A Genomewide Association Study of Response to Lithium for Prevention of Recurrence in Bipolar Disorder
Abstract
Objective: Lithium remains a first-line treatment for bipolar disorder, but the mechanisms by which it prevents the recurrence of mood episodes are not known. The authors utilized data from a genomewide association study to examine associations between single nucleotide polymorphisms (SNPs) and the outcome of lithium treatment in two cohorts of patients with bipolar I disorder or bipolar II disorder. Method: The hazard for mood episode recurrence was examined among 1,177 patients with bipolar I disorder or bipolar II disorder, including 458 individuals treated with lithium carbonate or citrate, who were participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) cohort. SNPs showing the greatest evidence of association in Cox regression models were then examined for association with positive lithium response among 359 bipolar I or II disorder patients treated with lithium carbonate or citrate in a second cohort from the University College London. Results: The strongest association in the STEP-BD cohort (minimum p=5.5×10 –7 ) was identified for a region on chromosome 10p15 (rs10795189). Of the regions showing suggestive evidence (p<5× 10 –4 ) of association with lithium response, five were further associated with positive lithium response in the University College London cohort, including SNPs in a region on chromosome 4q32 spanning a gene coding for the glutamate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA) receptor GRIA2 . Conclusions: Multiple novel loci merit further examination for association with lithium response in bipolar disorder patients, including one region that spans the GRIA2 gene, for which expression has been shown to be regulated by lithium treatment.
Recurrent mood episodes are a hallmark of bipolar disorder, with as many as 50% of patients experiencing recurrence within 1 year after resolution of an acute episode (1 – 4) . Multiple pharmacotherapies, including lithium and lamotrigine as well as some antipsychotic medications, have demonstrated efficacy in delaying recurrence of mood episodes in bipolar disorder (5 – 9) . However, the molecular mechanisms by which these drugs exert their therapeutic effects have not been established, despite intensive study implicating multiple pathways (10) , particularly for lithium.
Some evidence suggests that response to lithium treatment is familial (11) and associated with a family history of bipolar disorder (12) . However, initial candidate gene investigations of lithium response have not yet yielded replicated findings (13 – 15) . Limited understanding of the mechanism of action of bipolar pharmacotherapies impedes further candidate gene association studies and argues for a more unbiased genomewide approach. Indeed, recent transcriptional profiling studies (16 , 17) have identified changes following lithium treatment in genes not previously connected with lithium response.
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study (3 , 18) was an effectiveness study that examined real-world outcomes in a large cohort of patients receiving guideline-based, nonrandomized treatment. Results from a genomewide association study of the STEP-BD cohort have recently been reported (19 , 20) . We utilized these genomewide data to examine association with risk for recurrence among patients treated with lithium. We then examined the regions that showed the greatest evidence of association in a second cohort of bipolar I and II disorder patients who were drawn from clinical populations at University College London.
Method
Participants
The STEP-BD study was a multicenter cohort study conducted in the United States at 15 sites between 2001 and 2006. Methods for the STEP-BD study as a whole have been described previously (3 , 18) . In brief, study participation was offered to all bipolar patients seeking outpatient treatment at one of the participating study sites. Entry criteria were 1) meeting DSM-IV criteria for bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified, cyclothymia, or schizoaffective disorder bipolar type and 2) the ability to provide informed consent. For individuals ages 15 to 17, written assent from a parent or guardian was also required. Hospitalized individuals were eligible to enter following discharge.
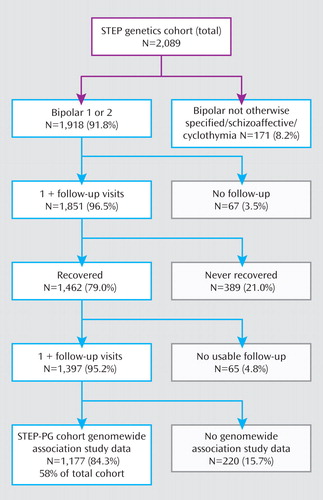
From the 4,361 participants in the STEP-BD study, 2,089 agreed to provide blood samples for DNA extraction and cell line generation through the STEP Genetic Repository for Participants. The present analysis is based on all subjects who entered STEP-BD with a diagnosis of bipolar I or II disorder who were part of the genetic study and were included in a bipolar genomewide association study (19) . Features of this cohort have been reported elsewhere (19) . Subjects with bipolar disorder not otherwise specified, schizoaffective disorder bipolar type, or cyclothymia represented <9% of the total STEP-BD sample and were excluded from the present analysis because of the difficulty in considering their course and treatment response in episodic terms.
Assessments
Bipolar diagnosis was determined using mood and psychosis modules from the Structured Clinical Interview for DSM-IV, as incorporated in the Affective Disorders Evaluation, and confirmed using the Mini-International Neuropsychiatric Interview (18 , 21) . Comorbid axis I diagnoses were also determined using the Mini-International Neuropsychiatric Interview. Additional details of patients’ retrospective course upon entering STEP-BD, including the proportion of time in the preceding year with significant depressive, manic, and anxious symptoms as well as the number of episodes of each type, were collected by the clinician using the Affective Disorders Evaluation.
Intervention
Study clinicians in STEP-BD were trained to use model practice procedures, which included published pharmacotherapy guidelines (18 , 22) , but could prescribe any treatment or combination of treatments they felt were indicated. The use of guideline-concordant care was substantially higher than expected under naturalistic conditions, suggesting that such training yielded the desired effect (18 , 22) . Patients also received education in the collaborative care approach, which has been described previously (18 , 23) , using a self-teaching workbook and videotape.
Outcomes and Phenotype Definition
Because STEP-BD was intended to mimic clinical practice, participants were seen by a study clinician as frequently as clinically indicated. At baseline and each follow-up visit, clinicians assigned current mood status based on the Clinical Monitoring Form, which assesses DSM-IV mood criteria for depressive, manic, hypomanic, or mixed states within the prior 14 days (18) .
Primary outcome definitions utilized in the present analysis were those of the STEP-BD study, selected for consistency with the National Institute of Mental Health Treatment of Depression Collaborative Research Program (24 – 26) as well as the McLean-Harvard First-Episode Mania Study (1) . Recovery was defined as two or fewer syndromal features of a mood episode for at least 8 weeks, and recurrence was defined as meeting full DSM criteria for a mood episode on any single subsequent visit. The presence of subsyndromal mood symptoms during follow-up was not considered recurrence.
The at-risk cohort was defined as individuals who achieved euthymia for at least 8 weeks during prospective follow-up, consistent with STEP-BD clinical reports and previous bipolar cohort studies as well as the McLean-Harvard First-Episode Mania Study (1 , 3 , 24 – 26) . Derivation of the STEP-BD recurrence cohort is illustrated in a Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 1 . The outcome of interest was defined as time to recurrence of a mood episode.
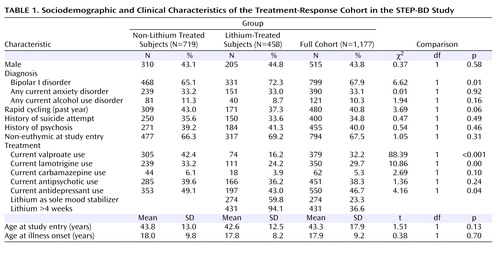
The primary analysis examined all subjects receiving treatment with lithium carbonate or lithium citrate, alone or in combination with other pharmacotherapies, at the first prospectively observed study visit during which they were in remission, an approach analogous to an intent-to-treat analysis ( Table 1 ). A subset of these patients who were receiving lithium for at least 4 weeks of follow-up (N=431) was also examined to address the possibility that differences in outcomes might arise from differential tolerability during the initial phase of treatment. Finally, to understand treatment-independent factors that might influence recurrence risk, a second analysis examined all subjects in the prospective cohort (N=1,177).
The University College London sample consisted of bipolar disorder patients of United Kingdom or Irish background. Individuals with not more than one grandparent of other Western European ancestry were also included. The U.K. National Health Service Multicenter Research Ethics Committee approval was obtained, and all subjects signed a consent form after reading an information sheet (20) . All subjects were interviewed using the Schedule for Affective Disorders and Schizophrenia—Lifetime Version and were assigned a Research Diagnostic Criteria diagnosis. A global assessment of response to lithium treatment was also determined by the interviewing psychiatrist based on the rate and severity of bipolar episodes before and after lithium treatment. These data were reviewed by a research psychiatrist (N.B.) in order to characterize subjects according to the following three response groups: “good,” “intermediate,” and “poor.” It was possible to characterize 359 genotyped subjects receiving lithium, alone or in combination with other medications, in this manner. For analyses presented in the present study, poor (negative) responders were contrasted with the other two response groups (positive responders), based on the determination by the evaluating psychiatrist that the former was the most clearly distinguished from the latter two.
Genotyping and Genotype Imputation
DNA was extracted from lymphoblastoid cell lines at the Rutgers Cell and DNA Repository. For the University College London sample, DNA was extracted from blood samples. Genotyping was performed using the Affymetrix GeneChip Human Mapping 500K Array Set by the Genetic Analysis Platform at the Broad Institute of Harvard and Massachusetts Institute of Technology. Details of data cleaning and genotype imputation have been described by Ferreira et al. (20) .
Statistical Analysis
In the STEP-BD cohort, Cox regression was used to examine the association between the hazard of recurrence and individual single nucleotide polymorphisms (SNPs), with the Efron method for resolving ties. Time-to-event or censoring was defined as the number of days following the first recovered visit (i.e., baseline) to the first recurrence of a major depressive, hypomanic, manic, or mixed episode, with results censored after recurrence, dropout, completion of 2 years of follow-up, or presence of a gap between visits >120 days.
All regression models also incorporated as a covariate the clinical state at entry into the STEP-BD study (euthymic or symptomatic), since individuals who enter a symptomatic state and subsequently recover remain more likely to experience recurrence (3) . To account for potential population stratification effects, we also included as covariates the first two quantitative indices of ancestry based on a multidimensional scaling analysis of the pair-wise identify-by-state distance matrix for all individuals. Analyses that included additional indices of ancestry (i.e., the third, fourth, and subsequent components from the multidimensional scaling analysis) were not meaningfully different.
Quintile-quintile plots comparing observed to expected p values based on Cox regression results were generated (see the data supplement accompanying the online version of this article). For each analysis, the genomic inflation factor lambda was calculated from the ratio of median observed to expected chi square values.
For any SNPs demonstrating significant association with recurrence at a p value <5×10 –4 (selected a priori as four orders of magnitude less than an accepted threshold for genomewide significance), we performed additional testing. First, we confirmed that the proportional hazards assumption was met using formal testing (implemented in the postestimation command estat phtest in Stata) and visual inspection of scaled identify-by-state plotted against log (time). Second, we examined the possible confounding effects of sociodemographic and clinical features previously associated with outcome (3) , including 1) depressive, manic, or anxious symptoms in the pre-baseline year; 2) rapid cycling in the preceding year; 3) sex; and 4) residual mood symptoms, by repeating the Cox regression with adjustment for each of these predictors individually. Third, we repeated the Cox regression restricting the cohort to those patients with at least 4 weeks of treatment (N=431). Fourth, we repeated the regression adding terms for concomitant antidepressant or antipsychotic treatment. Finally, to clarify the specificity of association for lithium treatment, we examined gene-by-treatment effects in the Cox regression by incorporating a term for treatment status (“on” or “off” lithium at entry).
For initial analyses of genetic association, PLINK v1.0 (27) and the coxph module of R were utilized. Stata 10.0 (StataCorp, College Station, Tex.) was used for follow-up analyses.
Replication Analysis
For the regions demonstrating the greatest evidence of association with lithium response in the STEP-BD cohort, we examined association of these SNPs with positive versus negative response in the University College London cohort. Findings were interpreted as consistent if a SNP exhibited a hazard ratio >1 (indicating association with greater recurrence risk) in the STEP-BD cohort and an odds ratio >1 (indicating association with negative lithium outcome) in the University College London cohort or vice versa.
Results
We examined approximately 1.4 million SNPs directly genotyped on the Affymetrix Gene Chip or imputed using PLINK, as described previously (20) , with a minor allele frequency ≥5% in a total of 1,177 bipolar I and bipolar II patients. Assuming a 55% recurrence rate and an alpha of 5×10 –4 , power was >80% to detect a hazard ratio of ≤0.71 in the full cohort and 0.58 in the 458 lithium-treated patients.
Sociodemographic and clinical features of the lithium-treated cohort were generally similar to those of the nonlithium-treated patients ( Table 1 ). Lithium-treated patients were more likely to have a diagnosis of bipolar I disorder and less likely to be receiving co-treatment with valproate, lamotrigine, or an antidepressant. Among the 458 lithium-treated patients, 274 (59.8%) were receiving lithium as their sole mood stabilizing medication, and 431 (94.1%) continued to receive lithium treatment for at least 4 weeks following remission.
Association Analyses
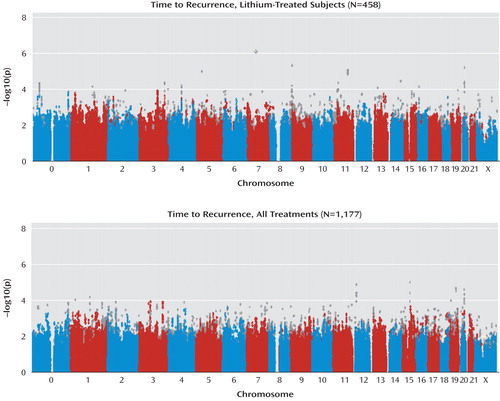
The genomic inflation factor (lambda) was 1.00 for the lithium-treated cohort and 1.00 for the full cohort, which, as expected, was less than that of the STEP-BD case-control analyses (19) because all subjects in the present study represent “cases” drawn from the same population. Genomewide association results for the lithium cohort are shown in Figure 1 and are also available online (pngu.mgh.harvard.edu/perlis/AJP_lithium_GWAS/). No SNPs met the threshold for genomewide significance (p=5×10 –8 ) correcting for approximately 1 million independent common SNPs (28) . The SNP with the greatest evidence for association was located on the chromosomal region 10p15 (minimum p=5.5×10 –7 for rs10795189). Three additional regions were associated with a p value <5×10 –6 (21q21, 12q22, and 6p21, 19 with a p value <5×10 –5 , and 140 with a p value <5×10 –4 .
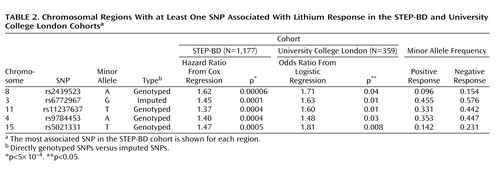
We next examined the regions with a p value <5×10 –4 for association with lithium response in a second cohort (University College London cohort) ( Table 2 [also see the data supplement accompanying the online version of this article]). In the University College London cohort (N=359), power was >80% to detect a genotypic risk ratio of 1.3, with an alpha of 0.05 for minor allele frequencies ≥10%. Of the regions examined, nine yielded a p value <0.05 in this cohort (versus seven expected by chance). Of these nine, five (8q22, 3p22, 11q14, 4q32, and 15q26) displayed the same direction of effect as displayed in the STEP-BD cohort (i.e., protective or risk inducing). These regions are illustrated in the data supplement accompanying the online version of this article.
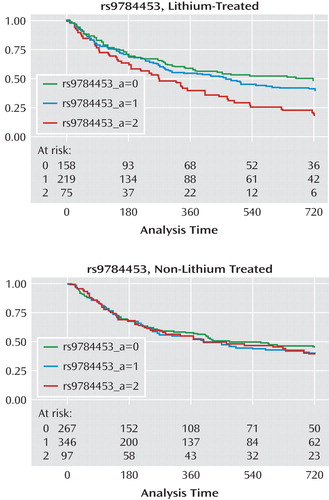
When terms for gene-by-treatment interactions were included in the Cox model in the STEP-BD cohort, significant interactions (p<0.05) were identified for all regions except 3p22 and 15q26. Figure 2 illustrates time to recurrence for rs9784453 on region 4q32 among lithium-treated (Kaplan-Meier log-rank: p=0.001) and non-lithium-treated (Kaplan-Meier log-rank: p=0.72) patients.
In no case did incorporation of clinical or sociodemographic covariates, including indicator variables for clinical sites, lead to a change in a hazard ratio >10%, indicating that these covariates did not confound the observed associations. For example, in the STEP-BD clinical cohort, we previously reported that bipolar I disorder versus bipolar II disorder was not significantly associated with recurrence risk (3) . In the present study, incorporating bipolar subtype into the model yielded a change of <1% in the resulting hazard ratio, and both subtype and the subtype-by-genotype interaction terms yielded a p value >0.05 for regions 8q22, 3p22, 11q14, 4q32, and 15q26. Likewise, limiting the analysis to the 431 subjects who continued treatment with lithium for at least 4 weeks after remission failed to meaningfully alter these results, suggesting that differences in short-term tolerability did not confound the genetic associations. Finally, including terms in the model for co-treatment with antidepressant or antipsychotic medications also did not suggest confounding.
Bipolar Liability Regions Implicated by Previous Investigations
We examined the regions implicated in a recent meta-analysis of genomewide association study data for bipolar disorder (20) , since such regions might a priori be more likely to influence treatment response. Results from this analysis are available in the data supplement accompanying the online version of this article.
Overall Time to Recurrence Analysis
Finally, we examined association with time to recurrence among the entire treatment cohort, independent of lithium treatment status. Genomewide association results are illustrated in Figure 3 as well as the data supplement accompanying the online version of this article (results are also available online [http://www.massgeneral.org/chgr/faculty_perlis/publications/lithium_gwas.htm]). No SNPs met the threshold for genomewide significance (for rs1493902 on 16q12: minimum p value=2.7×10 –6 ). Overall, there were two regions associated with a p value of <5×10 –6 and 147 regions associated with a p value of <5×10 –4 .
Discussion
In the present study, which to our knowledge is the first genomewide association study of lithium response in bipolar disorder, no SNP met the threshold for genomewide association. However, these results may still be useful in laying the groundwork for subsequent genetic and proteomic investigations by helping to prioritize association results. Of the regions with a p value of <5×10 –4 in the STEP-BD cohort, five showed consistent evidence of association in a second cohort of lithium-treated patients (University College London cohort). Among these five regions is the gene coding for a subunit of the ligand-gated ionotropic glutamate receptor, GluR2/GLURB, which binds to alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA). Notably, a recent report suggested that GRIA2 is one of the genes downregulated by chronic lithium treatment in a human neuronal cell line (17) , and chronic lithium or valproate treatment causes decreased synaptic expression of GluR2 in hippocampal neurons (29) . In a magnetic resonance spectroscopy study, hippocampal glutamate concentrations were significantly increased in euthymic chronically lithium-treated patients relative to healthy comparison subjects (30) . Thus, our results add to a convergent body of evidence suggesting the importance of glutamate in bipolar disorder and the mechanism by which lithium may affect glutamatergic neurotransmission.
Among the other regions with consistent association in the STEP-BD and University College London cohorts were those containing 1) Syndecan-2 ( SDC2 ), which codes for a cell-surface proteoglycan that has been shown to play a central role in the formation of dendritic spines in the hippocampus (31) ; 2) synaptic vesicle glycoprotein-2B ( SV2B ), a protein of unknown function expressed primarily in the hippocampus (32) ; and 3) the human homologue of Drosophila odd Oz (odz)-4 ( ODZ4 ), implicated in brain patterning (33) . Taken together our results also suggest that response to lithium is likely influenced by multiple genes of modest effect, similar to complex genetic diseases themselves.
We did not find significant evidence of association with treatment response for variation in regions previously implicated in a meta-analysis of genomewide association studies of bipolar disorder (20) . This is perhaps not surprising given that the genes in these regions account for a very small proportion of attributable risks of bipolar disorder. However, we cannot exclude the possibility that lithium acts up- or downstream of these genes.
The STEP-BD cohort presents both limitations and strengths for pharmacogenetic study. A primary limitation is the absence of detailed retrospective data to allow contrast between pre- and posttreatment course. As a result, we were unable to utilize the criteria for good versus poor lithium response, as developed by Grof and colleagues (11) , which relies on knowledge of prelithium course. Further limitations of the STEP-BD cohort are that treatments were not always initiated at the onset of a mood episode, treatment assignment was not randomized, and medications were often administered in combination. These features make assignment of longer-term outcomes to a given medication complex. Of note, this is a problem in bipolar disorder clinical practice as well, in which it can be difficult for the clinician and patient to “assign” benefit to a given pharmacotherapy, particularly if it is part of a combination regimen. Therefore, we made simplifying assumptions based on when clinicians typically initiate and discontinue new treatments (5) . Specifically, for time-to-recurrence, we assumed that if a patient was receiving a treatment at baseline, an outcome could be assigned to that medication. This approach corresponds to intent-to-treat in clinical trials. We did not require a specific threshold lithium level, since trough levels were not available for the majority of subjects, although STEP-BD clinician education in evidence-based practice included the importance of adequate lithium dosing.
On the other hand, the STEP-BD study has advantages for pharmacogenetic analysis. First, because of the availability of detailed prospective assessments, our confidence in the reliability of outcomes is higher than that available from typical retrospective assessment. Second, the STEP-BD study’s effectiveness design should yield greater generalizability and ecological validity than, for example, randomized controlled trials. Such trials typically exclude individuals with anxiety or substance use comorbidity, despite the prevalence of these comorbidities in clinical practice (34) . Finally, the total number of patients with time to recurrence available far exceeds that of other reported cohorts to date, and the availability of multiple treatment groups allows for examination of overlap in association.
In addition, little is known about the homogeneity of response between bipolar I disorder and bipolar II disorder (35) , but modern studies suggest similar efficacy for lithium in both subgroups (3 , 5 , 35) . We therefore elected to pool these two groups but adjust our associations by bipolar subtype. Among the SNPs showing the greatest evidence of association with lithium response, we did not detect evidence of gene-by-bipolar subtype interactions.
A notable feature of the present analysis is the availability of a replication cohort. The optimal replication cohort would have allowed the examination of an identical phenotype (i.e., time to recurrence). A quantitative survival phenotype as such should yield greater statistical power than deriving a categorical outcome. However, to our knowledge, no such cohort is extant. Alternatively, we could have derived a “positive response” phenotype more similar to that of the University College London cohort or other lithium-response cohorts. We elected not to categorize time to recurrence outcomes for the STEP-BD cohort in terms of “good” or “poor” response because to do so would have entailed an arbitrary distinction. Still, the lack of correspondence between the responsiveness phenotype in the two cohorts suggests that failure to “replicate” should be interpreted cautiously.
Taken together, our results do suggest a number of regions meriting further investigation. They further highlight the importance of collecting adequate replication cohorts with detailed longitudinal outcomes if the effect of genetic variation on lithium response is to be understood. An international consortium for the study of lithium genetics will be useful in this regard (http://www.conligen.org/). Finally, our results suggest the potential utility of pharmacogenetic investigation in bipolar disorder for elucidating mechanisms of action of established mood stabilizing medications.
1. Tohen M, Zarate CA Jr, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ: The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry 2003; 160:2099–2107Google Scholar
2. Carlson GA, Bromet EJ, Driessens C, Mojtabai R, Schwartz JE: Age at onset, childhood psychopathology, and 2-year outcome in psychotic bipolar disorder. Am J Psychiatry 2002; 159:307–309Google Scholar
3. Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME: Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2006; 163:217–224Google Scholar
4. Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM: Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161:1537–1547Google Scholar
5. Hirschfeld RM, Bowden CL, Gitlin MJ, Keck PE, Perlis RH, Suppes T, Thase ME: Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159(April suppl):1–50Google Scholar
6. Keck PE Jr, Perlis RH, Otto MW, Carpenter D, Ross R, Docherty JP: Expert Consensus Guideline Series: Treatment of Bipolar Disorder, 2004. White Plains, NY, Expert Knowledge Systems, 2004Google Scholar
7. Tohen M, Calabrese JR, Sachs GS, Banov MD, Detke HC, Risser R, Baker RW, Chou JC, Bowden CL: Randomized, placebo-controlled trial of olanzapine as maintenance therapy in patients with bipolar I disorder responding to acute treatment with olanzapine. Am J Psychiatry 2006; 163:247–256Google Scholar
8. Tohen M, Greil W, Calabrese JR, Sachs GS, Yatham LN, Oerlinghausen BM, Koukopoulos A, Cassano GB, Grunze H, Licht RW, Dell’Osso L, Evans AR, Risser R, Baker RW, Crane H, Dossenbach MR, Bowden CL: Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry 2005; 162:1281–1290Google Scholar
9. Leverich GS, Post RM, Keck PE Jr, Altshuler LL, Frye MA, Kupka RW, Nolen WA, Suppes T, McElroy SL, Grunze H, Denicoff K, Moravec MK, Luckenbaugh D: The poor prognosis of childhood-onset bipolar disorder. J Pediatr 2007; 150:485–490Google Scholar
10. Quiroz JA, Gould TD, Manji HK: Molecular effects of lithium. Mol Interv 2004; 4:259–272Google Scholar
11. Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O’Donovan C, Alda M: Is response to prophylactic lithium a familial trait? J Clin Psychiatry 2002; 63:942–947Google Scholar
12. Grof P, Alda M, Grof E, Zvolsky P, Walsh M: Lithium response and genetics of affective disorders. J Affect Disord 1994; 32:85–95Google Scholar
13. Mamdani F, Alda M, Grof P, Young LT, Rouleau G, Turecki G: Lithium response and genetic variation in the CREB family of genes. Am J Med Genet B Neuropsychiatr Genet 2008; 147B:500–504Google Scholar
14. Masui T, Hashimoto R, Kusumi I, Suzuki K, Tanaka T, Nakagawa S, Suzuki T, Iwata N, Ozaki N, Kato T, Takeda M, Kunugi H, Koyama T: A possible association between missense polymorphism of the breakpoint cluster region gene and lithium prophylaxis in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:204–208Google Scholar
15. Silberberg G, Levit A, Collier D, St Clair D, Munro J, Kerwin RW, Tondo L, Floris G, Breen G, Navon R: Stargazin involvement with bipolar disorder and response to lithium treatment. Pharmacogenet Genomics 2008; 18:403–412Google Scholar
16. McQuillin A, Rizig M, Gurling HM: A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics 2007; 17:605–617Google Scholar
17. Seelan RS, Khalyfa A, Lakshmanan J, Casanova MF, Parthasarathy RN: Deciphering the lithium transcriptome: microarray profiling of lithium-modulated gene expression in human neuronal cells. Neuroscience 2008; 151:1184–1197Google Scholar
18. Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF: Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003; 53:1028–1042Google Scholar
19. Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM: Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13:558–569Google Scholar
20. Ferreira MA, O"Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St Clair D, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40:1056–1058Google Scholar
21. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33; quiz 34–57Google Scholar
22. Dennehy EB, Bauer MS, Perlis RH, Kogan JN, Sachs GS: Concordance with treatment guidelines for bipolar disorder: data from the Systematic Treatment Enhancement Program for Bipolar Disorder. Psychopharmacol Bull 2007; 40:72–84Google Scholar
23. Nierenberg AA, Ostacher MJ, Borrelli DJ, Iosifescu DV, Perlis RH, Desrosiers A, Armistead MS, Calkins AW, Sachs GS: The integration of measurement and management for the treatment of bipolar disorder: a STEP-BD model of collaborative care in psychiatry. J Clin Psychiatry 2006; 67(suppl 11):3–7Google Scholar
24. Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB: A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003; 60:261–269Google Scholar
25. Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB: The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59:530–537Google Scholar
26. Keller MB, Lavori PW, Coryell W, Endicott J, Mueller TI: Bipolar I: a five-year prospective follow-up. J Nerv Ment Dis 1993; 181:238–245Google Scholar
27. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575Google Scholar
28. International HapMap Consortium: A haplotype map of the human genome. Nature 2005; 437:1299–1320Google Scholar
29. Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R, Yuan P, Chen G, Zhuo M, Manji HK: The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci 2008; 28:68–79Google Scholar
30. Colla M, Schubert F, Bubner M, Heidenreich JO, Bajbouj M, Seifert F, Luborzewski A, Heuser I, Kronenberg G: Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol Psychiatry 2008 [Epub ahead of print]Google Scholar
31. Ethell IM, Irie F, Kalo MS, Couchman JR, Pasquale EB, Yamaguchi Y: EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron 2001; 31:1001–1013Google Scholar
32. Bajjalieh SM, Peterson K, Linial M, Scheller RH: Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A 1993; 90:2150–2154Google Scholar
33. Ben-Zur T, Feige E, Motro B, Wides R: The mammalian Odz gene family: homologs of a Drosophila pair-rule gene with expression implying distinct yet overlapping developmental roles. Dev Biol 2000; 217:107–120Google Scholar
34. Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, Sachs GS, Nierenberg AA, Thase ME, Pollack MH: Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2004; 161:2222–2229Google Scholar
35. Suppes T, Dennehy EB: Evidence-based long-term treatment of bipolar II disorder. J Clin Psychiatry 2002; 63(suppl 10):29–33Google Scholar