. We extracted demographic information, ADHD diagnostic criteria and subtype, psychiatric comorbidities, medication status, three-dimensional coordinates, tasks, and contrasts.
Meta-Analytic Technique
We conducted an activation likelihood estimation meta-analysis using GingerALE, version 2.1.1 (www.brainmap.org/ale/). Activation likelihood estimation allows the detection of quantitative interstudy consistencies in activation by generating maps of activation likelihood estimates. In fMRI studies, the precise localization of specific activation coordinates is limited by substantial intersubject anatomical variability. Within studies, this is imperfectly addressed by Gaussian smoothing. Accordingly, activation foci are best considered as localization probability distributions centered at the reported coordinates. Based on this logic, in activation likelihood estimation meta-analysis, foci are first transformed into probability distributions using three-dimensional Gaussian functions with width expressed in millimeters at half the maximum value (referred to as full width at half maximum). Second, a whole-brain map is created by assigning each voxel a value equal to the probability that at least one of the activation points will be found within the voxel. This value is referred to as the activation likelihood estimation for each voxel. Third, to differentiate the voxels in the map that represent signal (i.e., nonrandom clustering of foci) from those that represent noise (i.e., random clustering), activation likelihood estimation values are compared with a null hypothesis distribution generated by permutation analysis (see www.brainmap.org/ale/).
For our meta-analysis, coordinates reported in Talairach space were transformed to Montreal Neurological Institute (MNI) coordinates using the icbm2tal (Lancaster) transformation (13). Moreover, since nearby coordinates cannot be assigned unequivocally to different regions, when coordinates associated with multiple contrasts from the same task were less than 12 mm apart, we excluded all but one (e.g., for a set of four coordinates within 12 mm of each other, all but the fourth peak were excluded). Statistical significance was determined using a permutation test (5,000 permutations) of randomly generated foci, corrected for multiple comparisons. Per Eickhoff et al. (14), full width at half maximum was calculated based on the number of participants in each study. The threshold for final activation likelihood estimation maps was set at p<0.05 using the false discovery rate with an extent threshold greater than 200 mm3 (the GingerALE default) and overlaid onto the MNI 152 template. As recommended (15), anatomical labels were assigned after direct examination of anatomy (16).
We performed focused meta-analyses of studies contrasting 1) children (age<18 years) with ADHD and comparison children across all tasks; 2) adults (age≥18 years) with ADHD and comparison adults across all tasks; 3) all stimulant-naive participants with ADHD (regardless of age) and comparison subjects (studies were included in this subanalysis only if all participants were stimulant naive); 4) all comorbidity-free individuals with ADHD (regardless of age) and comparison subjects; all individuals with ADHD (children and adults) and comparison subjects in 5) inhibition tasks, 6) working memory tasks, and 7) vigilance and attention tasks. As shown in Table 1, the number of retrieved studies with relevant foci was insufficient to perform separate meta-analyses of studies assessing 1) paradigms other than inhibition, working memory, or vigilance/attention; 2) individual tasks in children and adults separately; 3) individual paradigms in participants who were stimulant naive or without psychiatric comorbidities; and 4) ADHD > comparison subjects for working memory or vigilance and attention tasks. Additionally, for comparability with the previous meta-analysis (4), we performed a meta-analysis across all pertinent studies reporting results of between-group contrasts, regardless of participant characteristics and the specific paradigm tested (an “omnibus” meta-analysis).
| ADHDc | Comparison Subjectsd | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Age | |||||||||||||||
| First Authorb | N | M | Mean | SD | N | M | Mean | SD | ADHD Type | Psychiatric Comorbidities | Treatment With Stimulants | Withdrawal From Stimulants | Task(s) | Contrast(s) | Number of Foci | Correction for Multiple Comparisonse |
| Banich | 23 | 14 | 20 | 1.7 | 23 | 13 | 19 | 0.9 | Co | No | 20 lifetime, 14 current | 24 hours | Color word Stroop (variant) | Six contrasts included in the meta-analysisf | 20 (total) | Cluster-wise protection; voxel level threshold p=0.01 (AFNI AlphaSim) |
| Bayerl | 30 | 16 | 31 | 0.6 | 30 | 16 | 31 | 8.4 | Co | No | All naive | N/A | 2-back working memory | C>A | 1 | Cluster-size thresholding (50 voxels); voxel level p=0.001 uncorrected (SPM99) |
| Booth | 12 | 8 | 9.4 | 1.2 | 12 | 7 | 9.3 | 12 | 8 Co, 4 I | No | All current | 48 hours | Go/no-go selective attention | C>A; C>A | 17; 3 | Cluster-size thresholding (10 voxels); voxel level p<0.001 uncorrected (SPM99) |
| Braet | 20 | 17 | 14 | 2.1 | 38 | 31 | 13 | 1.9 | NS | NS | Some (NS) current | 24 hours | Sustained Attention to Response Test (go/no-go) | C>A: successful inhibition | 5 | NS for second-level analysis (AFNI) |
| Cao | 12 | 12 | 13 | 1.7 | 13 | 13 | 13 | 1.2 | 7 Co, 5 I | 5 ODD, 2 CD | 3 current | 2 weeks | Cued target detection | C>A: intrinsic alerting effect; C>A: phasic alerting effect; C>A: alerting effect | 3; 4; 3 | Cluster-size thresholding (10 voxels); voxel level p<0.001 uncorrected (SPM2) |
| Cerullo | 10 | 7 | 14 | 2 | 13 | 7 | 15 | 1.9 | NS | 2 ODD, 2 tics | 8 current | Day of the scan | CPT-X | C>A; A>C | 1; 2 | Cluster-size thresholding (137 voxels); voxel-level p<0.05 uncorrected (AFNI) |
| Cubillo (2010) | 11 | 11 | 29 | 1 | 14 | 14 | 28 | 2 | 6 Co, 2 HI, 3 I | 1 AD, 3 MD, 1 CD, 2 SUD, 1 ND | All naive | N/A | Tracking stop | C>A: successful stop-go trials; C>A: unsuccessful stop-go trials; C>A: switch task | 2; 1; 2 | Cluster-mass threshold; voxel-wide significance: p<0.05; cluster threshold p<0.01 (XBAM) |
| Cubillo (2011) | 11 | 11 | 29 | 1 | 15 | 15 | 28 | 3 | 6 Co, 2 HI, 3 I | 1 AD, 3 MD, 1 CD, 2 SUD, 1 ND | All naive | N/A | Simon task | C>A: incongruent-congruent; C>A: oddball-congruent | 1; 1 | Cluster-mass threshold; voxel-wide significance: p<0.05; cluster threshold p<0.01 (XBAM) |
| Cubillo (2012) | 11 | 11 | 29 | 1 | 15 | 15 | 28 | 3 | 6 Co, 2 HI, 3 I | 1 AD, 3 MD, 1 CD, 2 SUD, 1 ND | All naive | N/A | Rewarded CPT | C>A: effect of attention; C>A: effect of reward | 3; 2 | Cluster-mass threshold; voxel-wide significance: p<0.05; cluster threshold 0.01 (XBAM) |
| Dibbets (2009) | 16 | 16 | 29 | 6.4 | 13 | 13 | 29 | 6.4 | All Co | No | 14 current | 24 hours | Modified go/no-go | A>C: overall activation | 1 | Cluster-size thresholding (3 voxels); voxel level p<0.05 (SPM2) |
| Dibbets (2010) | 15 | 15 | 29 | 6.2 | 14 | 14 | 29 | 6.4 | All Co | No | 14 current | 24 hours | Switch | C>A; A>C | 6; 6 | Cluster-level thresholding (1,000 iterations; p<0.05) (Brainvoyager QX) |
| Dillo | 15 | 11 | 21–42 | N/A | 15 | 11 | 21–46 | N/A | 5 Co, 7 I, 3 HI | No | Some (NS) current | 3 weeks | Go/no-go | A>C | 2 | N/S (SPM2) |
| Durston (2003) | 7 | 6 | 8.5 | 1.6 | 7 | 6 | 8.7 | 1.5 | 4 Co, 3 I | ODD or CD (NS) | All current | 24 hours | Go/no-go | C>A: no-go > go; A>C: no-go > go | 1; 8 | Cluster-size thresholding (3 voxels); voxel-level p<0.05 (NeuroImaging Software) |
| Durston (2007) sample 1 | 10 | 8 | 12 | 2.6 | 10 | 8 | 12 | 2.1 | 5 Co, 2 I, 3 HI | 4 ODD | 5 current | 24 hours | Variant go/no-go | C>A: unexpected stimulus-unexpected time | 1 | Cluster-size thresholding (5 voxels); voxel-level p<0.05 (SPM2) |
| Durston (2007) sample 2 | 12 | 12 | 15 | 2.3 | 12 | 12 | 15 | 2.1 | 9 Co, 3 HI | 4 ODD | 9 current | 24 hours | Variant go/no-go | C>A: expected stimulus-unexpected time; C>A: unexpected stimulus-expected time | 1; 2 | Cluster-size thresholding (5 voxels); voxel-level p<0.05 (SPM2) |
| Hale | 10 | 9 | 35 | 8.1 | 10 | 9 | 27 | 4.1 | 3 Co, 7 I | 1 GAD + SoP + SP, 1 SP | 4 current | 2 weeks | WAIS forward/ backward digit span | C>A: backward; A>C: forward; A>C: backward | 5; 7; 3 | Cluster-size thresholding (25 voxels); voxel level p<0.001 uncorrected (SPM2) |
| Karch | 8 | 7 | 38 | 7.8 | 8 | 7 | 38 | 6.6 | All Co | No | All naive | N/A | Auditory go/no-go (modified) | C>A | 7 | Cluster-size thresholding; p<0.001 uncorrected; threshold: 10 voxels (BrainVoyager) |
| Kobel (2009) | 14 | 14 | 10 | 1.3 | 12 | 12 | 11 | 1.6 | 9 Co, 5 I | 5 ODD-CD, 4 GAD | All current | 24 hours | n-back | C>A: averaged 2- and 3-back | 5 | Family-wise error correction (p<0.05); cluster size: 10 voxels (SPM5) |
| Kobel (2010) | 14 | 14 | 10 | 1.3 | 12 | 12 | 11 | 1.6 | 9 Co, 5 I | 5 ODD-CD, 4 GAD | All current | No | n-back | C>A: activation in 3-back task | 1 | Correction applied but type of correction NS (SPM 5) |
| Konrad | 16 | 16 | 10 | 1.9 | 16 | 16 | 10 | 1.3 | 9 Co, 6 I, 1 HI | 5 ODD, 3 AD | All naive | N/A | Attention Network Test (modified) | C>A: alerting; C>A: executive control; A>C: alerting; A>C: reorienting; A>C: executive control | 1; 2; 1; 3; 1 | Cluster-size thresholding (10 voxels); p<0.001 uncorrected (SPM2) |
| Kooistra | 10 | 10 | 22 | NS | 10 | 10 | 22 | NS | NS | N/A | No after age 16 years | N/A | Go/no-go | C>A: go; A>C: go | 16; 2 | Corrected cluster significance threshold: p=0.01 (FSL) |
| Krauel | 12 | 12 | 15 | 0.7 | 12 | 12 | 15 | 1.3 | 6 Co, 5 I, 1 HI | 3 ODD, 2 CD | 5 current | 24 hours | Recognition memory | C>A: neutral; A>C: emotional; A>C: neutral | 1; 4; 4 | Cluster-size thresholding (10 voxels); voxel-level p<0.001 uncorrected (SPM99) |
| Mostofsky | 11 | 8 | 10 | 1.2 | 11 | 8 | 10 | 1.4 | 9 Co, 2 I | No | 8 current | 24 hours | Sequential finger tapping | C>A | 2 | Cluster-size thresholding (84 voxels); voxel-level p<0.001 uncorrected (SPM99) |
| Passarotti (2010, J Intl Neuropsychol Soc) | 15 | 12 | 13 | 2.6 | 14 | 7 | 14 | 2.4 | All C | No | 5 current | Over a 3-week period | Emotional valence Stroop | C>A: negative > neutral; C>A: positive > neutral words; A>C: negative > neutral words; A>C: positive > neutral words | 2; 2; 4; 5 | Contiguity threshold: p<0.01 uncorrected; experiment-wise type 1: p <0.02 corrected (AFNI AlphaSim) |
| Passarotti (2010, J Am Acad Child Adolesc Psychiatry) | 14 | 9 | 13 | 2.3 | 19 | 9 | 14 | 3.1 | All Co | No | NS | At least 4 days | Affective 2-back working memory | C>A: angry > neutral faces; A>C: angry > neutral faces; A>C: happy > neutral faces | 13; 1; 12 | Contiguity threshold: p<0.01 uncorrected; experiment-wise type 1: p<0.02 corrected (AFNI AlphaSim) |
| Passarotti (2010, Psychiatry Res) | 11 | 6 | 13 | 2.7 | 15 | 7 | 14 | 3.1 | All Co | No | 6 current | 3 weeks | Response inhibition | C>A; A>C | 7; 3 | Contiguity threshold: p<0.01 uncorrected; experiment-wise type 1: p<0.02 corrected (AFNI AlphaSim) |
| Prehen-Kristensen | 12 | NS | 13 | 1.8 | 12 | NS | 14 | 2 | 12 Co, 2 I | 5 ODD | All current | 48 hours | Delayed-match-to-sample paradigm | C>A | 15 | Cluster-size thresholding (5 voxels); voxel-level p<0.05 (SPM5) |
| Rubia (1999) | 7 | 7 | 16 | NS | 9 | 9 | 15 | NS | All Co | CD (NS) | NS | 1 week | Stop; delay | C>A; C>A | 4; 2 | Permutation; voxel-wise probability type I error: 0.05 |
| Rubia (2005) | 16 | 16 | 13 | 2.1 | 21 | 21 | 14 | 1.6 | All Co | 5 CD | All naive | N/A | Stop | C>A: successful > unsuccessful inhibition; C>A: unsuccessful inhibition > baseline go | 2; 2 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) |
| Rubia (2007) | 17 | 17 | 14 | 2 | 18 | 18 | 13 | 2 | All Co | No | All naive | N/A | Oddball | C>A: successful oddball > standard; C>A: standard > oddball | 3; 2 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) |
| Rubia (2008) | 20 | 20 | 13 | 1.5 | 20 | 20 | 14 | 2 | All Co | No | All naive | N/A | Tracking stop | C>A: successful > failed stop; C>A: failed stop > go; C>A: go > stop | 1; 1; 1 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.03 (cluster comparison) |
| Rubia (2009, Am J Psychiatry) | 18 | 18 | 13 | 1 | 16 | 16 | 13 | 3 | All Co | No | All naive | N/A | Rewarded CPT | C>A: effect of attention; C>A: effect of reward; A>C: effect of attention | 2; 1; 9 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) |
| Rubia (2009, J Child Psychol Psychiatry) | 20 | 20 | 13 | 1.4 | 20 | 20 | 14 | 1.9 | All Co | No | All naive | N/A | Simon | C>A: incongruent > oddball; C>A: oddball > congruent | 2; 2 | Cluster level difference; <1 false activated cluster at p<0.05 (both voxel and cluster comparison) |
| Rubia (2009, Neuropsychopharmacology) | 13 | 13 | 13 | 1.4 | 13 | 13 | 13 | 1.8 | All Co | 1 CD | All naive | N/A | Rewarded CPT | C>A: attention; C>A: reward; A>C: reward | 13; 2; 4 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.02 (cluster comparison) (XBAM) |
| Rubia (2009, Philos Trans R Soc Lond B Biol Sci) | ||||||||||||||||
| Study 1 | 10 | 10 | 14 | 2 | 10 | 10 | 15 | 4 | All Co | 1 CD | 4 naive, 6 current | 36 hours (N=6) | Delay discounting | C>A | 6 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.006 (cluster comparison) |
| Study 2 | 12 | 12 | 13 | 1 | 12 | 12 | 13 | 1 | All Co | 1 CD | All naive | N/A | Time discrimination | C>A; A>C | 1; 2 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.006 (cluster comparison) |
| Rubia (2010, Hum Brain Mapp, p 1823) | 14 | 14 | 13 | 1.1 | 20 | 20 | 14 | 1.9 | All Co | No | All naive | N/A | Switch | C>A | 2 | Cluster level difference; <1 false activated cluster at p<0.05 for both voxel and cluster comparison (XBAM) |
| Rubia (2010, Hum Brain Mapp, p 287) | 18 | 18 | 14 | 1.1 | 20 | 20 | 15 | 1.1 | All Co | No | All naive | N/A | Visual tracking stop; Meiran switch | C>A: successful-stop; C>A: failed stop; C>A | 1; 2; 3 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.003 (cluster comparison) (XBAM) |
| Rubia (2011, Biol Psychiatry) | 12 | 12 | 13 | 1 | 13 | 13 | 13 | 1 | All Co | No | All naive | N/A | Visual tracking stop | C>A: successful inhibition; C>A: inhibition failure | 13; 13 | Threshold-free cluster enhancement (p<0.05) |
| Rubia (2011, Neuropsychopharmacology) | 12 | 12 | 13 | 1 | 13 | 13 | 13 | 1 | All Co | No | All naive | N/A | Simon | C>A: Simon > oddball condition | 4 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) (XBAM) |
| Rubia (2011, Hum Brain Mapp) | 18 | 18 | 14 | 2 | 20 | 20 | 16 | 1 | All Co | No | All naive | N/A | Simon | C>A: oddball > congruent; C>A: incongruent > oddball | 2; 2 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) (XBAM) |
| Schulz | 5g | 5 | 18 | 1.3 | 5 | 5 | 18 | 1.4 | 1 Co, 3 I, 1 HI | 1 CD | 4 lifetimeh | N/A | Go/no-go | C>A: correct no-go > correct go; A>C: correct no-go > correct go | 2; 3 | Cluster-size thresholding (120 voxels); voxel level p<0.01 uncorrected (SPM99) |
| Sheridan | 10 | 0 | 15 | 2 | 10 | 0 | 15 | 1.3 | 6 Co; 4 I | 2 ODD, 2 SP | 5 lifetimei, 2 current | 24 hours | Delay match-to-sample | C>A: activation at high load period | 2 | Gaussian field theory; p=0.05 corrected at cluster level (fMRIstat program) |
| Silk (2008) | 12 | 12 | 11 | 1.5 | 12 | 12 | 11 | 1.5 | All Co | No | All naive | N/A | Raven’s Standard Progressive Matrices | C>A | 45 | Clusters of voxels (z>2.33) with cluster level p<0.05 corrected (FSL) |
| Silk (2005) | 7 | 7 | 14 | 1.8 | 7 | 7 | 15 | 1.8 | All Co | No | All naive | N/A | Mental rotation | C>A; A>C | 8; 4 | Voxel level p<0.001 uncorrected; cluster level p<0.05 corrected |
| Smith (2006) | ||||||||||||||||
| Study 1 | 17 | 17 | 13 | 2 | 18 | 18 | 14 | 2 | All Co | 5 CD | All naive | N/A | Go/no-go | C>A: successful no-go stimuli | 1 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) |
| Study 2 | 14 | 14 | 13 | 1.8 | 27 | 27 | 14 | 1.8 | All Co | 5 CD | All naive | N/A | Switch | C>A: switch | 9 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) |
| Smith (2008) | 21 | 21 | 13 | 1.6 | 17 | 17 | 14 | 2.1 | All Co | 3 CD/ODD | All naive | N/A | Time discrimination | C>A: time discrimination > temporal order judgment | 2 | Cluster level difference; <1 false activated cluster at p<0.05 (voxel comparison); p<0.01 (cluster comparison) (XBAM) |
| Spinelli (2011, J Am Acad Child Adolesc Psychiatry) | 13 | 9 | 11 | 1.4 | 17 | 8 | 11 | 1.2 | 10 Co, 3 I | 3 ODD, 1 SP | 2 current | 24 hours | Go/no-go | A>C: preerror versus precorrect inhibition | 2 | Spatial extent cluster size threshold to achieve a corrected statistical threshold of p=0.05 (SPM5) |
| Spinelli (2011, J Child Psychol Psychiatry) | 13 | 9 | 11 | 1.4 | 17 | 8 | 11 | 1.2 | 10 Co, 3 I | 3 ODD, 1 SP | 2 current | 24 hours | Go/no-go | A>C: posterror versus postcorrect inhibition trials | 9 | Spatial extent cluster size threshold to achieve a corrected statistical threshold of p=0.05 (SPM5) |
| Strohle | 10 | 10 | 32 | 8.1 | 10 | 10 | 32 | 9.9 | 4 Co, 4 I, 2 HI | No | All naive | N/A | Monetary Incentive Delay | C>A: anticipation of gain > nongain; A>C: outcome of gain > nongain | 1; 7 | p<0.05 false discovery rate-corrected (SPM2) |
| Tamm (2004) | 10 | 10 | 16 | 1.4 | 12 | 12 | 16 | 0.8 | All Co | No | 5 current | 18 hours | Modified go/no-go | C>A: “A–B” contrast; A>C: “A–B” contrast | 1; 1 | Deactivation mask (z=1.67; p<0.05) (SPM99) |
| Tamm (2006) | 14 | 14 | 14–18 | N/A | 12 | 12 | 14–18 | N/A | All Co | No | 5 current | 18 hours | Oddball | C>A: activation during the oddball event | 4 | NS |
| Valera (2005) | 20 | 12 | 34 | 11.8 | 20 | 12 | 33 | 11.0 | NS | No | 10 lifetime | 24 hours | Variant of visual n-back test | C>A: 2-back minus X-task contrast | 2 | p=0.001 (uncorrected) with extent threshold determined by Gaussian random field theory (SPM99) |
| Valera (2010, Biol Psychiatry) | 21 | 15 | 34 | 10.1 | 19 | 12 | 33 | 11.0 | 5 Co, 12 I | No | 9 current | 24 hours | Paced and unpaced finger tapping | C>A: paced finger tapping; C>A: unpaced finger tapping | 24; 17 | p<0.05, family-wise error corrected, with extent threshold of 20 contiguous voxels (SPM5) |
| Valera (2010, Am J Psychiatry) | 44 | 23 | 37 | 11.0 | 49 | 23 | 33 | 10.0 | 13 Co, 17 I, 1 HI | No | 18 current | 24 hours | 2-back | C>A: 2-back visual memory task | 2 | p<0.05, corrected (Gaussian random field theory) (SPM2) |
| Vance | 12 | 12 | 11 | 1.5 | 12 | 12 | 10 | 1.3 | All Co | No | All naive | N/A | Mental rotation | C>A | 4 | Cluster-level threshold p<0.05, corrected (FSL) |
| Wolf | 13 | 13 | 22 | 4.4 | 12 | 12 | 22 | 4.7 | 9 Co, 2 I, 2 HI | No | All lifetime | 6 at least 6 weeks, 6 for 3 days | Cognitive activation | C>A: increasing working memory load | 5 | p<0.05, false discovery rate-corrected (SPM5) |
To test whether results of the focused meta-analyses differed statistically, we performed subtraction analyses using the contrast studies procedure in GingerALE. We compared ADHD adults with ADHD children; stimulant-naive individuals with stimulant-treated individuals; and participants with comorbid mental disorders with comorbidity-free participants in the contrasts comparison subjects > ADHD and ADHD > comparison subjects. For “all participants, inhibition tasks” compared with “all participants, working memory tasks,” “all participants, inhibition tasks” compared with “all participants, vigilance/attention tasks,” and “all participants, working memory tasks” compared with “all participants, vigilance/attention tasks,” we could only examine the contrast comparison subjects > ADHD.
Activation Liklihood Estimation Results in Relation to Neuronal Networks
We related the ADHD hypo- and hyperactivated regions from our meta-analysis to seven reference networks defined by Yeo et al. (9) on the basis of a data-driven analysis of resting state functional imaging data collected from 1,000 participants. Those seven robustly replicable networks, which are limited to cortical regions, include the frontoparietal, the dorsal and ventral attentional, the somatomotor, the visual, the limbic, and the default networks. We first determined the network in which each voxel of the ADHD-related hypo- or hyperactivated regions was located by computing the number of significant voxels from the comparison subjects > ADHD and ADHD > comparison subjects contrasts that overlapped each of the network masks (downloaded from http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011). We then performed a chi-square analysis contrasting the proportions of hypo- and hyperactivated voxels in the seven networks.
Results
Studies Included in the Meta-Analysis
Figure 1 summarizes the search results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (17). Details of included and excluded studies are provided in section A2 of the online data supplement. The search yielded 55 eligible studies, 16 of them assessing adults and 39 assessing children. Additional characteristics of the studies are summarized in Table 1. While we endeavored to count individuals from the same sample only once, the total number of participants reported in Table 1 (741 with ADHD and 801 comparison subjects) is approximate because some research groups reported results with partially overlapping samples. References of the included studies are provided in section A3 of the online data supplement.
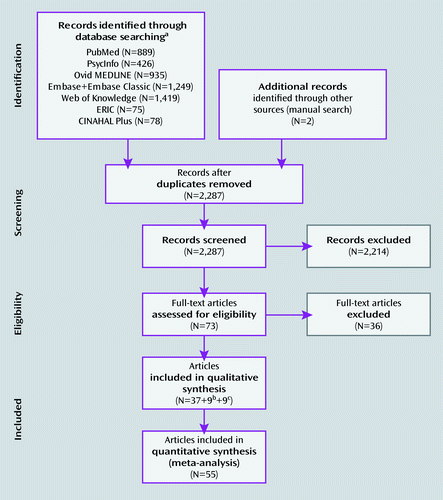
a Up to January 27, 2011.
b From updated search (June 30, 2011).
c From Dickstein et al. (4).
Activation Likelihood Estimation Results
The meta-analysis focused on children (Table 2 and Figure 2) revealed significant ADHD-related hypoactivation in frontal regions and putamen bilaterally and in right parietal and right temporal regions. ADHD-related hyperactivation was observed in the right angular gyrus, middle occipital gyrus, posterior cingulate cortex, and midcingulate cortex. In the meta-analysis restricted to adults (Table 2 and Figure 2), a different pattern emerged. Adults with ADHD showed significant hypoactivation relative to comparison subjects in the right central sulcus, precentral gyrus, and middle frontal gyrus. ADHD-related hyperactivation was observed in a region with a peak in the right angular and middle occipital gyri.
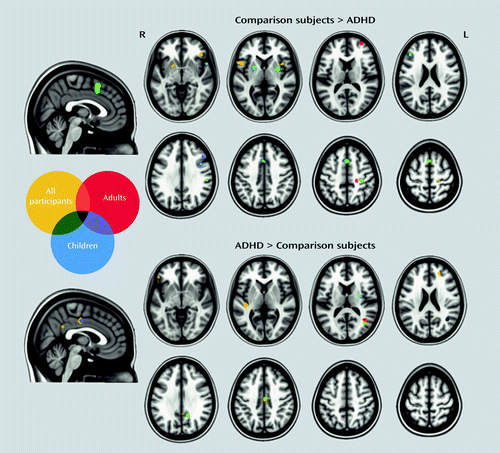
aR=right; L=left. The figure reports results for meta-analyses focused on adults or children and for the omnibus meta-analysis. “All participants” refers to the omnibus meta-analysis.
| Weighted Centera | Maximum Activation Likelihood Estimate Valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Contrast, Analysis, and Cluster Number | Volume (mm3) | x | y | z | Extrema Value | x | y | z | Anatomical Labelb |
| Comparison subjects > participants with ADHD | |||||||||
| Children, all tasksc | |||||||||
| 1 | 1,864 | −0.35 | 15.56 | 48.61 | 0.0211 | 0 | 16 | 54 | Medial superior frontal gyrus/supplementary motor area (BA 6) R, L (frontoparietal/ventral attention) |
| 0.0186 | 0 | 14 | 44 | Paracingulate gyrus (BA 32) R, L (ventral attention) | |||||
| 2 | 688 | 31.42 | 0.19 | 3.33 | 0.0148 | 32 | 0 | 4 | Putamen R |
| 3 | 464 | −21.92 | 2.37 | 4.1 | 0.0119 | −20 | 4 | 4 | Putamen L |
| 4 | 424 | 33.7 | −27.47 | 50.75 | 0.0136 | 34 | −28 | 52 | Postcentral gyrus R (somatomotor) |
| 5 | 400 | 42.23 | 9.52 | 29.36 | 0.0134 | 42 | 10 | 30 | Inferior frontal sulcus; inferior precentral sulcus R (frontoparietal) |
| 6 | 384 | 57.29 | −25.00 | 28.42 | 0.0145 | 58 | −26 | 28 | Inferior parietal lobule (supramarginal gyrus; BA 40) R (ventral attention) |
| 7 | 368 | 55.66 | 12.64 | −6.64 | 0.0136 | 56 | 12 | −6 | Superior temporal gyrus (BA 22) R (somatomotor) |
| 8 | 280 | −41.26 | 31.46 | 23.46 | 0.0131 | −42 | 32 | 24 | Middle frontal gyrus (BA 9/46) L (frontoparietal) |
| 9 | 248 | 47.46 | 25.85 | 31.84 | 0.0115 | 48 | 26 | 32 | Middle frontal gyrus (BA 9/46) R (frontoparietal) |
| Adults, all tasksc | |||||||||
| 1 | 400 | 21.7 | −27.31 | 50.84 | 0.0106 | 22 | −28 | 50 | Central sulcus/precentral gyrus R |
| 2 | 232 | 34.38 | 56.96 | 13.62 | 0.0103 | 34 | 56 | 14 | Middle frontal gyrus (BA 10) R (frontoparietal) |
| Participants with ADHD > comparison subjects | |||||||||
| Children, all tasksd | |||||||||
| 1 | 680 | 39.72 | −58.51 | 18.51 | 0.0135 | 40 | −58 | 20 | Angular gyrus; middle occipital gyrus R |
| 0.0079 | 40 | −66 | 14 | Angular gyrus; middle occipital gyrus R (visual) | |||||
| 2 | 400 | 15.02 | −50.94 | 31.01 | 0.0101 | 16 | −52 | 32 | Posterior cingulate cortex; subparietal sulcus R |
| 3 | 392 | 31.22 | −7.23 | 16.7 | 0.0107 | 32 | −8 | 16 | White matter R (suboperculum) |
| 4 | 352 | 4.67 | −13.72 | 42.61 | 0.0101 | 4 | −14 | 42 | Midcingulate cortex R (ventral attention) |
| Adults, all tasksd | |||||||||
| 1 | 872 | 40.39 | −58.08 | 16.91 | 0.0152 | 40 | −58 | 18 | Angular gyrus; middle occipital gyrus R |
In stimulant-naive participants (see Table S1 and Figure S1 in the online data supplement), ADHD-related hypoactivation was observed in several frontal regions bilaterally, the right superior temporal gyrus, right posterior cingulate cortex, right postcentral gyrus, the putamen bilaterally, and the right thalamus. Only one significant cluster of ADHD-related hyperactivation was observed, with a peak located in the right superior longitudinal fasciculus underlying the insula.
When considering comorbidity-free participants (see Table S1 and Figure S1 in the online data supplement), ADHD-related hypoactivated regions were located in the frontal regions and the putamen bilaterally, the right superior temporal gyrus, and the right occipital pole. ADHD-related hyperactivation was observed in the left inferior frontal gyrus, left Heschl’s gyrus, and several right posterior regions.
In analyses limited to specific tasks (see Table S1 in the online data supplement), ADHD-related hypoactivation in studies examining inhibition paradigms included several frontal regions bilaterally as well as the right superior temporal gyrus, the left inferior occipital gyrus, the right thalamus, and the midbrain. The contrast ADHD > comparison subjects yielded two regions with peaks in the deep right parieto-occipital cortex and right intermediate frontal sulcus. The analysis restricted to working memory tasks revealed significant ADHD-related hypoactivation in the left inferior frontal gyrus and anterior insula and in the right middle frontal gyrus. In vigilance or attention tasks, we observed significant ADHD-related hypoactivation only in the right paracingulate gyrus.
The omnibus meta-analysis (Figure 2; see also Table S2 in the online data supplement) largely recapitulated the results of the meta-analysis focused on children, with additional ADHD-related hypoactivations in the inferior frontal gyri, right central sulcus, and right posterior parietal lobe and hyperactivations in Heschl’s gyrus as well as additional loci in inferior frontal gyri.
When we performed planned subtraction analyses (adults compared with children; stimulant-naive compared with stimulant-treated individuals; comorbid compared with comorbidity-free participants; and comparisons among specific tasks), no significant differences that survived whole-brain false discovery rate correction were found. However, with an exploratory threshold set at p<0.05, uncorrected, we observed differences in the following analyses: adults with ADHD compared with children with ADHD for the comparison subjects > ADHD, all tasks contrast; stimulant-naive individuals compared with stimulant-treated individuals for the comparison subjects > ADHD, all tasks contrast, and the ADHD > comparison subjects, all tasks contrast; comorbid participants compared with participants without comorbidity for the comparison subjects > ADHD, all tasks contrast and the ADHD > comparison subjects, all tasks contrast (see Table S3 in the online data supplement).
Results in Relation to Neuronal Networks
Figure 3 shows the number of voxels located in each of the seven networks described by Yeo et al. (9), expressed as a percentage of the total number of significant voxels.
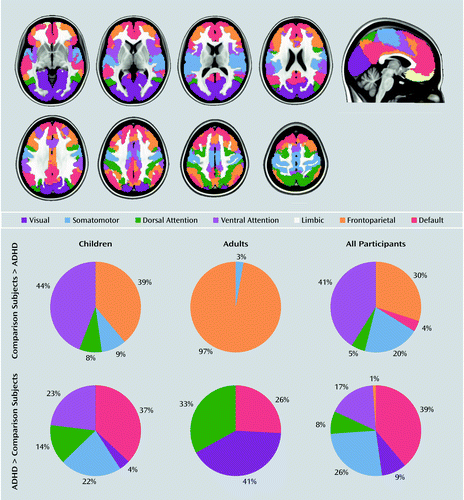
a Number of significant voxels in the contrast comparison subjects > ADHD: children=3,320; adults=272; omnibus=6,024. Number of significant voxels in the contrast ADHD > comparison subjects: children=888; adults=464; omnibus=2,720. The regions presented in the upper panel are in relation to the Yeo et al. (9) seven networks.
Given the lack of data for the contrast ADHD > comparison subjects for most of the specific tasks, we do not report assignment to the canonical networks for the meta-analyses restricted to specific tasks.
Discussion
The ADHD fMRI literature has grown substantially since an initial meta-analysis examined 16 studies (4). Using stringent selection criteria, we included 55 articles in meta-analyses that focused on clinical characteristics (previous history of stimulant treatment and presence of comorbid psychiatric disorders) or specific neuropsychological constructs in children or adults with ADHD.
Abnormally activated regions in individuals with ADHD relative to comparison subjects can be interpreted within a systems neuroscience perspective (i.e., as dysfunctional nodes within large-scale neuronal networks) (7). While the number of definable neural networks can vary substantially, we selected the seven networks identified by Yeo et al. (9) as being heuristically appropriate for the study of ADHD. These include the frontoparietal, dorsal and ventral attention, sensorimotor, visual, limbic, and default networks.
Meta-Analyses
Children.
Brain regions hypoactivated in children with ADHD relative to comparison subjects were prevalent in the frontoparietal and ventral attention networks (Figure 3). The canonical frontoparietal network (9) includes the lateral frontal pole, dorsal anterior cingulate, dorsolateral anterior prefrontal cortex, lateral cerebellum, anterior insula, and inferior parietal lobe. This network supports goal-directed executive processes and guides decision making by integrating information from the external world with internally elaborated representations (18). Deficiencies in performing goal-directed executive processes have been considered pivotal in early theoretical models of ADHD (5).
The ventral attention network and its interplay with the dorsal attention network have come under closer examination in cognitive neuroscience (18), although their potential dysfunction in ADHD has been infrequently considered. The ventral attention network includes the temporoparietal junction, the supramarginal gyrus, frontal operculum, and anterior insula; the dorsal system is anchored in the intraparietal sulcus and the frontal eye fields (18). While the dorsal attention network underpins the selection of sensory stimuli based on internal goals or personal expectations, the ventral network supports attentional reorienting to salient and behaviorally relevant external stimuli (18). A recent preliminary study reported deficient ventral attention network engagement in adults with ADHD, suggesting that this may underlie an ADHD-related deficit in adaptive switching to external salient stimuli (19). This, along with our finding of ventral attention network hypoactivation, is in line with the theoretical framework proposed by Nigg and Casey (6), which emphasizes that learning when and in what contexts to expect an event is critical for planning and maintaining appropriate behaviors. In their model, difficulties in detecting regularities or irregularities in the environment lead to problems in modulating behaviors according to changes in the environment, which manifest as ADHD symptoms. We speculate that hypoactivation in the ventral attention network underpins ADHD-related deficits in detecting regularities and irregularities in the environment. We also observed hyperactivation of regions in the ventral attention network. Since the suppression of this network is necessary to prevent shifts of attention to irrelevant objects (20), its hyperactivation might underpin distractibility, one of the cardinal symptoms of ADHD.
We note that the dorsal attention system was relatively underrepresented among ADHD-related hypoactivated regions, although hypofunction of this system has been proposed in ADHD (7). In part, our results may reflect the substantial prevalence of inhibition-related tasks that are subserved predominantly by the ventral, rather than the dorsal, attention network (18).
We also identified peaks of ADHD-related hypoactivation in the right somatomotor system and in the putamen bilaterally. Together with the cluster of hypoactivation in the medial superior frontal gyrus and supplementary motor area, these regions are consistent with abnormal function of the pyramidal motor system, which would be expected in ADHD given the salience of motoric hyperactivity. Remarkably, interindividual differences in locomotor activity have rarely been examined in ADHD using neuroimaging measures. Using transcranial magnetic stimulation, robustly abnormal intracortical inhibition has been reported in the motor system in children with ADHD (21).
Besides hypoactivation, we also observed several regions of ADHD-related hyperactivation, predominantly in the default network. This network underlies self-referential cognitive processes that are typically suppressed during the performance of externally oriented attentionally demanding tasks (22). Spontaneous activity fluctuations in the default network tend to be anticorrelated with fluctuations in “task positive” networks (i.e., networks that are activated during active tasks), such as the frontoparietal and dorsal attention networks (22). According to the default-mode hypothesis of ADHD (22), the default network might be inadequately regulated by other task-active systems and might consequently intrude on or disrupt ongoing cognitive performance, contributing to fluctuations in attention that characterize ADHD. The studies whose coordinates contributed to the hyperactivated default network clusters in our meta-analysis did not systematically report whether this hyperactivation reflected weaker task-related deactivation in ADHD relative to comparison subjects, or stronger activation in ADHD relative to comparison subjects. However, amelioration of inadequate default network deactivation in ADHD by methylphenidate was recently reported by two separate groups (12, 23).
We also observed ADHD-related hyperactivation in the somatomotor and visual systems. This is in line with the hypothesis that individuals with ADHD compensate for impaired function in the prefrontal and anterior cingulate cortex by overreliance (relative to comparison subjects) on brain regions associated with visual, spatial, or motoric processing (24).
Adults.
The meta-analysis restricted to adults yielded fewer regional group differences compared with that restricted to children. This may be accounted for by the smaller number of retained studies (N=16) and consequently lower statistical power. Almost all the hypoactivated voxels were found in the frontoparietal system, which is consistent with the persistence of executive dysfunction in adults with ADHD (25). Hypoactivation in the somatomotor system was less prominent in adults than in children, in line with clinical observations that motoric hyperactivity decreases with age (26). In the visual and dorsal attention systems, adults with ADHD exhibited proportionally more hyperactivation than children, suggesting the hypothesis that these networks may carry the bulk of the compensatory load in adults (24).
Stimulant-naive individuals.
Although early imaging studies of ADHD were confounded by previous stimulant treatment history (27), recent meta-analytic evidence has confirmed that brain structural changes are present in stimulant-naive participants with ADHD (28) and suggested that treatment with stimulants may even normalize structural abnormalities (29). Here, we extend those observations by finding significant differences between stimulant-naive individuals with ADHD and comparison subjects, which indicates that ADHD neuronal dysfunctions are also not likely accounted for by previous stimulant treatment.
The thalamus, despite its central location in multiple brain circuits, has been generally overlooked in the ADHD neuroimaging literature (30). We observed thalamic hypoactivation in stimulant-naive individuals with ADHD and also in the meta-analysis of inhibition tasks. Given the role of the thalamus in alertness/arousal via thalamo-cortical projections (31), thalamic abnormalities in ADHD may be related to arousal dysmodulation, which has long been considered a core component of ADHD pathophysiology (6).
Comorbidity-free individuals.
The pattern of ADHD-related hypo- or hyperactivation in comorbidity-free individuals generally overlapped with that observed in the meta-analyses restricted to children or inhibition tasks. One exception was the inclusion of the default network among hypoactivated regions, although, as in the other meta-analyses, it was proportionally more represented among the hyperactivated regions.
Specific tasks.
The regions identified in analyses limited to inhibition tasks generally overlapped with those seen in the meta-analysis focused on children, which is not surprising since inhibition tasks were the most represented paradigm among those analyzed in children. In the analysis focused on studies of vigilance and attention, only a cluster in the ventral attention system emerged as significant. The limited number of retained studies on vigilance and attention may have limited the chance to detect other significant regions. Similarly, a region in the frontoparietal network, which is involved in executive functions such as working memory, was significantly hypoactivated in ADHD in the working memory analysis.
Omnibus meta-analysis.
Besides analyses limited to children or adults, we performed an omnibus meta-analysis in which all ages and tasks were combined, as done previously by Dickstein et al. (4). The omnibus results largely overlapped those of the meta-analysis focused on children, which was expected because 70% of the included studies were conducted in children. Still, the larger number of studies and greater statistical power allowed us to detect a substantially larger number of significantly different voxels than in the sum of the two age-limited meta-analyses (see Figure 3). This yielded additional putatively dysfunctional regions in ADHD, including the superior frontal gyrus/supplementary motor area, the putamen, and the superior temporal gyrus, that had not emerged in the previous meta-analysis (4).
Subtraction analyses.
Despite specificities in each focused meta-analysis, subtraction analyses corrected for multiple comparisons showed that age, clinical characteristics, or type of task did not moderate our results. However, these negative results must be interpreted cautiously. Although definitive standards for statistical power in subtraction analyses do not exist, the informal consensus in the GingerALE users forum (www.brainmap.org/ale/) is that between-analysis subtractions with fewer than 10 studies provide inadequate statistical power. Therefore, while the number of studies was sufficient for focused meta-analyses within subgroups, we were likely underpowered to carry out reliable subtraction analyses across these subgroups. Indeed, when we relaxed the statistical threshold and considered noncorrected results, we did detect differences in some subtraction analyses. Those results are not discussed further as they likely contain too many type I errors. However, we report them in Table S3 in the online data supplement since they may be useful for comparison with future analyses.
General Overview
Our results also provide meta-analytic support to views positing ADHD as a disorder characterized not only by functional deficiencies but also by possible compensatory mechanisms, such as hyperactivation in visual regions. Such putative compensatory mechanisms can be difficult to observe through clinical measures alone, but become evident through neuroimaging (33). Awareness and documentation of brain compensatory mechanisms may eventually yield a clinical benefit from neuroimaging. This would be analogous to the use of neurocognitive assessments to identify particular strengths to best formulate a comprehensive clinical treatment plan.
We failed to find support for the involvement of regions related to motivation and emotion, such as the ventral striatum (34), orbitofrontal, or amygdala/hippocampus (35), despite increasing recognition of motivational and emotional dysfunction in models of ADHD (36). We cannot rule out type II error, as three studies assessing reward (37–39) and two on emotional processing (40, 41) did not meet our inclusion criteria. In addition, the orbitofrontal cortex and the medial temporal lobes are challenging brain regions to examine with fMRI because of susceptibility artifact and signal dropout. We also note the lack of apparent involvement of the cerebellum, which has been implicated in ADHD by multiple volumetric and functional studies and by theoretical models emphasizing the role of cerebellar dysfunction in contributing to deficits in monitoring the frequency and timing of events (6). In our meta-analysis, several peaks in the cerebellum did not reach our cluster size threshold for significance. We suspect that high intersubject variability in cerebellar geometry relative to stereotaxic space mitigated the emergence of cerebellar findings across studies.
Limitations
Several limitations of our meta-analysis should be taken into account. The first relates to selection criteria. To minimize confounding factors such as differences in diagnostic procedures or analytical approaches, we excluded approximately half the screened studies. Still, the studies included were heterogeneous, for example, with respect to the method used to correct for multiple comparisons. This is notable because activation likelihood estimation does not take into account interstudy differences in statistical thresholds. Second, separate meta-analyses could not be performed by ADHD subtype or sex, because separate results for male and female participants and ADHD subtypes are not usually reported in the literature. This is unfortunate since patterns of fMRI activation in ADHD can differ by sex (42) and, possibly, by subtype (43). Third, the meta-analytic approach we adopted allows a quantitative summary of positive results but cannot take into account negative findings. Effect sizes in fMRI may be confounded by many factors, such as movement covariates, and there is no agreement on how they should be handled. Thus, activation likelihood estimation should be considered a summary of the spatial distribution of positive results, rather than a true meta-analysis. Fourth, it was generally not possible to determine the extent to which overlapping samples were reported across studies. Thus, the total number of participants represents an upper bound. The open sharing of fMRI data (e.g., via www.OpenfMRI.org) should obviate this problem in the future. Finally, fMRI data are fundamentally limited. Besides only indirectly reflecting neuronal activity (44), fMRI data cannot define an absolute or quantitative baseline state of activation (45), as they always depend on the differences in signal between two conditions. Positron emission tomography provides absolute quantification (e.g., see 46, 47), but current ethical constraints limit its application to adults.
Conclusions
Moving beyond models of ADHD focused on a limited set of brain regions, the maturing fMRI literature in ADHD reveals dysfunctions in regions belonging to multiple neuronal networks involved in higher-level cognitive and sensorimotor functions. Our results were not ascribable to stimulant treatment history or presence of comorbidities. The systems neuroscience perspective we adopted is in line with the NIH Research Domain Criteria framework (48), which conceptualizes mental disorders in terms of dysfunctions of brain circuits to inform future nosological systems beyond a symptoms-based approach. Future work aimed at understanding the interplay among large-scale neural networks and their links to ADHD symptom dimensions should illuminate the pathophysiology of this common and vexing disorder.
1 : The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 2007; 164:942–948Link, Google Scholar
2 : Persistence of attention-deficit/hyperactivity disorder into adulthood: what have we learned from the prospective follow-up studies? J Atten Disord 2003; 7:93–100Crossref, Medline, Google Scholar
3 : Attention deficit hyperactivity disorder: the most studied and yet most controversial diagnosis. Ment Retard Dev Disabil Res Rev 1999; 5:163–168Crossref, Google Scholar
4 : The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 2006; 47:1051–1062Crossref, Medline, Google Scholar
5 : Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 1997; 121:65–94Crossref, Medline, Google Scholar
6 : An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 2005; 17:785–806Crossref, Medline, Google Scholar
7 : Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 2012; 16:17–26Crossref, Medline, Google Scholar
8 : Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci 2009; 31:36–49Crossref, Medline, Google Scholar
9 : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106:1125–1165Crossref, Medline, Google Scholar
10 : Structural and functional brain imaging in adult attention-deficit/hyperactivity disorder. Expert Rev Neurother 2010; 10:603–620Crossref, Medline, Google Scholar
11 : Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother 2007; 7:1337–1356Crossref, Medline, Google Scholar
12 : An fMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry 2009; 166:1286–1294Link, Google Scholar
13 : Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007; 28:1194–1205Crossref, Medline, Google Scholar
14 : Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009; 30:2907–2926Crossref, Medline, Google Scholar
15 : In praise of tedious anatomy. Neuroimage 2007; 37:1033–1041, discussion 1050–1058Crossref, Medline, Google Scholar
16 : Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010; 53:1–15Crossref, Medline, Google Scholar
17 : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700Crossref, Medline, Google Scholar
18 : The reorienting system of the human brain: from environment to theory of mind. Neuron 2008; 58:306–324Crossref, Medline, Google Scholar
19 : Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 2011; 49:1889–1896Crossref, Medline, Google Scholar
20 : Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3:201–215Crossref, Medline, Google Scholar
21 : Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology 2011; 76:615–621Crossref, Medline, Google Scholar
22 : Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 2007; 31:977–986Crossref, Medline, Google Scholar
23 : Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry 2011; 52:761–771Crossref, Medline, Google Scholar
24 : Is there evidence for neural compensation in attention deficit hyperactivity disorder? a review of the functional neuroimaging literature. Clin Psychol Rev 2006; 26:445–465Crossref, Medline, Google Scholar
25 : Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev 2006; 26:466–485Crossref, Medline, Google Scholar
26 : Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 2000; 157:816–818Link, Google Scholar
27 : Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002; 288:1740–1748Crossref, Medline, Google Scholar
28 : Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand 2012; 125:114–126Crossref, Medline, Google Scholar
29 : Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 2011; 168:1154–1163Link, Google Scholar
30 : Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry 2005; 57:1273–1284Crossref, Medline, Google Scholar
31 : The cholinergic system, EEG and sleep. Behav Brain Res 2011; 221:499–504Crossref, Medline, Google Scholar
32 : Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 1997; 36:381–393Crossref, Medline, Google Scholar
33 : The dynamic approach to neurodevelopmental psychiatric disorders: use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and schizophrenia. Behav Brain Res 2002; 130:47–56Crossref, Medline, Google Scholar
34 : Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biol Psychiatry 2009; 66:972–977Crossref, Medline, Google Scholar
35 : Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63:795–807Crossref, Medline, Google Scholar
36 : Editorial: ADHD as a reinforcement disorder: moving from general effects to identifying (six) specific models to test. J Child Psychol Psychiatry 2011; 52:917–918Crossref, Medline, Google Scholar
37 : Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2009; 65:7–14Crossref, Medline, Google Scholar
38 : Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:720–724Crossref, Medline, Google Scholar
39 : Reward processing in male adults with childhood ADHD—a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology (Berl) 2011; 215:467–481Crossref, Medline, Google Scholar
40 : Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry 2010; 167:61–69Link, Google Scholar
41 : Childhood methylphenidate treatment of ADHD and response to affective stimuli. Eur Neuropsychopharmacol 2011; 21:646–654Crossref, Medline, Google Scholar
42 : Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry 2010; 167:86–94Link, Google Scholar
43 : Event-related fMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J Neuroimaging 2009; 19:205–212Crossref, Medline, Google Scholar
44 : What we can do and what we cannot do with fMRI. Nature 2008; 453:869–878Crossref, Medline, Google Scholar
45 : Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001; 2:685–694Crossref, Medline, Google Scholar
46 : Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 1994; 33:858–868Crossref, Medline, Google Scholar
47 : Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med 1990; 323:1361–1366Crossref, Medline, Google Scholar
48 : Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar



