Childhood Motor Coordination and Adult Schizophrenia Spectrum Disorders
Abstract
Objective: The authors examined whether motor coordination difficulties assessed in childhood predict later adult schizophrenia spectrum outcomes. Method: A standardized childhood neurological examination was administered to a sample of 265 Danish children in 1972, when participants were 10–13 years old. Adult diagnostic information was available for 244 members of the sample. Participants fell into three groups: children whose mothers or fathers had a psychiatric hospital diagnosis of schizophrenia (N=94); children who had at least one parent with a psychiatric record of hospitalization for a nonpsychotic disorder (N=84); and children with no parental records of psychiatric hospitalization (N=66). Psychiatric outcomes of the offspring were assessed through psychiatric interviews in 1992 when participants were 31–33 years of age, as well as through a scan of national psychiatric registers completed in May 2007. Results: Children who later developed a schizophrenia spectrum disorder (N=32) displayed significantly higher scores on a scale of coordination deficits compared with those who did not develop a mental illness in this category (N=133). Conclusions: Results from this study provide further support for the neurodevelopmental hypothesis of schizophrenia and underscore the potential role of cerebellar and/or basal ganglia abnormalities in the etiology and pathophysiology of schizophrenia.
A substantial amount of research has documented minor neurological abnormalities in people with schizophrenia. Neurological soft signs are frequently cited abnormalities that have been defined as “nonlocalizing neurological abnormalities that cannot be related to impairment of a specific brain region or are not believed to be part of a well-defined neurological syndrome” ( 1 , p. 959). Soft signs commonly observed in adults with schizophrenia include motor incoordination, motor sequencing impairment, sensory integrative dysfunction, and eye movement abnormalities (2 – 7) . Since neurological soft signs are common not only among people with schizophrenia but also among their first-degree relatives, it has been proposed that these signs reflect a genetically transmitted biological marker of risk for the disorder (4 , 8 – 11) .
Additionally, a widely cited meta-analysis examining neurocognitive deficits in schizophrenia relative to comparison subjects reported neuromotor abnormalities as the second-largest effect of 22 assessed domains (12) . The majority of studies revealing neuromotor dysfunction in schizophrenia are based on examination of individuals with full-blown schizophrenia in adulthood. Studying patients already diagnosed with schizophrenia makes it impossible to determine whether dysfunction precedes schizophrenia or is a byproduct of disease onset or treatment. Although a few studies have uncovered a link between motor abnormalities and schizophrenia among neuroleptic-naive individuals (13) and in individuals experiencing their first episode of psychosis (14) , studies examining individuals before illness onset provide the most direct evidence for preexisting neurological dysfunction preceding psychosis (8 , 10 , 15 – 18) .
Motor incoordination stands out as perhaps the most frequently reported category of soft signs in schizophrenia patients as well as in those at risk for the disease (19) . Motor coordination deficits have frequently been cited as significant discriminators of the schizophrenia neurodevelopmental diathesis (20 , 21) and have been referred to as “the most common childhood neuromotor deviation” (11 , p. 68). Similarly, the authors of a comprehensive review of prospective high-risk projects stated that “motor uncoordination was the most common finding differentiating high-risk children from controls in many high-risk studies” (21 , p. 251).
However, relatively few prospective studies (8 , 15) have specifically examined whether childhood coordination deficits predict the development of schizophrenia spectrum disorders in adulthood. In published prospective studies of neuromotor functioning, the number of individuals who developed a schizophrenia spectrum disorder was exceedingly small. Additionally, the measures of neuromotor deviations have varied and have not always been standardized. Some studies employed composite examination ratings, combining not only multiple motor findings but also cognitive factors (18) . Unfortunately, summing disparate types of examination data into total scores makes it difficult to extract information with neurological localizing significance.
The aim of this study was to investigate motor coordination at ages 10–13 years, as assessed through a thorough neurological examination, and the risk of subsequently developing schizophrenia spectrum disorders. In light of the existing literature, we tested the hypothesis that motor coordination deficits in childhood predict schizophrenia spectrum disorders in adulthood, relative to either a nonpsychotic mental illness or no diagnosis in adulthood.
Method
Participants
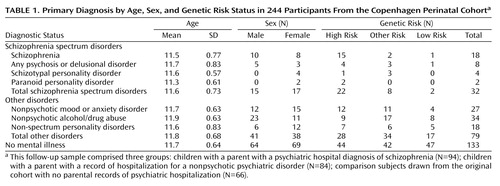
Participants were drawn from the Copenhagen Perinatal Cohort, which comprised 9,125 individuals born between September 1, 1959, and December 31, 1961, at Rigshospitalet, in Copenhagen, Denmark (22) . To identify high-risk children and comparison subjects, in 1961 the lifetime record of parental psychiatric admissions was checked through the Danish psychiatric record for the parents of the birth cohort. In 1972, 265 children 10–13 years old from this cohort were intensively examined at Psykologisk Institute, Kommunehospitalet (23) .
In 1992, when the offspring were 31–33 years of age, their psychiatric status was ascertained. A psychiatrist administered the Structured Clinical Interview for DSM-III-R (24) and the psychosis section of the Present State Examination (25) . In addition, Danish psychiatric hospital records for the participants were examined. A detailed coding scheme was used to yield DSM-III-R diagnoses. An additional attempt to ascertain diagnostic status was made through scanning of the Danish Psychiatric Central Registry between 1994 and 2007. Previous research suggests that scanning national registers is a valid method for obtaining psychiatric diagnoses (26) . As a function of this process, we identified six additional participants who met ICD-10 criteria for schizophrenia, schizotypy, paranoid psychosis, or delusional disorder and 10 who met criteria for a nonpsychotic disorder. By May 2007, 32 participants were identified who met criteria for a disorder within the schizophrenia spectrum. The use of different diagnostic systems was inevitable given the longitudinal nature of the present study. A number of recent studies use different diagnostic systems interchangeably for the spectrum (27) , and research suggests high diagnostic agreement across diagnostic systems for the spectrum (28) as well as comparable prevalence estimates for the spectrum between diagnostic systems (29) . Given these findings, we do not anticipate significant inconsistencies between diagnostic systems used in this study.
On the basis of the interviews and/or hospital records, we obtained adult diagnostic outcomes for 244 of the 265 participants (follow-up rate, 92%). The follow-up rate did not significantly differ by risk status (94/102 high-risk participants; 84/89 other-risk participants; 66/74 low-risk participants). The follow-up sample comprised three groups: children whose mother or father had a psychiatric hospital diagnosis of schizophrenia (N=94); children with a parent with a psychiatric record of hospitalization for a nonpsychotic disorder (N=84); comparison subjects drawn from the original cohort with no parental records of psychiatric hospitalization (N=66). In the original design of the study, an effort was made to match the comparison subjects to the high-risk subjects on the basis of gender, social class, and parents’ age (all participants were Caucasian). Although the majority of participants did not change risk status over time, some did so as a result of lifetime parental psychiatric hospitalization records ascertained through scanning of the Danish Psychiatric Central Registry in 2007. Given a shift in the original matching protocol, to assess whether groups were still equivalent based on demographic information, groups were compared on the above-mentioned demographic characteristics. No significant differences between risk groups were found. (Further consideration of this issue is taken into account in a subsequent statistical analysis.) Diagnostic outcome and risk status information are presented in Table 1 .
Participants received a complete description of the study and provided written informed consent. All procedures were in accordance with the ethical standards set forth with the committee on human experimentation of the Psykologisk Institute, Kommunehospitalet, and with the Helsinki Declaration of 1975.
The 1972 Neurological Examination
All 265 children were examined between ages 10 and 13 at the Psykologisk Institute in Copenhagen by an experienced child neurologist (N.M.) who was blind to information about the parents’ psychiatric status. A detailed description of the 1972 neurological examination has been provided elsewhere (20) . In brief, the examination consisted partly of subtests drawn from traditional adult neurological examinations, from subtests known from pediatric neurological examination procedures, and from motor performance tests described in the literature at the time (30) . We have previously reported results of tests of minor physical anomalies, ocular alignment, and laterality from the comprehensive battery used in the current study (22 , 31 , 32) . For this study, we were particularly interested in measures of coordination, an area with known associations with schizophrenia. A total of 13 coordination tasks were administered in the 1972 assessment. These tasks roughly covered the same areas of motor coordination functioning as described in the review by Boks et al. (3) and are consistent with major neurological batteries (33 , 34) . Four of these were excluded because of zero variance (left and right finger to nose and left and right heel to knee). The remaining nine tests were considered as measures of coordination: left diadochokinesia, right diadochokinesia, left finger opposition test, right finger opposition test, left speeded finger opposition test, right speeded finger opposition test, right index finger and right foot tap, right and left index finger and right foot tap, and right hand-left hand opens-closes.
Statistical Analyses
The coordination variables were generally scored on a continuum from normal to abnormal. The metric for certain tests varied, so we created a “coordination scale” by individually standardizing and summing the nine coordination measures; higher scores indicate poorer performance. To address rare instances of missing data (13 missing data points from a total of 2,385 possible), within-group mean substitution was employed. The distributional properties of the resulting coordination scale were such that they appeared not likely to violate assumptions of normality in analyses (skewness=1.20, SE=0.16; kurtosis=0.94, SE=0.31), and the internal consistency of the scale was high (α=0.89).
The primary analysis was a multinomial logistic regression performed to assess the ability of genetic risk and childhood coordination to predict adult diagnostic outcome. The dependent variable was diagnostic outcome (schizophrenia spectrum disorder, other mental disorder, no mental illness), with genetic risk and coordination serving as independent variables (predictors). To increase power for the primary analysis, genetic risk status was dichotomized into high-risk and non-high-risk. The potential interaction between genetic risk and coordination was also assessed. Participants shifted groups from the original case-control design, and therefore analyses were performed controlling for variables for which the original groups were matched (parental age, marital status, social class, and sex).
Results
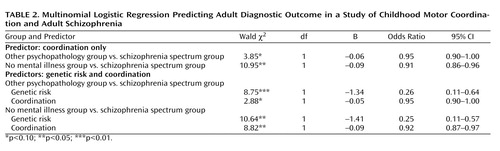
Our primary hypothesis was that the coordination scale assessed in childhood would predict those who would later develop schizophrenia spectrum disorders and those who would not. The average coordination scale score was highest for the spectrum disorders group (mean=3.37, SD=8.0), followed by the other psychopathology group (mean=0.38, SD=6.40) and the no mental illness group (mean=–1.03, SD=6.03). Given the importance of genetic risk factors for developing schizophrenia spectrum disorders, as well as the role of genetic risk in the design of the project, risk status was considered an independent variable in addition to coordination in predicting outcome. As there were only two individuals in the low-risk group who developed a spectrum disorder, the low-risk and other-risk groups were combined. A multinomial logistic regression was conducted to test the ability of genetic risk and the childhood coordination scale to predict adult diagnostic outcome. The overall model was significant (χ 2 =23.21, df=4, p<0.001) and yielded a Nagelkerke pseudo R 2 =0.11. Both the coordination scale and genetic risk status emerged as significant predictors ( Table 2 ). (We also ran the multinomial logistic regression considering all three levels of genetic risk, which yielded similar findings.) We also assessed a possible interaction between coordination and genetic risk and found no significant difference between the model with and without the interaction terms. We were also interested in whether coordination deficits were mediated by genetic risk. No evidence to support a mediational effect was found. To assess for mediation, given that the odds ratio in logistic regression is a measure of effect size (35) , we obtained the estimates for the mediation effects by subtracting the coefficients associated with coordination when the logistic model contained both coordination and genetic risk as predictors in the equation, from the coefficient of the model when coordination was the only predictor in the logistic model. Then we obtained the odds ratios of the coefficients of mediation effects by taking the exponent. For both spectrum disorders compared to no mental illness and spectrum disorders compared to other psychopathology, the odds ratios were close to 1, indicating almost no effect size (spectrum versus no mental illness, mediation effects odds ratio=0.99; spectrum versus other psychopathology, mediation effects odds ratio=0.99).
The project was originally designed as a case-control individually matched study of children with a parent with schizophrenia, a parent with a nonpsychotic disorder, and parents with no history of mental illness. Some participants, however, shifted risk groups as parental diagnostic status changed over time (i.e., some parents developed a mental illness after the birth of their child and after the initial risk groups were established). As a result, we conducted a second multinomial logistic regression controlling for variables for which the original groups were matched (sex, maternal age, paternal age, social status, and marital status). None of the controlling variables were significant, and the results for risk and coordination remained consistent with our original analysis.
Individual Coordination Scale Items
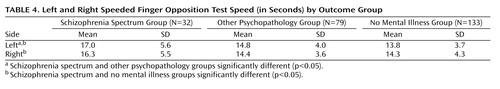
To provide a comprehensive view of our findings, Table 3 summarizes how each outcome group scored in terms of categories in which the individual coordination scale items were scored (“normal,” “suggestive,” and “definitive”). The odds ratios of having a normal versus non-normal score between the spectrum disorders and no mental illness groups are also presented. Table 4 displays results for the two continuously scored coordination variables, left and right speeded finger opposition test.
Discussion
Coordination Scale
Results from this study suggest childhood differences in coordination between those who do and do not develop a schizophrenia spectrum disorder in adulthood. Coordination deficits appeared specific to the spectrum group, as participants who eventually developed a schizophrenia spectrum disorder exhibited significantly poorer premorbid coordination scores compared to those who did not develop a mental illness, and nearly significantly (p=0.08) poorer premorbid coordination than those who developed a nonpsychotic mental illness in adulthood. These results were found while incorporating genetic risk, and they held when controlling for demographic variables. Our primary analyses involved a coordination scale consisting of several individual tests of coordination. The aggregate scale provided increased statistical power to detect differences relative to a single item and yielded an effect size in the high range (Cohen’s d=0.62) when comparing the spectrum disorders group to the no mental illness group.
The finding of elevated coordination deficits among those who eventually developed schizophrenia spectrum disorders is consistent with the overall findings in this research domain as well as with the few existing prospective studies. As in the reports of the Israeli High-Risk Survey (36) , the Swedish High-Risk Survey (9) , and the New York High-Risk Project (10) , motor abnormalities detected in infancy and/or childhood were associated with an increased risk of subsequent schizophrenia spectrum disorders. Findings from this current study diverge, however, from a landmark study by Walker et al. (17) in that the differences in motor functioning observed among infants in their study group were not observable beyond age 2. Substantial methodological differences may account for this inconsistency. Walker et al. rated motor function on the basis of the coding of spontaneous behaviors visible on home movies. In contrast, assessment of infant and child coordination in our study involved formal, highly structured, hands-on examinations performed by a pediatric neurologist. We believe that this approach is more likely to detect neurological soft signs.
This study included several other methodological advantages over previous studies, including an assessment of coordination blind to risk group and eventual diagnostic outcome; an analysis of a composite scale as well as of individual items; a familial psychiatric risk comparison group; and a relatively high number of individuals with a schizophrenia spectrum disorder at follow-up through middle age (ages 46–48). The unique methodological advantages we incorporated in this long-term longitudinal prospective study, along with previous studies documenting movement abnormalities beyond infancy (6 , 15) , together increase confidence in the conclusion that coordination deficits frequently antedate the diagnosis of schizophrenia spectrum disorders, perhaps by as much as two decades, and are detectable at a variety of developmental stages.
The results of this study clearly suggest direct effects between outcome and coordination and between outcome and genetic risk. The analyses did not support an interaction between genetic risk and coordination or a model whereby coordination deficits are mediated by genetic risk. Although it is important not to overinterpret null findings (especially in light of our imperfect measure of genetic risk), these findings suggest that coordination predicts over and above, and is independent of, genetic risk for schizophrenia. Possible neural and environmental explanations as to how coordination deficits might relay to adult schizophrenia outcome are described below.
Possible Mechanisms
Multiple motor systems, including the corticospinal/pyramidal, supplemental motor, basal ganglionic/extrapyramidal, and cerebellar systems, and their associated networks likely contribute to motor task performance in our coordination battery (37 , 38) . That being said, motor incoordination is classically attributed to dysfunction of the cerebellum and/or basal ganglia. This neurological understanding, originally derived from clinical-pathological correlations, has been confirmed by functional MRI studies and other strategies (see, for example, references 5 , 7 , 39 , 40) . Therefore, we consider it likely that dysfunctions in the cerebellum, basal ganglia, or both play a role in the observed coordination deficits in those who developed a schizophrenia spectrum disorder.
Rather than abnormalities in specific brain structures such as the cerebellum or the basal ganglia, several authors argue that disruptions of specific pathways (frontocerebellar dysfunction, striatal pathology) are responsible for neuromotor dysfunction in schizophrenia (19 , 41) . For example, Mittal et al. (40) suggest that, similar to minor physical anomalies, movement dysfunction potentially reflects subcortical brain dysfunction resulting from prenatal insults. This conclusion appears compatible with our own results, documenting coordination deficits well before symptom onset. Thus, it seems reasonable that both positions are true. That is, individuals with schizophrenia may exhibit both intrinsic dysfunction and neuroanatomical atypicality of the cerebellum and/or basal ganglia and disruption of the patterns of connectivity through which the cerebellum and perhaps the basal ganglia exert modifying effects on motor output. Regardless of the precise mechanisms and timing, the findings from this study implicate neural substrate involvement (structural, pathways, or both) in schizophrenia, early in the course of illness, prior to the emergence of psychotic symptoms.
Given the likely role of the cerebellum and other related circuits in coordination deficits, our findings might be viewed in the context of Andreasen and colleagues’ unitary model of “dysmetria” (42) . Andreasen et al. suggest that dysfunction in the cerebellum and cortico-cerebellar-thalamo-cortical circuits might be a unifying explanation for diverse motor, cognitive, and psychiatric symptoms of the illness. Recent studies suggest that cerebellar dysfunction might underlie some of the core features of the disease (e.g., cognitive abnormalities) (43) , and our findings provide further evidence that such cerebellar dysfunction precedes illness onset. Andreasen et al. suggest that the presence of coordination deficits indicates underlying abnormalities in basic cognitive processes (e.g., perception, associations) that could lead to misinterpretation of external and internal stimuli. Misinterpretation might account for schizophrenia symptoms, ultimately taking the form of psychotic processes (e.g., hallucinations, delusions, thought disorder, and negative symptoms).
Previous studies from this project have reported other neurodevelopmental markers and precursors to schizophrenia spectrum disorders that provide additional information about regions of possible disruption as well as timing of possible insults (e.g., increased minor physical anomalies, ocular disalignment, and atypical laterality) (22 , 31 , 32) . These findings, together with those from this study, support the presence of dysfunction of the cortico-cerebellar-thalamo-cortical circuit and perhaps other neural networks and processes (e.g., hemispheric asymmetry) early in life (possibly originating in the first and second trimesters of gestation), well before the onset of more downstream hallmark symptoms (i.e., delusions and hallucinations). However, additional studies are needed to pinpoint the specific regions or pathways, as well as the timing of disruption and developmental processes, that are responsible for the diverse symptomatic manifestations of schizophrenia, using more advanced techniques over time (43) .
From a diathesis-stress perspective (emphasizing environmental stress), research suggests that poor coordination is associated with a number of social, academic, and emotional consequences (44) . Additionally, there is evidence to support detrimental effects of coordination abnormalities over time (45) . Beyond the neurodevelopmental implications of our findings discussed above, it is reasonable to speculate that poor coordination in childhood engenders at least some taxing psychosocial encounters that may contribute to stress within a diathesis-stress framework. It is also reasonable to speculate that these stressful events further exacerbate preexisting coordination deficits as well as neurological vulnerabilities, resulting in a self-sustaining, iterative, and perhaps progressively detrimental process between coordination and stress.
This study suffers from some notable limitations. Despite a relatively large number of individuals who developed a schizophrenia spectrum disorder, the total number of people in this group limited the statistical power for some analyses. This is particularly true of the number of individuals who developed a spectrum disorder who were not in the high-risk group, and it might also have contributed to the finding of only a trend-level difference between the schizophrenia spectrum disorders group and other psychopathology group. Another concern, shared by all research on high-risk groups, is the issue of generalizability to those individuals who develop a spectrum disorder but who do not have a parent with schizophrenia. It is likely, however, that genetic influences play a role in most cases of schizophrenia, even if the parents fail to manifest the disorder phenotypically (46) . Finally, only one neurologist performed the neurological examinations, preventing an evaluation of interrater reliability. The neurologist was, however, highly trained and was functioning under strict research procedures and conditions.
Despite these limitations, given the strengths and uniqueness of this study, the findings advance the understanding of the development of schizophrenia in several ways. Detecting coordination deficits prospectively adds considerable support to the notion that coordination dysfunction precedes schizophrenia and may be a meaningful expression of an underlying biomarker. Applying what is known about the mechanisms of coordination deficits to the etiology of schizophrenia offers possible clues to how early neural deficits mediate the development of the disorder.
1. Chan RCK, Gottesman II: Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev 2008; 32:957–971Google Scholar
2. Bachmann S, Bottmer C, Schröder J: Neurological soft signs in first-episode schizophrenia: a follow-up study. Am J Psychiatry 2005; 162:2337–2343Google Scholar
3. Boks PM, Russo S, Knegtering R, van den Bosch RJ: The specificity of neurological signs in schizophrenia: a review. Schizophr Res 2000; 43:109–116Google Scholar
4. Bombin I, Arango C, Buchanan RW: Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull 2005; 31:962–977Google Scholar
5. Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schröder J: Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res 2005; 140:239–250Google Scholar
6. Compton MT, Bollini AM, McKenzie Mack L, Kryda AD, Rutland J, Weiss PS, Bercu Z, Esterberg ML, Walker EF: Neurological soft signs and minor physical anomalies in patients with schizophrenia and related disorders, their first-degree biological relatives, and non-psychiatric controls. Schizophr Res 2007; 94:64–73Google Scholar
7. Dazzan P, Morgan KD, Orr KG, Hutchinson G, Chitnis X, Suckling J, Fearon P, Salvo J, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM: The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain 2004; 127:143–153Google Scholar
8. Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray RM: School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry 1999; 56:457–463Google Scholar
9. McNeil TF, Harty B, Blennow G, Cantor-Graae E: Neuromotor deviation in offspring of psychotic mothers: a selective developmental deficiency in two groups of children at heightened psychiatric risk? J Psychiatr Res 1993; 27:39–54Google Scholar
10. Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II: Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry 2000; 157:1416–1422Google Scholar
11. Erlenmeyer-Kimling L: Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet 2000; 97:65–71Google Scholar
12. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Google Scholar
13. Wolf A, O’Disriscoll G: Motor deficits and schizophrenia: the evidence from neuroleptic-naive patients and populations at risk. J Psychiatry Neurosci 1999; 24:304–314Google Scholar
14. Dazzan P, Murrary RM: Neurological soft signs in first-episode psychosis: a systematic review. Br J Psychiatry 2002; 181:s5–s57Google Scholar
15. Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, Cannon TD: Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000; 26:367–378Google Scholar
16. Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S: Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry 2004; 161:2021–2027Google Scholar
17. Walker EF, Savoie T, Davis D: Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Google Scholar
18. Hans SL, Marcus J, Nuechterlein KH, Asarnow RF, Styr B, Auerbach JG: Neurobehavioral deficits at adolescence in children at risk for schizophrenia: the Jerusalem Infant Development Study. Arch Gen Psychiatry 1999; 56:741–748Google Scholar
19. Boks MPM, Liddle PF, Burerhof JGM, Knegtering R, van den Bosch RJ: Neurological soft signs discriminating mood disorders from first-episode schizophrenia. Acta Psychiatr Scand 2004; 110:29–35Google Scholar
20. Marcus J, Hans SL, Mednick SA, Schulsinger F, Michelsen N: Neurological dysfunctioning in offspring of schizophrenics in Israel and Denmark: a replication analysis. Arch Gen Psychiatry 1985; 42:753–761Google Scholar
21. Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK: Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res 2003; 60:239–258Google Scholar
22. Schiffman J, Ekstrom M, LaBrie J, Schulsinger F, Sorensen H, Mednick S: Minor physical anomalies and schizophrenia spectrum disorders: a prospective investigation. Am J Psychiatry 2002; 159:238–243Google Scholar
23. Mednick SA, Mura E, Schulsinger F, Mednick B: Perinatal conditions and infant development in children with schizophrenic parents. Soc Biol 1971; 18:S103–S113Google Scholar
24. Spitzer RL, Williams JBW, Gibbon M, First MB: User’s Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
25. Wing JK, Cooper JE, Sartorius N: The Measurement and Classification of Psychiatric Symptoms: An Instructional Manual for the PSE and CATEGO Programs. New York, Cambridge University Press, 1974Google Scholar
26. Dalman CH, Broms J, Cullberg J, Allebeck P: Young cases of schizophrenia identified in a national inpatient register: are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol 2002; 37:527–531Google Scholar
27. Hwang R, Shinkai T, DeLuca V, Muller DJ, Ni X, Macciardi F, Potkin S, Lieberman JA, Meltzer HY, Kennedy J: Association study of 12 polymorphisms spanning the dopamine D 2 receptor gene and clozapine treatment response in two treatment refractory/intolerant populations. Psychopharmacology 2005; 181:179–187 Google Scholar
28. Jakobsen KD, Frederiksen JN, Parnas J, Werge T: Diagnostic agreement of schizophrenia spectrum disorders among chronic patients with functional psychoses. Psychopathology 2006; 39:269–276Google Scholar
29. Lindstrom E, Widerlov B, Von Knorring L: The ICD-10 and DSM-IV diagnostic criteria and the prevalence of schizophrenia. Eur Psychiatry 1997; 12:217–223Google Scholar
30. Rutter M, Graham P, Yule W: A Neuropsychiatric Study of Childhood. Philadelphia, JB Lippincott, 1970Google Scholar
31. Schiffman J, Maeda JA, Hayashi K, Michelsen N, Sorensen HJ, Ekstrom M, Abe KA, Chronicle EP, Mednick SA: Premorbid childhood ocular alignment abnormalities and adult schizophrenia-spectrum disorder. Schizophr Res 2006; 81:253–260Google Scholar
32. Schiffman J, Pestle S, Mednick S, Ekstrom M, Sorensen H, Mednick S: Childhood laterality and adult schizophrenia spectrum disorders: a prospective investigation. Schizophr Res 2005; 72:151–160Google Scholar
33. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989; 27:335–350Google Scholar
34. Chen EYH, Shapleske J, Luque R, McKenna PJ, Hodges JR, Calloway P, Hymas NFS, Dening TD, Berrios GE: The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res 1995; 56:183–204Google Scholar
35. Monahan PO, McHorney CA, Stump TE, Perkins AJ: Odds ratio, delta, ETS classification, and standardization measures of DIF magnitude for binary logistic regression. Journal of Educational and Behavioral Statistics 2007; 32:92–109Google Scholar
36. Marcus J, Hans SL, Nagler S, Auerbach JG, Mirsky AF, Aubrey A: Review of the NIMH Israeli Kibbutz-City Study and the Jerusalem Infant Development Study. Schizophr Bull 1987; 13:425–438Google Scholar
37. Chan RC, Rao H, Chen EE, Ye B, Zhang C: The neural basis of motor sequencing: an fMRI study of healthy subjects. Neurosci Lett 2006; 398:189–194Google Scholar
38. Whitty PF, Owoeye O, Waddington JL: Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull 2009; 35:415–424Google Scholar
39. Mittal VA, Hasenkamp W, Sanfilipo M, Wieland S, Angrist B, Rotrosen J, Duncan EJ: Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophr Res 2007; 94:37–44Google Scholar
40. Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF: The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry 2007; 61:1179–1186Google Scholar
41. Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV: Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophr Res 2002; 57:281–291Google Scholar
42. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M: Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908–920Google Scholar
43. Andreasen NC, Pierson R: The role of the cerebellum in schizophrenia. Biol Psychiatry 2008; 64:81–88Google Scholar
44. Cummins A, Piek JP, Dyck MJ: Motor coordination, empathy, and social behaviour in school-aged children. Dev Med Child Neurol 2005; 47:437–442Google Scholar
45. Losse A, Henderson SE, Elliman D, Hall D, Knight E, Jongmans M: Clumsiness in children: do they grow out of it? a 10-year follow-up study. Dev Med Child Neurol 1991; 33:55–68Google Scholar
46. Cannon TD, Mednick SA, Parnas J, Shulsinger F, Praesthold J, Vestergaard A: Developmental brain abnormalities in schizophrenia: contributions of genetic and perinatal factors: reply (letter). Arch Gen Psychiatry 1996; 52:157–159Google Scholar