Treatment-Resistant Depression and Mortality After Acute Coronary Syndrome
Abstract
Depression is a risk factor for morbidity and mortality in patients with coronary heart disease, especially following acute coronary syndrome. Evidence from recent clinical trials suggests that treatment-resistant depression may be associated with a particularly high risk of mortality or cardiac morbidity in patients following acute coronary syndrome. This article reviews this evidence and considers possible explanations for this relationship. Directions for future research are also considered, with particular emphasis on efforts to elucidate the underlying mechanisms and to develop more efficacious treatments for depression in patients with coronary heart disease.
Depression is a risk factor for morbidity and mortality in coronary heart disease. A meta-analysis of 22 studies found that major depression more than doubles the risk of mortality after an acute myocardial infarction (1) . There has been less research on the prognostic importance of depression after hospitalization for unstable angina, but in one study, depression increased the risk of nonfatal myocardial infarction or cardiac death more than fourfold after an episode of unstable angina (2) . Thus, depression is a significant risk factor in both forms of acute coronary syndrome.
It has been clear for some time that the risk of mortality is not uniformly high among depressed acute coronary syndrome patients, but little progress has been made in differentiating between high- and low-risk forms of depression following acute coronary syndrome. However, converging evidence from several recent studies suggests the possibility that treatment-resistant major depression may be a distinctly high-risk form of depression in patients following acute coronary syndrome.
Treatment-Resistant Depression
Treatment resistance has been defined in a variety of ways in the depression literature (3) . Failure to respond to a single trial of monotherapy is the most inclusive definition, Thase and Rush’s five-stage model (4) is the most restrictive, and failure to respond to two or more monotherapies is one of the most common. About 50% of depressed patients have an adequate response to antidepressant therapy, and about 15% have a partial response, but between 20% and 35% are classified as nonresponders (5) . In addition, some patients actually become more severely depressed after the initiation of treatment. Thus, there is a wide range of responses to treatment of depression, extending from full remission to partial response, nonresponse, or deterioration.
Various approaches for overcoming treatment resistance have been tried, including sequential, combination, and augmentation therapies. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial is the largest effort to date to identify effective strategies for treating refractory depression under conditions similar to those encountered in clinical practice (6) . Over 4,000 outpatients with nonpsychotic unipolar depression were enrolled in STAR*D. Many of them had psychiatric or medical comorbidities. All of the participants were initially treated with citalopram and given a higher dose (55 mg/day) for a longer duration (12 weeks) than is usually provided in routine care. Remission of depression was defined as a score of ≤7 on the 17-item Hamilton Depression Rating Scale (HAM-D) or ≤5 on the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR). Response was defined as a ≥50% reduction in the QIDS-SR score.
Two popular strategies for treating depression nonresponsive to this regimen were tested in the STAR*D trial: switching to a different antidepressant or augmenting citalopram with a second drug or with cognitive therapy. About 25% of patients whose depression did not respond to citalopram experienced remission after switching to a second antidepressant (7) . A slightly higher percentage achieved remission after augmentation of citalopram with bupropion (7 , 8) . Thus, about 50% of the participants experienced remission, either during the initial citalopram-only phase or during the switching or augmentation phase. Response but not full remission was achieved in another 20%. Over 30% did not experience full remission even after trying two additional antidepressants or cognitive therapy for a total of up to four treatments. Those who did respond to either the third or fourth treatment had a high relapse rate (9) . Antidepressant trials often produce more favorable outcomes than these, but most of them exclude patients with psychiatric or medical comorbidities such as heart disease. Although the STAR*D findings are encouraging, they suggest that a substantial minority of patients do not experience remission, or respond only partially, even with state-of-the-art treatment for depression. Even prior to STAR*D, it was known that between 20% and 30% of depressed patients fail to respond even to multiple agents (5 , 10 , 11) .
Cardiac Events and Treatment-Resistant Depression
The ENRICHD Study
The Enhancing Recovery in Coronary Heart Disease (ENRICHD) study was a multicenter, randomized, controlled clinical trial designed to determine whether treating depression and low perceived social support reduces the risk of recurrent infarction and death after an acute myocardial infarction (12) . Patients with major or minor depression, and/or low perceived social support, were randomly assigned to usual care or an intervention that provided up to 6 months of cognitive behavior therapy (CBT). In addition, sertraline was given for up to 1 year to patients in the intervention arm who either had severe depression (HAM-D 17 score >25) at enrollment or whose score on the Beck Depression Inventory (BDI) did not improve at least 50% after 6 sessions of CBT. Among the depressed patients, 6-month mean BDI change scores were –8.6 (SD=9.2) and –5.8 (SD=8.1) in the intervention and usual care arms, respectively. However, there was no between-group difference in reinfarction-free survival during a median of 29 months of follow-up (13) .
However, among patients with major depression in the intervention group, those who did not experience treatment response had a higher risk of late mortality (i.e., death occurring ≥6 months after the acute myocardial infarction) relative to those whose depression responded to treatment (14) . Patients whose depression worsened by ≥10 BDI points despite treatment were 1.6 times more likely to die in the ensuing months than were those who merely failed to improve (i.e., no or minimal change in BDI score), and 2.5 times as likely to die as those who improved by 10 or more points on the BDI. These effects were independent of the baseline BDI score, antidepressant use, and established predictors of mortality following myocardial infarction, including left ventricular ejection fraction, age, and prior history of myocardial infarction. Curiously, although there was a strong relationship between change in depression and late mortality in the intervention arm, the relationship was not significant in the usual care arm ( Figure 1 ). Although fewer subjects in the intervention (15%) than the usual care (26%) arm failed to show any improvement in BDI score from baseline to 6 months (defined as a 6-month BDI score that was equal to or higher than the baseline BDI score), the mortality rate among nonimprovers was higher in the intervention arm (21%) than in the usual care arm (10%) of the trial.
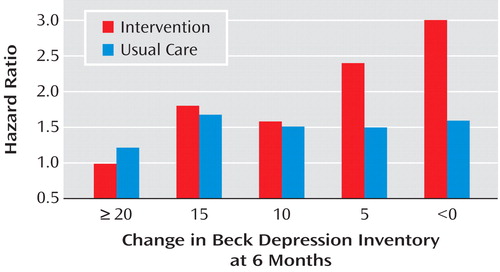
For patients in the intervention group, the lack of improvement occurred despite receiving 6 months of aggressive treatment. However, only about 15% of the usual care patients received any form of nonstudy treatment for their depression during the first 6 months. Even fewer of the patients in the usual care arm who did not experience improvement had received any depression treatment. Some of them might not have achieved remission even if they had been treated, but others might have responded very well to treatment had it been provided. Although the subgroup sample sizes were small and the effect was not significant, there was a stronger relationship between treatment nonresponse and late mortality among patients in the intervention group who received both sertraline and CBT than those who received only CBT. Similarly, among patients in the usual care group who took nonstudy antidepressants, there was a twofold difference in mortality between those with the best and worst treatment responses (14) . Relatively few usual care patients were treated, and the effect was not statistically significant. Nevertheless, these findings suggest that exposure to the ENRICHD intervention identified patients with a high-risk subtype of depression, i.e., depression that does not respond to standard antidepressant therapy.
Other Clinical Trials
There is evidence from other clinical trials supporting this conclusion. The Myocardial INfarction and Depression Intervention Trial (MIND-IT) compared 24 weeks of usual care versus mirtazapine versus placebo, followed by open-label citalopram among those whose illness was not responsive to treatment (15) . Like the ENRICHD study, MIND-IT failed to demonstrate the superiority of the study interventions over usual care with respect to cardiac event-free survival during an average of 27 months of follow-up (15) .
In a recent secondary analysis, de Jonge et al. (16) classified patients in the MIND-IT intervention group as those who experienced response (≥50% improvement on the HAM-D at 24 weeks) or nonresponse (<50%). They compared these two subgroups to patients in the usual care arm who did not receive any treatment for depression. The 18-month incidence of cardiac events was 26% among intervention group patients whose illness was nonresponsive to treatment, 11% in untreated control subjects, and 7% among intervention group patients who experienced response (p<0.001). These findings are strikingly similar to the ENRICHD outcomes. Also like ENRICHD, the MIND-IT findings could not be explained by between-group differences in the initial severity of medical illness. Specifically, patients with treatment-responsive and -nonresponsive depression in MIND-IT did not differ in age, left ventricular ejection fraction, Killip class, the Charlson Comorbidity Index, or in the prevalence of diabetes, cerebrovascular disease, peripheral vascular disease, hypercholesterolemia, smoking, or prior revascularization.
The Montreal Heart Attack Readjustment Trial (MHART) tested the efficacy of a 12-month, home-based nursing intervention targeting emotional distress in post-myocardial infarction patients (17) . Although depression per se was not the primary target of the intervention, over one third of the intervention patients had clinically significant depression (BDI >10) at baseline. Like MIND-IT and ENRICHD, the MHART intervention also failed to improve post-myocardial infarction survival.
A 5-year follow-up of the usual care arm of MHART showed that improvement in depression after 1 year was associated with lower cardiac mortality only in patients who had mild depression at baseline (18) . There was no relationship between change in depression and subsequent mortality among patients who had moderate to severe depression at baseline, the very patients who may be considered for treatment in clinical settings.
This report did not include comparable analyses of the outcomes within the intervention arm. In unpublished analyses, however, the MHART investigators found a relationship between BDI change from baseline to 3 months and 5-year survival in the intervention (p<0.0001) but not in the usual care arm (p=0.98) (Frasure-Smith, personal communication, 2004). Only 6% of the patients in the intervention arm who were in the highest quintile of improvement on the BDI died within the first year, compared to 17% of patients in the lowest quintile. Thus, there is a striking similarity between the MHART and ENRICHD findings.
The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) was designed to determine the safety and efficacy of sertraline in patients with a recent acute coronary syndrome. At the completion of the trial, the sertraline and placebo arms did not differ on the HAM-D in the overall sample. However, there was a statistically significant difference in HAM-D outcomes in the subgroup with severe, recurrent major depression. There was also a trend toward fewer cardiac events in the sertraline arm (19) .
The SADHART investigators recently completed a long-term follow-up (median 6.6 years) of the trial participants. They found a significant relationship between improvement in depression during the 24 weeks of treatment and survival in both the sertraline and placebo arms, even after adjusting for other mortality risk factors (20) . Using the Clinical Global Impression (CGI) scale to measure improvement in depression following treatment, they found that the patients in both the placebo and sertraline groups with the most improvement (N=130) had the lowest rate of mortality (11.5%). For those with moderate improvement (N=80), 22.5% died; and for those whose depression minimally improved, worsened, or stayed the same following treatment (N=148), 28.4% died during the follow-up interval (p=0.001).
Unlike ENRICHD and MHART, the control group in SADHART also showed a relationship between improvement in depression and survival. However, a placebo condition and a usual care or no-treatment control group are not equivalent. A review of the efficacy data reported for placebo-controlled antidepressant trials found that the average HAM-D difference between drug and placebo groups is just 2 points (range=0.89 to 3.21) (21 , 22) . Although the placebo is pharmacologically inert, the clinical management that is provided to a patient in a double-blinded study is often perceived as supportive and beneficial. Simply meeting with patients to discuss their depression symptoms, encouraging them to take the pills as prescribed, and to return for the next scheduled visit may be therapeutic. For example, in a study of 248 patients with coronary heart disease, Lespérance et al. (23) found greater depression improvement in patients who received only clinical management compared with those who received clinical management plus interpersonal psychotherapy, a recognized treatment for depression in psychiatric patients.
The relationship between treatment-resistant depression and cardiac mortality and morbidity extends beyond traditional treatments for depression. Milani and Lavie (24) studied 522 coronary heart disease patients in a cardiac rehabilitation aerobic exercise program. The participants were assessed for depression symptoms before and after the program. A comparison group (N=179) was assessed at baseline but not at follow-up. The mortality rate among the depressed patients who completed the training but who remained depressed was significantly higher (22%) than that of the nondepressed participants (5%) and of the initially depressed patients whose mood improved following the exercise program (8%, p=0.0004). Thus, patients whose depression did not respond to exercise training had a three- to fourfold higher risk of dying than depression responders and nondepressed patients. Exercise training can therefore be added to the list of depression interventions, including sertraline, mirtazapine, citalopram, cognitive behavior therapy, and stress management, in which nonresponse is associated with an increased risk of mortality.
Milani and Lavie’s (24) findings are consistent with our hypotheses. However, they do not reveal whether patients with persistent, untreated depression (a subgroup which includes both potential treatment responders and potential nonresponders) are also at increased risk for mortality because they did not assess depression in the comparison patients during follow-up.
Taken together, these findings, especially those from the ENRICHD, MIND-IT, SADHART, and MHART clinical trials, suggest that unsuccessful treatment of depression after hospitalization for acute coronary syndrome identifies a high-risk patient subgroup. This may help to explain the failure of ENRICHD and the other clinical trials to improve survival. Although depression may have improved slightly more on average in the patients who received the intervention than in those who received usual care, the patients who were at the highest risk for cardiac events did not improve despite treatment, and they were at higher risk for cardiac events after treatment than were the responders. Because the participants in these trials were randomly assigned to treatment or control conditions, patients with potentially treatment-resistant depression were probably equally distributed between the groups. In the usual care groups, in which most patients did not receive any nonstudy treatment for depression, the potential nonresponders cannot be easily distinguished from potential responders who were simply never treated.
Clinical Characteristics of Depression Treatment Nonresponse
What are the characteristics of patients whose illness does not respond well to standard treatments for depression? There have been many efforts to identify predictors of poor response to treatment in medically well, depressed psychiatric patients. Misdiagnosis, suboptimal treatment, intolerance to the side effects of the drug, and poor adherence to the treatment regimen are clearly responsible for poor response to antidepressant treatments in many cases (25 , 26) . Some psychiatric comorbidities, especially cognitive dysfunction, substance abuse, anxiety disorders, and personality disorders, also negatively affect depression treatment outcome in patients with unipolar major depression (27 , 28) . Having specific medical comorbid conditions, such as thyroid dysfunction and rheumatoid arthritis, diminishes antidepressant efficacy (25 , 26) , but merely having one or more chronic medical illnesses is not reliably associated with a poorer response. Long duration of the present episode has consistently been a strong predictor of a poor response (10 , 11 , 25 , 26) , as has a family history of depression (28) . Except for psychosis, the clinical characteristics of the depressive episode do not consistently predict treatment response (25) .
The explanation for why depression that is unresponsive to treatment is associated with a greater risk for cardiac-related morbidity and mortality is not immediately apparent. However, a number of biological markers that have been shown to predict poor response to depression treatment in psychiatric patients have also been identified as risk markers for cardiac events. These have also been suggested as possible mechanisms underlying the effect of depression on mortality in patients with coronary heart disease.
Inflammatory Markers and Depression Treatment Resistance
Elevated inflammatory markers consistent with an acute phase immunological response (29 – 34) , HPA axis and autonomic nervous system dysregulation (35 – 38) , low thyroid hormone levels (38) , and low plasma levels of omega-3 free fatty acids (39) , have all been associated with poor response to a variety of antidepressants. In separate studies, they have also been identified as risk factors for cardiac morbidity and mortality.
Several sleep abnormalities that have cardiovascular effects have also been linked to antidepressant treatment resistance, including poor subjective sleep quality, abnormal sleep architecture, especially shortened REM latency (40) , and both central and obstructive sleep apnea (41) . Poor sleep quality (42) , sleep apnea (43) , subclinical hypothyroidism (44 , 45) , and low plasma levels of omega-3 free fatty acids (46) have also been associated with alterations in autonomic nervous system activity and/or elevated inflammatory markers, suggesting possible connections to other cardiac risk markers and depression treatment resistance.
Few studies have examined relationships between cardiac risk factors and treatment-resistant depression in patients with coronary heart disease. Shimbo and his colleagues (32) classified patients with a recent acute coronary syndrome into two groups: those having no depressive symptoms (≤4 on the BDI), and those with at least mild depression (BDI ≥10). They found that elevated C-reactive protein at baseline tended (p=0.10) to be more common in patients who remained depressed after 3 months (95%) than in those whose depression improved (79%) or in those who were never depressed (73%). At 3 months, C-reactive protein was elevated in 71%, 54%, and 31% of each respective group. This was a natural history study, and many of the depressed patients presumably did not receive any depression treatment. For the patients who did receive treatment, we would expect to see larger differences between the groups, with higher C-reactive protein at baseline in true treatment nonresponders, and improvement in C-reactive protein and other risk markers in patients whose depression improved with treatment.
Vascular Depression
It is possible that patients with coronary heart disease who do not respond to depression treatment have a qualitatively different form of depression than patients whose depression improves with treatment. Cerebrovascular comorbidity is common in elderly patients with advanced coronary heart disease, and there is evidence that some cases of late-onset depression may be caused or exacerbated by cerebrovascular disease, a condition referred to as “vascular depression” (47) . Neuroimaging studies of first-episode depressions occurring late in life have found evidence of structural and functional brain abnormalities in some of these patients (48) .
Although the patients in ENRICHD or MIND-IT did not undergo neuroimaging studies, there was little evidence in either study to support overt or subclinical cerebrovascular disease as an explanation for poor response to treatment. Contrary to what would have been expected if patients with cerebrovascular depression had comprised a large proportion of those with treatment nonresponse, the ENRICHD study revealed no differences between the improved and unimproved groups in age at enrollment, history of hypertension or stroke, age at time of the initial depressive episode (mid-30s, on average), the number of prior major depression episodes, the duration of the current episode, or the proportion of patients with a family history of depression. Nevertheless, the possible contribution of subclinical cerebrovascular disease to nonresponse in these studies cannot be ruled out. Similarly, all of the trials reviewed here, especially ENRICHD, SADHART, and MIND-IT, controlled for predictors of cardiac mortality and morbidity, and the ENRICHD therapists’ ratings of their patients’ physical ability to participate in the intervention during the course of treatment did not differ between experiencing treatment response versus nonresponse, but it remains possible that a deterioration in cardiac function that was not predicted by the conventional risk factors was responsible for the poor response to treatment and subsequent death.
Initial Versus Recurrent Major Depressive Episode
Another series of secondary analyses of clinical trials of depression treatment and survival following acute coronary syndrome have focused on whether the depressive episode was either the first ever or a recurrent episode, and whether it preceded or followed the acute cardiac event. Most of these studies have found that first depressive episodes and those that begin after the index cardiac event are more predictive of mortality and morbidity than are episodes that are recurrent or that precede the onset of the myocardial infarction (49 – 53) . For example, participants in the ENRICHD clinical trial with a first episode of major depression had poorer survival (18.4% all-cause mortality) than those with recurrent major depression (11.8%), and both groups had significantly poorer survival than did the nondepressed participants (3.4% all-cause mortality). Moreover, Glassman et al. (54) found that patients with either recurrent major depression or a depressive episode with onset preceding an acute coronary syndrome responded better to sertraline than did those with an initial episode of depression or whose episode began after the acute event. Lespérance et al. (23) reported a similar finding for citalopram in patients with medically stable coronary artery disease. Thus, the findings of treatment response and survival may be linked to whether the depression is a recurrent or an initial episode of major depression. However, no relationship between improvement in BDI scores and recurrent versus first depression was observed in a recent analysis of the ENRICHD clinical trial (49) . Nevertheless, the relationship between response to depression treatment, depression history, and survival deserves more careful study.
Future Directions
Although of obvious importance to the field of psychiatry, remarkably little is known about the biology of treatment-resistant depression. There is, however, growing interest in identification of the genes associated with the treatment mechanisms, response, or tolerance to antidepressants (55 – 58) . Some of the genetic factors identified through pharmacogenetic research, such as the serotonin transporter gene polymorphism (55 , 57) , may also have a role in promoting coronary disease (59) . This area clearly needs further study.
Most studies of treatment-resistant depression have focused primarily on pharmacological treatments, although some of the same factors that predict poor response to antidepressants have also been associated with poor response to psychotherapy (60) . However, a better understanding of the factors predicting poor response to psychotherapy is also needed. Furthermore, there are few depression treatment studies of any type in patients with coronary heart disease. Factors associated with treatment resistance may differ in this subgroup of depressed patients. For the purpose of improving medical outcomes following acute coronary syndrome, more research focusing on depressed patients with coronary heart disease is also needed.
If specific cardiac risk markers are found to predict treatment resistance in patients with coronary heart disease, it might be possible to improve depression and possibly even survival in these patients by aggressively treating the cardiovascular risk factors, or by choosing depression treatments that also modify these risk factors. Research on this question could provide a direction for developing new antidepressants or other depression treatment strategies for these patients. For example, if subclinical hypothyroidism is found to be commonly associated with depression treatment resistance in patients with coronary heart disease, then patients with low levels of thyroid hormone who do not respond to depression treatment could receive T3 augmentation of standard antidepressants. If elevated inflammatory markers are associated with treatment resistance in coronary heart disease patients, for example, then patients who do not respond to antidepressants could be tried on an anti-inflammatory drug or on specific cytokine antagonists along with standard depression treatment.
What should practitioners do in the meantime? Nonresponse to depression treatment should be considered a cardiac risk marker in patients with coronary disease. These patients should be followed more closely and perhaps be given more aggressive cardiological care than might otherwise seem warranted. Every effort should be made to intervene in all other modifiable risk factors and to treat all comorbid medical disorders to the extent possible. More aggressive treatment for depression may also be warranted, but further research is needed to develop more effective ways to treat coronary heart disease patients with depression that does not respond to first-line interventions.
1. van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van de Berg MP: Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822Google Scholar
2. Lesperance F, Frasure-Smith N, Juneau M, Theroux P: Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360Google Scholar
3. Keller MB: Issues in treatment-resistant depression. J Clin Psychiatry 2005; 66(suppl 8):5–12Google Scholar
4. Thase ME, Rush AJ: When at first you do not succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry 1997; 58(suppl 13):23–29Google Scholar
5. Fava M, Davidson KG: Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996; 19:179–200Google Scholar
6. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team: Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163:28–40Google Scholar
7. Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ; STAR*D Study Team: Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354:1243–1252Google Scholar
8. Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M; STAR*D Study Team: Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006; 354:1231–1242Google Scholar
9. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M: Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Google Scholar
10. Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J: Long-term outcome of episodes of major depression: clinical and public health significance. JAMA 1984; 252:788–792Google Scholar
11. Paykel ES: Epidemiology of refractory depression, in Refractory Depression: Current Strategies and Future Directions. Edited by Nolen WA, Zohar J, Roose SP, Amsterdam JD. Chichester, UK, Wiley, 1994, pp 3–18Google Scholar
12. The ENRICHD Investigators: Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD): study design and methods. Am Heart J 2000; 139:1–9Google Scholar
13. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N; Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD): Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116Google Scholar
14. Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, Cornell C, Saab PG, Kaufmann PG, Czajkowski SM, Jaffe AS; ENRICHD Investigators: Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004; 66:466–474Google Scholar
15. van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, Schins A, Tulner D, van den Berg MP, Ormel J; MIND-IT investigators: Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry 2007; 190:460–466Google Scholar
16. de Jonge P, Honig A, van Melle JP, Schene AH, Kuyper AM, Tulner D, Schins A, Ormel J; MIND-IT Investigators: Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry 2007; 164:1371–138Google Scholar
17. Frasure-Smith N, Lesperance F, Prince RH, Verrier P, Garber RA, Juneau M, Wolfson C, Bourassa MG: Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479Google Scholar
18. Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG: Five-year risk of cardiac mortality in relation to initial severity and 1-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105:1049–1053Google Scholar
19. Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr., Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M; Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group: Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709Google Scholar
20. Glassman AH, Bigger JT, Gaffney M: Psychiatric characteristics associated with long-term mortality among 361 acute coronary syndrome patients with major depression: seven year follow-up of SADHART participants. Arch Gen Psychiatry (in press)Google Scholar
21. Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R: Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008; 358:252–260Google Scholar
22. Kirsch I, Moore TJ, Scoboria A, Nicholls SS: The emperor’s new drugs: an analysis of antidepressant medication data submitted to the US Food and Drug Administration. Prevention & Treatment 2002; 5Google Scholar
23. Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC; CREATE Investigators: Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379Google Scholar
24. Milani RV, Lavie CJ: Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120:799–806Google Scholar
25. Scott J: Predictors of nonresponse to antidepressants in Refractory Depression: Current Strategies and Future Directions. Edited by Nolen WA, Zohar J, Roose SP, Amsterdam JD. Chichester, UK, Wiley, 1994, pp 19–28Google Scholar
26. Souery D, Lipp O, Massat I, Mendlewicz J: The characterization and definition of treatment-resistant mood disorders, in Treatment-Resistant Mood Disorders. Edited by Amsterdam JD, Hornig M, Nierenberg AA. Cambridge, UK, Cambridge University Press, 2001, pp 3–29Google Scholar
27. Rush AJ, Wisniewski SR, Warden D, Luther JF, Davis LL, Fava M, Nierenberg AA, Trivedi MH: Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry 2008; 65:870–880Google Scholar
28. Fagiolini A, Kupfer DJ: Is treatment-resistant depression a unique subtype of depression? Biol Psychiatry 2003; 53:640–648Google Scholar
29. Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E: Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26:1167–1170Google Scholar
30. Lanquillon S, Krieg JC, ing-Abu-Shach U, Vedder H: Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22:370–379Google Scholar
31. Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H: Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997; 9:853–858Google Scholar
32. Shimbo D, Rieckmann N, Paulino R, Davidson KW: Relation between C reactive protein and depression remission status in patients presenting with acute coronary syndrome. Heart 2006; 92:1316–1318Google Scholar
33. Vedder H, Lanquillon S, Krieg J-C: Cytokine secretion and treatment response in major depression. Biol Psychiatry 2000; 47(Aug suppl 1):S89Google Scholar
34. Yu YW, Chen TJ, Hong CJ, Chen HM, Tsai SJ: Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology 2003; 28:1182–1185Google Scholar
35. Christensen P, Lolk A, Gram LF, Kragh-Sorensen P, Pedersen OL, Nielsen S: Cortisol and treatment of depression: predictive value of spontaneous and suppressed cortisol levels and course of spontaneous plasma cortisol. Psychopharmacology (Berl) 1989; 97:471–475Google Scholar
36. de Guevara MS, Schauffele SI, Nicola-Siri LC, Fahrer RD, Ortiz-Fragola E, Martinez-Martinez JA, Cardinali DP, Guinjoan SM: Worsening of depressive symptoms 6 months after an acute coronary event in older adults is associated with impairment of cardiac autonomic function. J Affect Disord 2004; 80:257–262Google Scholar
37. Fraguas R Jr, Marci C, Fava M, Iosifescu DV, Bankier B, Loh R, Dougherty DD: Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Res 2007; 151:169–172Google Scholar
38. Wokowitz OM, Reus VI: Psychoneuroendocrine aspects of treatment-resistant mood disorders, in Treatment-Resistant Mood Disorders. Edited by Amsterdam JD, Hornig M, Nierenberg AA. Cambridge, UK, Cambridge University Press, 2001, pp 49–79Google Scholar
39. Peet M, Horrobin DF: A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002; 59:913–919Google Scholar
40. Buysse DJ, Hall M, Begley A, Cherry CR, Houck PR, Land S, Ombao H, Kupfer DJ, Frank E: Sleep and treatment response in depression: new findings using power spectral analysis. Psychiatry Res 2001; 103:51–67Google Scholar
41. Szuba MP, Fernando AT, Groh-Szuba G: Sleep abnormalities in treatment-resistant mood disorders, in Treatment-Resistant Mood Disorders. Edited by Amsterdam JD, Hornig M, Nierenberg AA. Cambridge, UK, Cambridge University Press, 2001, pp 96–110Google Scholar
42. Motivala SJ, Sarfatti A, Olmos L, Irwin MR: Inflammatory markers and sleep disturbance in major depression. Psychosom Med 2005; 67:187–194Google Scholar
43. Keyl C, Lemberger P, Rodig G, Dambacher M, Frey AW: Changes in cardiac autonomic control during nocturnal repetitive oxygen desaturation episodes in patients with coronary artery disease. J Cardiovasc Risk 1996; 3:221–227Google Scholar
44. Christ-Crain M, Meier C, Guglielmetti M, Huber PR, Riesen W, Staub JJ, Müller B: Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis 2003; 166:379–386Google Scholar
45. Sahin I, Turan N, Kosar F, Taskapan C, Gunen H: Evaluation of autonomic activity in patients with subclinical hypothyroidism. J Endocrinol Invest 2005; 28:209–213Google Scholar
46. Leaf A, Weber PC: Cardiovascular effects of n-3 fatty acids. N Engl J Med 1988; 318:549–557Google Scholar
47. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M: “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922Google Scholar
48. Krishnan KR, Hays JC, Blazer DG: MRI-defined vascular depression. Am J Psychiatry 1997; 154:497–501Google Scholar
49. Carney RM, Freedland KE, Steinmeyer B, Blumenthal JA, de Jonge P, Davidson KW, Czajkowski SM, Jaffe AS: History of depression and survival following acute myocardial infarction. Psychosom Med (in press)Google Scholar
50. de Jonge P, van den Brink RH, Spijkerman TA, Ormel J: Only incident depressive episodes after myocardial infarction are associated with new cardiovascular events. J Am Coll Cardiol 2006; 48:2204–2208Google Scholar
51. Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, Creed F: New onset depression following myocardial infarction predicts cardiac mortality. Psychosom Med 2008; 70:450–455Google Scholar
52. Grace SL, Abbey SE, Kapral MK, Fang J, Nolan RP, Stewart DE: Effect of depression on 5-year mortality after an acute coronary syndrome. Am J Cardiol 2005; 96:1179–1185Google Scholar
53. Parker GB, Hilton TM, Walsh WF, Owen CA, Heruc GA, Olley A, Brotchie H, Hadzi-Pavlovic D: Timing is everything: the onset of depression and acute coronary syndrome outcome. Biol Psychiatry 2008 Jul 2Google Scholar
54. Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR: Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288Google Scholar
55. Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, McMahon FJ, Manji H, Lipsky RH: Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry 2007; 64:783–792Google Scholar
56. Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S: The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol Psychiatry 2008; 63:1103–1110Google Scholar
57. Murphy GM, Jr., Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF: Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry 2004; 61:1163–1169Google Scholar
58. Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ: Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry 2007; 164:1181–1188Google Scholar
59. Carney RM, Freedland KE: Depression and coronary heart disease: more pieces of the puzzle. Am J Psychiatry 2007; 164:1307–1309Google Scholar
60. Buysse DJ, Tu XM, Cherry CR, Begley AE, Kowalski J, Kupfer DJ, Frank E: Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry 1999; 45:205–213Google Scholar



