Association of the Alzheimer's Gene SORL1 With Hippocampal Volume in Young, Healthy Adults
Abstract
Objective:
Alzheimer's disease is among the most common neurodegenerative disorders. The SORL1 (sortilin receptor 1) gene is associated with the disease, but different variants seem to contribute. The authors used a gene-wide approach to test whether SORL1 is associated with volume of the hippocampus, one of the first structures to be affected by Alzheimer's disease in young, healthy individuals, in an attempt to map potential pathways from gene to disease.
Method:
Individuals were genotyped using an array-based method, and a total of 117 single nucleotide polymorphisms (SNPs) in and surrounding SORL1 were included in the analysis. Through the use of a brain segmentation protocol, SNP-by-SNP and gene-wide associations with bilateral hippocampal volume were assessed in two large, independent samples consisting of 446 (discovery cohort) and 490 (replication cohort) healthy young individuals.
Results:
Significant association of the SORL1 gene with hippocampal volume was observed in both the discovery and replication samples as well as in the combined sample. The gene-wide association was independent of the apolipoprotein E genotype and resistant to removal of four significantly associated single SNPs.
Conclusions:
This study provides the first evidence that the SORL1 gene is associated with differences in hippocampal volume in young, healthy adults. It is demonstrated that gene-wide analysis techniques may overcome power problems caused by allelic heterogeneity in association studies. The results support the hypothesis that the SORL1 gene contributes to an increased risk for Alzheimer's disease through effects on hippocampal volume.
Amyloid beta plays an important role in a cascade of events that eventually lead to Alzheimer's-type dementia (1). Amyloid beta production results from proteolytic cleavage of the amyloid precursor protein via secretases (beta-secretase followed by gamma-secretase). Sorting mechanisms that cause amyloid precursor protein and beta- and gamma-secretases to colocalize in the same membranous compartment play an important role in the regulation of amyloid beta production. The SORL1 (sortilin receptor 1) gene (gene ID: 6653), which codes for a protein involved in sorting amyloid precursor protein and, in this fashion, influences the production of amyloid beta, has been studied extensively for its involvement in Alzheimer's disease (2). Single nucleotide polymorphisms (SNPs) in SORL1 that are associated with Alzheimer's disease have been reported (3). Subsequent replication efforts have resulted in a mixture of negative and positive findings (4–7) (for a review, see reference 2), but a recent meta-analysis confirms several SNPs in different haplotype blocks of SORL1 to be significantly associated with risk for Alzheimer's disease (8). This fits well with the presence of multiple functional domains in SORL1, which could contribute to the disease in different ways (6) and might also suggest allelic heterogeneity.
Understanding the role of disease genes in the healthy brain may help to reduce the heterogeneity observed in Alzheimer's disease, especially if genetically less complex (intermediate) phenotypes are studied (9). One interesting intermediate phenotype is brain morphology. Alzheimer's disease is associated with structural changes in the brain, starting with anterior medial temporal lobe atrophy centered in and around the hippocampus (10, 11). Atrophy of a large network of brain structures, including the hippocampus, has been linked to amyloid beta deposition very early in the disease process (12), and associations between risk genotypes of the well-established risk factor for Alzheimer's disease, APOE (apolipoprotein E), and hippocampal volume in both patients (13) and healthy young individuals (14) have been found. Associations between the SORL1 genotype and brain atrophy have also been described. Cuenco et al. (6) reported an association of a SORL1 haplotype block with atrophy of the medial temporal lobe in a sample of Alzheimer's disease patients and their siblings (mean age: 71 years) (6). An independent sample of end-stage Alzheimer's disease patients subsequently showed association of the SORL1 genotype with atrophy of the hippocampus at autopsy. These findings suggest a role of SORL1 in the disease that might be mediated through hippocampal volume.
It appears that most genetic variants involved in (late-onset) Alzheimer's disease only have small effects (15–17). In this sense, considering different SNPs within the same analysis, relative to a single-SNP or haplotype analysis, can increase the explained phenotypic variance (18) and thereby boost the power of genetic studies. Given that different variants in SORL1 appear to contribute to Alzheimer's disease (8), this gene seems to be a prime candidate for a study of the combined effects of all its observed variation. Therefore, we used a gene-wide analysis to investigate the effect of SORL1 variants in determining hippocampal volume of young healthy adults in two independent large samples from the Brain Imaging Genetics study.
Method
Sample
The present study is part of the Brain Imaging Genetics project being conducted at the Radboud University Nijmegen Medical Centre (Nijmegen, the Netherlands). Saliva and structural magnetic resonance imaging (MRI) data have been collected for 936 healthy, highly educated adults of Caucasian origin between 18 and 36 years of age (mean age: 22.76 years [SD=3.34]; right-handed, 100%; female, 58.4%) and with no self-reported neurological or psychiatric history. Demographic characteristics of the sample are presented in Table 1. The study was approved by the regional medical ethics committee, and all participants gave written informed consent.
| Age (Years) | |||||
|---|---|---|---|---|---|
| Cohort | N | Mean | SD | Gender (Male/Female) | Single Nucleotide Polymorphisms Analyzed |
| Discovery (scanned with 1.5-T) | 446 | 22.9 | 3.54 | 258/188 | 117 |
| Replication (scanned with 3.0-T) | 490 | 22.6 | 3.14 | 291/199 | 115 |
| Combined | 936 | 22.8 | 3.34 | 549/387 | 117 |
| Apolipoprotein E subsample | 735 | 22.8 | 3.28 | 299/436 | 117 |
TABLE 1. Demographic Characteristics of Young, Healthy Adults in the Brain Imaging Genetics Project
Genotyping
Genetic analyses were performed at the Department of Human Genetics of the Radboud University Nijmegen Medical Centre. DNA was isolated from saliva using Oragene containers (DNA Genotek, Ottawa, Ontario, Canada) according to the protocol supplied by the manufacturer.
Affymetrix GeneChip SNP, 6.0 (Affymetrix Inc., Santa Clara, Calif.), arrays were used for genome-wide genotyping of SNPs, as previously described (19). The call-rate threshold was set at 90% for the arrays. One hundred sixty-two SNPs within the SORL1 gene and 100 kilo base pairs up- and downstream the gene (capturing regulatory sequences) were selected for a total of 936 participants (discovery cohort, N=446; replication cohort, N=490).
The APOE genotype was assessed using Taqman analysis (Taqman assay C_3084793_20 for APOE rs429358 and assay C_904973_10 for APOE rs7412 [Applied Biosystems, Nieuwerkerk a/d IJssel, the Netherlands]), and the two SNP genotypes were subsequently combined to confirm the APOE alleles of interest (ε2, ε3, or ε4). Genotyping was carried out in a volume of 10 μl, containing 10 ng of genomic DNA, 5 μl of Taqman Master Mix (2X [Applied Biosytems]), 0.375 μl of the Taqman assay, and 3.625 μl of purified water. The amplification protocol consisted of an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of denaturation at 92°C for 15 seconds and annealing and extension at 60°C for 60 seconds. Allele-specific fluorescence was subsequently measured on an Applied Biosystems 7500 Fast Real-Time PCR System. Taqman genotyping assays were validated before use, and 5% duplicates and blanks were taken along as quality controls during genotyping. Genotyping results were only considered valid if duplicates and blanks were called correctly and genotypes could be called for at least 95% of the sample tested. Participants were classified for the APOE genotype as having an e4 allele (ε2ε4, ε3ε4, or ε4ε4) or having no ε4 allele (ε2ε2, ε2ε3, or ε3ε3).
Neuroimaging Procedures
MRI data were acquired at the Donders Centre for Cognitive Neuroimaging (Nijmegen, the Netherlands). In the discovery cohort, images were acquired with 1.5-T Siemens Sonata and Avanto scanners (Siemens, Erlangen, Germany) using small variations in a standard T1-weighted three-dimensional magnetization-prepared rapid gradient echo sequence (TR=2,300 msec; TI=1,100 msec; TE=3.03 msec; sagittal slices=192; field of view=256 mm). These variations included the following for TR/TI/TE/saggital slices, respectively: 2,730/1,000/2.95/176, 2,250/850/2.95/176, 2,250/850/3.93/176, 2,250/850/3.68/176. The use of generalized autocalibrating partially parallel acquisition imaging with an acceleration factor of 2 was also included. In the replication cohort, images were acquired with 3.0-T Siemens Trio and TrioTim scanners (Siemens, Erlangen, Germany) using small variations in a standard T1-weighted three-dimensional magnetization-prepared rapid gradient echo sequence (TR=2,300 msec, TI=1,100 msec, TE=3.93 msec, sagittal slices=192, field of view=256 mm). These variations included the following for TR/TI/TE/saggital slices, respectively: 2,300/1,100/3.03/192, 2,300/1,100/2.92/192, 2,300/1,100/2.96/192, 2,300/1,100/2.99/192, 1,940/1,100/3.93/ 176, 1,960/1,100/4.58/176. The use of generalized autocalibrating partially parallel acquisition imaging with an acceleration factor of 2 was also included. Slight variations in these imaging parameters have been shown not to affect the reliability of morphometric results (20).
Brain Segmentation
All T1-weighted structural MRI data encompassed the entire brain and had a voxel-size of 1×1×1 mm3. Automated volumetry, as implemented in FIRST, version 1.2 (FMRIB's Integrated Registration and Segmentation Tool of FMRIB Software Library, version 4.1 [http://www.fmrib.ox.ac.uk/fsl/first/] [21–24]), was applied to segment bilateral hippocampal volume. Using MRI data from 68 individuals scanned twice, the test-retest reliability (Pearson's correlation) of the segmentation protocol for the hippocampus was determined (r>0.9). Volumes of bilateral hippocampus were summed for genetic analysis. To correct for total brain volume, raw DICOM (Digital Imaging and Communications in Medicine) MRI data were converted to NIfTI (Neuroimaging Informatics Technology Initiative) format using the conversion as implemented in SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5/). Normalizing, bias correcting, and segmenting into gray matter, white matter, and CSF were performed with the VBM 5.1 toolbox, version 1.19 (http://dbm.neuro.uni-jena.de/vbm/), in SPM using priors (default settings). This method incorporates an optimized voxel-based morphometry protocol (25, 26) as well as a model based on hidden Markov random fields developed to increase the signal-to-noise ratio (27). Total volume for gray and white matter as well as CSF was calculated by adding the resulting tissue probabilities. Total brain volume was defined as the sum of white and gray matter volume.
Analysis
Associations of the SORL1 gene with hippocampal volume were assessed using PLINK software, version 1.07 (http://pngu.mgh.harvard.edu/purcell/plink/ [28]). Quality control steps were performed for the genotype data. SNPs were excluded from the imaging genetics analysis if the call rate per SNP was less than 95%, the minor allele frequency was less than 1%, or the SNPs failed the Hardy-Weinberg equilibrium test at a threshold of p≤10–6(genome-wide). Participants were excluded from the analysis if the call rate per individual was lower than 95%.
Statistical analysis was performed using the “linear” command in PLINK. To decrease heterogeneity effects, we used a gene-wide analysis method with a statistical approach similar to that described by Hoh et al. (29). The analysis consisted of a SNP-by-SNP linear regression and the estimation of the effect of the complete gene. The SNP-by-SNP linear regression for association with hippocampal volume was performed using sex, age, total brain volume, and scanner protocols as covariates. To include the scanner protocols in the model, the protocols were grouped into four categories, and dummy variables were used to classify these categories, which also incorporated the variation of field strength. Multiple testing correction was performed by running 10,000 max(T) permutation tests using the “mperm” command and obtaining an empirical p value for each SNP. The association statistics of the observed and permuted data were saved using the “mperm-save-all” command and then added to create a Σstatistic per run for all SNPs at the same time (10,001 in total, one for the observed data and 10,000 for the permuted data). The empirical p value was then estimated by the number of times the observed Σstatistic was smaller than the permuted Σstatistic divided by the total number of permutations (10, 000). Results were considered significant at a p value <0.05.
First, we analyzed the cohort scanned at 1.5-T (discovery cohort, N=446). After quality control, 117 SNPs remained for analysis. Subsequently, replication was attempted in a second, independent cohort scanned at 3.0-T (replication cohort, N=490). After quality control, 115 SNPs remained for analysis. As a third step, a combined analysis (N=936) was performed that included 117 SNPs. To investigate the effect of linkage disequilibrium on our analysis, the combined analysis was repeated using a pruned data set, which was conducted using the “indep” command, with an r2 threshold of 0.80. In this analysis, 44 SNPs remained. To investigate lateralization effects, the combined group was also tested for association with right and left hippocampal volumes, separately. To make certain that our results were not driven by the APOE gene, we performed an initial analysis on a subgroup of 735 individuals for which APOE genotypic data were available. This was performed by adding ε4 status (carrier versus noncarrier) as a covariate in the linear regression analysis of the combined sample; 117 SNPs were included in this analysis.
We also investigated whether the effects of single SNPs significantly associated with hippocampal volume were causing the gene-wide effect. We therefore excluded the variants showing significant association in the single-SNP approach from the analysis, in addition to those SNPs in high linkage disequilibrium (r2>0.80) with them. Linkage disequilibrium was estimated using Haploview, version 4.2 (30). Following this pruning, 111 SNPs were available for analysis. In order to estimate the effect of the individual SNPs on hippocampal volume, we applied the “qt means” command.
We also performed a haplotype analysis for the SORL1 SNPs. Haplotypes were estimated using the “haplo.em” function in the Haplo Stats software package (31). Haplotype association analyses were performed in a three-SNP sliding window design scanning the effect of all available SORL1 SNPs, using the “haplo.score.slide” function, allowing adjustment for covariates. This analysis was corrected for multiple testing by applying the “simulate=TRUE” parameter in “haplo.score.slide” (31).
Results
Table 1 shows the general characteristics of the studied population. The analysis of bilateral hippocampal volume in the first cohort (N=446), scanned at 1.5-T, revealed a significant gene-wide association (p=0.04) for SORL1 . Replication was achieved in a second, independent cohort (N=490), scanned at 3.0-T, which also revealed significant association (p=0.01). The combined analysis of both cohorts (N=936) further increased significance of the finding (p=0.002). This association was not a result of spurious linkage disequilibrium because the effect was still significant after pruning (44 SNPs, r2≤0.8, p=0.01). The effects on hippocampal volume appeared global rather than lateralized, since associations for separate left and right hippocampal volumes were nonsignificant. To exclude the possibility that our results could be explained by indirect effects of the APOE genotype, we performed the same analysis on a subgroup of our sample for which APOE genotypic data were available (N=735), including APOE ε4-allele status as a covariate. This analysis showed that the APOE genotype had no effect on the results, leaving the association statistically significant (p=0.003).
Investigating the SORL1 SNP-by-SNP association showed linkage with bilateral hippocampal volume that survived permutation for four individual SNPs in the combined sample (Table 2 [also see Table 1 in the data supplement accompanying the online version of this article]). To investigate whether these four significantly associated SNPs explained the gene-wide association with hippocampal volume, we repeated the analysis excluding these four SNPs plus all SNPs that were in linkage disequilibrium with them (r2≥0.80). The association with hippocampal volume remained significant after exclusion of these SNPs (p=0.001; 111 SNPs analyzed), confirming the added value of the gene-wide approach. Importantly, the TT genotype of SNP rs668387(C>T), previously associated with Alzheimer's disease (3) and demonstrating the best association p value in the present study, showed the smallest mean volume of the hippocampus, which is consistent with expectation (Table 2).
| Discovery Cohort (N=446) | Replication Cohort (N=490) | Combined Cohort (N=936) | ||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Positionb | Unadjusted (p) | Empirical (p) | Unadjusted (p) | Empirical (p) | Unadjusted (p) | Empirical (p) | Type |
| rs17125342 | 120813518 | 0.55 | 1.0 | 0.03 | 0.73 | 0.04 | 0.88 | upstream |
| rs661057c | 120834164 | 0.94 | 1.0 | 0.01 | 0.39 | 0.05 | 0.89 | intron 2 |
| rs1784934 | 120843203 | 0.59 | 1.0 | 0.02 | 0.66 | 0.04 | 0.82 | intron 2 |
| rs3781826 | 120848256 | 0.0001 | 0.009 | 0.71 | 1.0 | 0.03 | 0.81 | intron 3 |
| rs676759 | 120864475 | 0.21 | 1.0 | 0.001 | 0.07 | 0.001 | 0.06 | intron 5 |
| rs560573 | 120866094 | 0.16 | 1.0 | d | d | 0.0004 | 0.02 | intron 6 |
| rs593769 | 120869213 | 0.16 | 1.0 | 0.0011 | 0.06 | 0.0006 | 0.03 | intron 6 |
| rs12364988 | 120872836 | 0.18 | 1.0 | 0.0024 | 0.13 | 0.0013 | 0.07 | exon 7 |
| rs668387c | 120873131 | 0.14 | 1.0 | 0.0003 | 0.02 | 0.0002 | 0.01 | intron 7 |
| rs923892 | 120873373 | 0.17 | 1.0 | 0.0003 | 0.02 | 0.0002 | 0.01 | intron 7 |
| rs666004 | 120903788 | 0.30 | 1.0 | 0.02 | 0.58 | 0.01 | 0.44 | intron 12 |
| rs7933552 | 120922038 | 0.98 | 1.0 | 0.00 | 0.19 | 0.03 | 0.75 | intron 15 |
| rs640479 | 120937693 | 0.09 | 1.0 | 0.06 | 0.93 | 0.01 | 0.53 | intron 22 |
| rs924746 | 120948247 | 0.02 | 0.70 | 0.09 | 1.0 | 0.01 | 0.32 | intron 24 |
| rs3781834 | 120951150 | 0.66 | 1.0 | 0.00 | 0.15 | 0.01 | 0.43 | intron 25 |
| rs4420280 | 120951269 | 0.04 | 0.83 | 0.12 | 1.0 | 0.01 | 0.47 | intron 25 |
| rs3781836 | 120953548 | 0.89 | 1.0 | 0.00 | 0.20 | 0.05 | 0.89 | intron 26 |
| rs1699103 | 120957136 | 0.08 | 0.97 | 0.16 | 1.0 | 0.03 | 0.77 | intron 26 |
| rs7116734 | 120957150 | 0.02 | 0.61 | 0.11 | 1.0 | 0.01 | 0.33 | intron 26 |
| rs1620003 | 120978203 | 0.07 | 0.96 | 0.15 | 1.0 | 0.03 | 0.71 | intron 33 |
| rs1629493 | 120982306 | 0.05 | 0.89 | 0.33 | 1.0 | 0.04 | 0.87 | intron 37 |
| rs1784931 | 120988148 | 0.06 | 0.93 | 0.19 | 1.0 | 0.03 | 0.72 | intron 40 |
| rs540860 | 121036098 | 0.28 | 1.0 | 0.03 | 0.78 | 0.02 | 0.67 | downstream |
| rs663175 | 121039037 | 0.44 | 1.0 | 0.02 | 0.62 | 0.03 | 0.72 | downstream |
| rs647905 | 121040148 | 0.40 | 1 | 0.02 | 0.68 | 0.03 | 0.72 | downstream |
| rs568599 | 121041221 | 0.33 | 1 | 0.02 | 0.52 | 0.01 | 0.53 | downstream |
| rs11218381 | 121067926 | 0.01 | 0.31 | 0.09 | 1 | 0.003 | 0.17 | downstream |
| rs10502260 | 121073107 | 0.01 | 0.53 | 0.52 | 1 | 0.03 | 0.80 | downstream |
| rs2887763 | 121073389 | 0.02 | 0.58 | 0.04 | 0.85 | 0.003 | 0.17 | downstream |
| rs10892771 | 121073472 | 0.04 | 0.87 | 0.02 | 0.53 | 0.003 | 0.15 | downstream |
TABLE 2. SORL1 Single Nucleotide Polymorphisms (SNPs) Associated With Bilateral Hippocampal Volume in Young, Healthy Adults in the Brain Imaging Genetics Projecta
In a haplotype analysis of 115 three-SNP haplotypes, from which seven overlapped with those reported previously (6), we found a total of 35 haplotypes associated with bilateral hippocampal volume (maximum simulated p value ≤0.05 [also see Table 2 and Figure 1 in the data supplement]). Consistent with the results of the gene-wide analysis, several regions of the gene showed association with hippocampal volume, including several haplotypes containing SNPs previously shown to be associated with Alzheimer's disease (6). Most significant associations were present at the 3′ of the gene and located downstream (see Figure 1 in the data supplement).
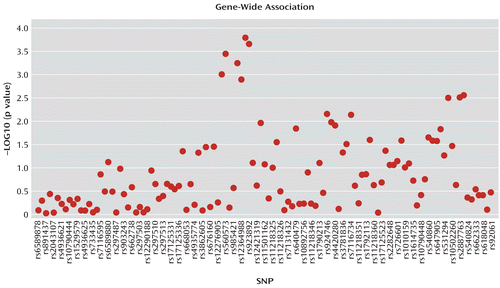
FIGURE 1. Gene-Wide and SNP-by-SNP Associations Between SORL1 Variants and Hippocampal Volume in Healthy, Young Adults in the Brain Imaging Genetics Studya
a The analysis included one cohort scanned at 1.5-T (N=446; p=0.04) and one scanned at 3.0-T (N=490; p=0.01). Gene-wide statistical significance was observed (p=0.002). SNP=single nucleotide polymorphism.
Discussion
We performed a gene-wide association analysis of the SORL1 gene and its linkage to hippocampal volume in young healthy adults in the Brain Imaging Genetics project (Figure 1). Our finding was replicated in an independent cohort and shown to be autonomous of the APOE genotype and linkage disequilibrium structure and was based on global volume rather than lateralization-dependent volume. Although association with hippocampal volume on a single SNP level was also discovered, exclusion of these statistically significant findings (and their linkage disequilibrium blocks) did not affect the significant association. This shows that our result was based on a gene-wide effect rather than single SNP effects.
The presence of several risk variants for Alzheimer's disease in SORL1 makes this gene an excellent candidate for a gene-wide association analysis. By taking into account all genotyped SNPs in the gene simultaneously, potential allelic heterogeneity (where an association with disease might not be detected because in the studied data set the biologically active SORL1 alleles are measured indirectly, via linkage disequilibrium, or not genotyped at all) can be addressed and power needed to detect the effect of genetic variants with small effect sizes might be increased, relative to single SNP analyses (32), as recently shown in a genome-wide association analysis of breast cancer (33).
The method used in the present study is only one of many possible approaches. Ruano et al. (34) recently used a similar analysis method successfully to identify a group of genes associated with cognitive ability. Additionally, Purcell et al. (35) used an approach in which variation across nominally associated loci was summed into quantitative scores that could be related to disease state in independent samples. Similarly, Yang et al. recently showed an association of common variants of very small effect size with height (18).
Several research groups are currently developing other multivariate approaches that might be even more powerful. The first examples are studies involving endophenotypic imaging findings to investigate genomic SNP factors using parallel independent component analysis aimed at identifying simultaneously independent components of each modality (imaging, genetics) and the relationships between them (36, 37).
In our study, the SORL1 SNP rs668387 showed the most significant association with decreased hippocampal volume (in SNP-by-SNP and haplotype analyses). This variant was previously found to be associated with Alzheimer's disease in a meta-analysis (8), suggesting detrimental effects of a smaller hippocampal volume. This finding is in line with the brain capacity theory by Mori et al. (38), which suggests that larger brain volumes provide an individual with more resistance to the consequences of neuronal degeneration. Since our findings are in young healthy individuals and detailed information on medical and family history of Alzheimer's disease was not available, the chain of events leading from SORL1 genetic variation to the disease cannot be clarified. Our findings could, alternatively, indicate that in the carriers of the genetic risk factors, atrophy has already started, which will ultimately, at a later age, contribute to the disease. However, we also cannot exclude the possibility that the volume difference found is a mere epiphenomenon and has nothing to do with the risk for Alzheimer's disease (39). Longitudinal studies will be necessary to distinguish between these possibilities.
Although atrophy of the hippocampus has been found in the early stages of Alzheimer's disease, atrophy of the entorhinal cortex can be found in individuals with mild cognitive impairment (40). We were unable to investigate the effects of the SORL1 genotype on the entorhinal cortex with the brain segmentation protocol utilized. This will be an interesting subject for further study.
In summary, this is the first study, to our knowledge, to provide evidence that the SORL1 gene is associated with structural differences in the hippocampal volume of young healthy adults. We show that gene-wide analysis techniques may overcome the difficulties caused by allelic heterogeneity. Our results support the hypothesis that the SORL1 gene contributes to an increased Alzheimer's disease risk through effects on hippocampal volume.
1. : Alzheimer's disease: the amyloid cascade hypothesis. Science 1992; 256:184–185Crossref, Medline, Google Scholar
2. : Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 2007; 39:17–23Crossref, Medline, Google Scholar
3. : The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 2007; 39:168–177Crossref, Medline, Google Scholar
4. : A study of the SORL1 gene in Alzheimer's disease and cognitive function. J Alzheimers Dis 2009; 18:51–64Crossref, Medline, Google Scholar
5. : Association of SORL1 gene variants with Alzheimer's disease. Brain Res 2009; 1264:1–6Crossref, Medline, Google Scholar
6. : Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol 2008; 65:1640–1648Crossref, Medline, Google Scholar
7. : No association of SORL1 SNPs with Alzheimer's disease. Neurosci Lett 2008; 440:190–192Crossref, Medline, Google Scholar
8. : Sequence variation in SORL1 and dementia risk in Swedes. Neurogenetics 2010; 11:139–142Crossref, Medline, Google Scholar
9. : Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006; 7:818–827Crossref, Medline, Google Scholar
10. : Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–259Crossref, Medline, Google Scholar
11. : Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 1984; 225:1168–1170Crossref, Medline, Google Scholar
12. : Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol 2010; 67:317–324Medline, Google Scholar
13. : APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 1999; 53:1825–1832Crossref, Medline, Google Scholar
14. : Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology 2006; 67:1221–1224Crossref, Medline, Google Scholar
15. : The genetics of adult-onset neuropsychiatric disease: complexities and conundra? Science 2003; 302:822–826Crossref, Medline, Google Scholar
16. : Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet 2009; 41:1088–1093Crossref, Medline, Google Scholar
17. : Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet 2009; 41:1094–1099Crossref, Medline, Google Scholar
18. : Common SNPs explain a large proportion of the heritability for human height. Nat Genet 2010; 42:565–569Crossref, Medline, Google Scholar
19. : Genetic variation in CACNA1C, a gene associated with bipolar disorder, influences brainstem rather than gray matter volume in healthy individuals. Biol Psychiatry 2010; 15:586–588Crossref, Google Scholar
20. : MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 2009; 46:177–192Crossref, Medline, Google Scholar
21. : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(suppl 1)S208–S219Crossref, Medline, Google Scholar
22. : Bayesian Statistical Models of Shape and Appearance for Subcortical Brain Segmentation (D.Phil thesis). Oxford, United Kingdom, University of Oxford, 2007Google Scholar
23. : Improved Surface Models for FIRST, in the Proceedings of the 14th Annual Meeting of the Organization for Human Brain Mapping,
24. : FIRST: FMRIB's Integrated Registration and Segmentation Tool, the Proceedings of the 13th Annual Meeting of the Organization for Human Brain Mapping,
25. : Voxel-based morphometry: the methods. Neuroimage 2000; 11:805–821Crossref, Medline, Google Scholar
26. : A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001; 14:21–36Crossref, Medline, Google Scholar
27. : Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging 2005; 24:1548–1565Crossref, Medline, Google Scholar
28. : PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575Crossref, Medline, Google Scholar
29. : Trimming, weighting, and grouping SNPs in human case-control association studies. Genome Res 2001; 11:2115–2119Crossref, Medline, Google Scholar
30. : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21:263–265Crossref, Medline, Google Scholar
31. : Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 2002; 70:425–434Crossref, Medline, Google Scholar
32. : Testing SNPs and sets of SNPs for importance in association studies. Biostatistics 2010; 12:18–32Crossref, Medline, Google Scholar
33. : Powerful SNP-set analysis for case-control genome-wide association studies. Am J Hum Genet 2010; 86:929–942Crossref, Medline, Google Scholar
34. : Functional gene group analysis reveals a role of synaptic heterotrimeric G proteins in cognitive ability. Am J Hum Genet 2010; 86:113–125Crossref, Medline, Google Scholar
35. : Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460:748–752Crossref, Medline, Google Scholar
36. : A parallel independent component analysis approach to investigate genomic influence on brain function. IEEE Signal Process Lett 2008; 15:413–416Crossref, Medline, Google Scholar
37. : A pilot multivariate parallel ICA study to investigate differential linkage between neural networks and genetic profiles in schizophrenia. Neuroimage 2010; 53:1007–1015Crossref, Medline, Google Scholar
38. : Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am J Psychiatry 1997; 154:18–24Link, Google Scholar
39. : Endophenotype: a conceptual analysis. Mol Psychiatry 2010; 15:789–797Crossref, Medline, Google Scholar
40. : Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 2004; 25:303–310Crossref, Medline, Google Scholar



