Silent Cerebrovascular Events and Alzheimer's Disease: An Overlooked Opportunity for Prevention?
For more than 100 years, since its earliest description, there has been debate about the cause of Alzheimer's disease and its overlap with cerebrovascular disease. Indeed, there was controversy about whether Dr. Alzheimer's first case exhibited arteriosclerosis or not (1). The dichotomy between Alzheimer's disease and vascular dementia became more established in the 1980s with the development of distinct diagnostic criteria and with the growing prominence of the amyloid hypothesis of Alzheimer's disease (2). Thus, even now, using DSM-IV-TR criteria, one cannot diagnose dementia due to Alzheimer's in the presence of significant cerebrovascular disease. One unintended consequence of this dichotomy has been that research in Alzheimer's disease and vascular dementia has taken separate paths. While lip service is paid to the fact that mixed dementia is more common than pure Alzheimer's disease, such patients are rarely included in biomarker or intervention trials.
Today, it is fair to say we are back at the same crossroads. After more than 25 years of testing hundreds of candidate treatments, we have several modestly effective symptomatic therapies but no treatment to slow Alzheimer's disease progression or delay its onset. The development of biomarkers to detect pathological and degenerative changes in the disease (e.g., amyloid positron emission tomography [PET] scans) has led to a better ability to monitor pathology in vivo, which in turn has allowed for the development of pathology-based research diagnostic criteria for both Alzheimer's disease and preclinical Alzheimer's disease (3). But the failure of several leading antiamyloid therapies (many of which made patients worse) has highlighted our incomplete knowledge of cause, the likely multifactorial nature of late-onset Alzheimer's disease, and the need for early intervention and better targets.
Accumulating epidemiological, pathological, and imaging evidence over several decades has suggested a role for cerebrovascular disease in the onset and progression of Alzheimer's disease (2, 4–11). Indeed, in the majority of cases, the brains of people with Alzheimer's disease have vascular microinfarcts (not usually detected by MRI), white matter lesions, or vessel wall alterations (2, 4). Other vascular changes in the brain noted in Alzheimer's disease include macroinfarcts, lacunes, atherosclerosis, and hemorrhages (2, 4–8). Experimentally induced cerebral microemboli in aged rats lead to increased beta-amyloid and hyperphosphorylated tau reactivity in both infarcted and adjacent brain areas (5), suggesting a possible link with Alzheimer's pathophysiology. Atherosclerosis of the Circle of Willis may be more severe in Alzheimer's disease (6), and its presence may determine the clinical expression of the disease (7). Imaging studies (single photon emission computed tomography, PET, or MRI) have demonstrated that reduced cerebral perfusion or silent infarcts in Alzheimer's disease correlate with future rates of decline (8).
Vascular risk factors, such as high blood pressure or cholesterol, obesity, elevated homocysteine, atherosclerosis, carotid stenosis, atrial fibrillation, diabetes, and coronary disease, have been linked to risk for Alzheimer's disease in many epidemiological studies (2, 4, 9–11), although there is some inconsistency from one study to another. A preliminary predictive score using demographic and vascular risk factors indicated that elderly individuals scoring above 23 (on a scale ranging from 0 to 60) had a 12-fold greater risk of developing Alzheimer's disease (10). Likewise, in middle-aged people, combining specific vascular risks could predict onset of dementia 20 years later, with a sensitivity of 0.77, a specificity of 0.63, and a negative predictive value of 0.98 (11).
Such findings have led to a neurovascular model of Alzheimer's disease, in which chronic hypoperfusion and uncoupling of blood flow to brain metabolic demands over decades trigger neurodegeneration and amyloidogenesis (2). Disruption of the blood-brain barrier has also been posited more recently (7). However, this theory remains secondary, in part because of disagreements about how to integrate vascular risks into the amyloid cascade (i.e., whether reduced blood flow is a cause or a consequence of cell death) and the failure of some vasoactive agents (statins, aspirin, nootropics) to improve cognition and because vascular disease is not invariant in Alzheimer's disease. (This can become a circular argument, since our ability to detect microvascular disease and soluble amyloid oligomers in vivo is limited.) Lastly, the genetic evidence that alterations in the amyloid cascade are critically involved in Alzheimer's pathophysiology is overwhelming (3), but the same is not yet true for the vascular hypothesis.
In this issue of the Journal, Purandare et al. report that silent cerebral microemboli are associated with faster progression of decline in both Alzheimer's disease and vascular dementia (12). In a longitudinal study, the authors assessed spontaneous microembolic signals using minimally invasive transcranial Doppler over a 1-hour period of monitoring in the middle cerebral artery, which supplies frontal, temporal, and parietal areas. At baseline, despite use of stringent diagnostic criteria, 40% of Alzheimer's patients and 37% of vascular dementia patients were found to have spontaneous cerebral emboli (almost triple the rate of matched comparison subjects). Over the next 2 years, emboli-positive patients deteriorated at double the rate of emboli-negative patients (a decline of 6.9 points compared with 3.4 points on the Mini-Mental State Examination), a difference equivalent to 1 year of natural progression. This was despite the fact that both Alzheimer's and vascular dementia patients were receiving therapies for their respective conditions (such as cholinergics and antiplatelet agents). Furthermore, functional abilities and behavior also deteriorated faster in emboli-positive patients.
The study has some limitations. There was no dose effect with regard to the number of emboli, and emboli size was not examined. Emboli were related to white matter disease but not to vascular risks, suggesting that their etiology was unclear (other than the presence of a patent foramen ovale in some 25% of Alzheimer's patients, which is the same percentage found in the general population). The monitoring of emboli occurred over 1 hour, and it is possible that the relatively short duration may have falsely classified some patients as emboli negative. Furthermore, emboli may not all have the same effect, since some brain areas are more critical to cognition. Clearly, this study should be viewed as preliminary, pending replication in larger samples of both Alzheimer's patients and at-risk individuals. Nevertheless, the large clinical difference in progression rates, if truly causally related to emboli, is a wake-up call for the field.
The study of silent cerebral embolic signals (detected by transcranial Doppler) or events (detected by MRI) has gained more urgency recently because common surgical procedures (e.g., stenting, hip replacement) are known to cause such emboli. For example, one study of “minimally invasive” transfemoral valve repair (13) found that 16 of 22 patients developed at least one new cerebral embolic lesion 3 months after the procedure, with diffusion-weighted MRI showing a total of 75 new lesions among the 16 patients. However, many issues remain unknown, such as the prevalence and long-term sequelae of silent cerebral emboli in otherwise healthy individuals, the genetic risk factors for such emboli, and the best approaches to monitoring and treating individuals with emboli.
Given the hundreds of millions of people worldwide who are affected by vascular risks, we can no longer afford to take a divided dichotomous stance. It's time for the best minds in vascular research to unite with the best minds in amyloid and tau protein research. Prospective field trials to clarify vascular links to Alzheimer's disease will in turn help us better apply readily available tools, such as carotid ultrasound, transcranial Doppler, MRI scans (e.g., arterial spin tagging, diffusion-weighted imaging), and echocardiograms in Alzheimer's disease evaluation. If brain damage is a hidden fingerprint of invasive procedures, such as surgery or angiography, then this would be another priority area for risk reduction. Up to one-half the population with vascular risk factors or silent cerebrovascular disease are thought not to be receiving optimal therapy. But the field also needs to develop more effective vascular interventions than those available today (2). Hence the opportunity for education, lifestyle modification, and clinical trials of novel protective strategies appears significant. Why don't we start today?
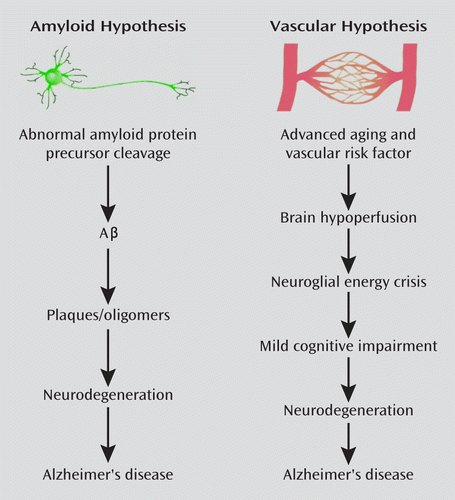
FIGURE 1. Two Theories of Alzheimer's Diseasea
a The original amyloid hypothesis has been modified to implicate soluble oligomers (rather than plaques) as the neurotoxic trigger. The vascular hypothesis posits that amyloidogenesis is a consequence of hypoperfusion. Some evidence suggests that the two insults may be synergistic, highlighting the need to better integrate both theories. (Figure adapted/modified with permission from de la Torre JC, “Is Alzheimer's Disease a Neurodegenerative or a Vascular Disorder? Data, Dogma, and Dialectics” [Lancet Neurol 2004; 3:184–190]. Copyright © Elsevier 2004).
1. : Auguste D and Alzheimer's disease. Lancet 1997; 349:1546–1549Crossref, Medline, Google Scholar
2. : Is Alzheimer's disease a neurodegenerative or a vascular disorder? data, dogma, and dialectics. Lancet Neurol 2004; 3:184–190Crossref, Medline, Google Scholar
3. : Dementia: new criteria but no new treatments. Lancet Neurol 2012; 11:4–5Crossref, Medline, Google Scholar
4. de la Torre JCKalaria RNNakajima KNagata K(eds): Alzheimer's Disease: Vascular Etiology and Pathology. New York, New York Academy of Sciences, 2002Google Scholar
5. : Accumulation of beta-amyloid in the brain microvessels accompanies increased hyperphosphorylated tau proteins following microsphere embolisms in aged rats. Neuroscience 2008; 2:414–427Crossref, Google Scholar
6. : Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia. Alzheimers Dement 2011; 7:436–444Crossref, Medline, Google Scholar
7. : Brain infarction and the clinical expression of Alzheimer's disease: the Nun Study. JAMA 1997; 277:813–817Crossref, Medline, Google Scholar
8. : Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab 2011; 31:1354–1370Crossref, Medline, Google Scholar
9. : The interaction between vascular disorders and Alzheimer's disease, in Alzheimer's Disease and Related Disorders: Etiology, Pathogenesis and Therapeutics. New York, John Wiley & Sons, 1999, pp 523–530Google Scholar
10. : A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol 2010; 67:835–841Crossref, Medline, Google Scholar
11. : Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 2006; 5:735–741Crossref, Medline, Google Scholar
12. : Association of cerebral emboli with accelerated cognitive deterioration in Alzheimer's disease and vascular dementia. Am J Psychiatry 2012; 169:300–308Link, Google Scholar
13. : Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 2010; 55:1427–1432Crossref, Medline, Google Scholar



