Psychostimulant Treatment and the Developing Cortex in Attention Deficit Hyperactivity Disorder
Abstract
Objective: While there has been considerable concern over possible adverse effects of psychostimulants on brain development, this issue has not been examined in a prospective study. The authors sought to determine prospectively whether psychostimulant treatment for attention deficit hyperactivity disorder (ADHD) was associated with differences in the development of the cerebral cortex during adolescence. Method: Change in cortical thickness was estimated from two neuroanatomic MRI scans in 43 youths with ADHD. The mean age at the first scan was 12.5 years, and at the second scan, 16.4 years. Nineteen patients not treated with psychostimulants between the scans were compared with an age-matched group of 24 patients who were treated with psychostimulants. Further comparison was made against a template derived from 620 scans of 294 typically developing youths without ADHD. Results: Adolescents taking psychostimulants differed from those not taking psychostimulants in the rate of change of the cortical thickness in the right motor strip, the left middle/inferior frontal gyrus, and the right parieto-occipital region. The group difference was due to more rapid cortical thinning in the group not taking psychostimulants (mean cortical thinning of 0.16 mm/year [SD=0.17], compared with 0.03 mm/year [SD=0.11] in the group taking psychostimulants). Comparison against the typically developing cohort without ADHD showed that cortical thinning in the group not taking psychostimulants was in excess of age-appropriate rates. The treatment groups did not differ in clinical outcome, however. Conclusions: These findings show no evidence that psychostimulants were associated with slowing of overall growth of the cortical mantle.
Psychostimulants represent the largest single class of psychotropic medication prescribed to children in the United States, with around 9% of all boys and 4% of girls receiving these medications for treatment of attention deficit hyperactivity disorder (ADHD) (1) . The long-term safety of psychostimulants is thus of great importance (2) . In two large randomized trials, psychostimulants were found to suppress growth rates during treatment, with a decrease of 1.3 cm per year in height and between 1.3 kg per year (for preschool age) and 2.5 kg per year (for school-age children) in weight from age-appropriate growth rates (3 , 4) . This finding raises the question of whether there might be similar effects on development of the brain.
A meta-analysis of neuroanatomic studies found that ADHD is characterized by reductions in gray and white lobar volumes, with some prefrontal cortical regions being particularly affected (5) . However, there have been few studies of the structural correlates of psychostimulant treatment. Castellanos et al. (6) found that prior psychostimulant treatment in children with ADHD was associated at study entry with greater white matter lobar volumes relative to those of stimulant-naive children with ADHD; volumes in the medication-treated group were also closer to the range of those in typically developing children, suggesting a neuroprotective effect of psychostimulants. Pliszka et al. (7) , in a study examining regions implicated in the pathogenesis of ADHD, similarly found that treatment with psychostimulants was associated with more normative volumes of the caudate and the anterior cingulate cortex (7) . While informative, these studies were cross-sectional, limiting inferences that can be made about the developmental effects of medication, and they examined change only within a priori defined regions of interest or at the level of entire lobes.
We recently reported evidence of a delay in cortical maturation in ADHD in a study examining the age at which cerebral cortical points reached their peak thickness—that is, the point at which childhood cortical thickening gave way to thinning (8) . Whereas typically developing children without ADHD reached peak cortical thickness in the frontal cortex around ages 7–8, in children with ADHD this developmental milestone was reached around ages 10–11. Throughout adolescence, both children with ADHD and healthy children show cortical thinning throughout nearly the entire cortex.
In this study, we examined whether treatment with psychostimulants affects these developmental trajectories. We selected a subset of patients from our cohort who underwent repeated neuroanatomic imaging and either received treatment with psychostimulants between scans or did not. We confined our examination to the age range of 9–20 years, which included most of those who were not taking psychostimulants during the interscan period. We compared the group not taking psychostimulants with both an age-matched group of ADHD youths who did receive psychostimulant treatment during the interscan period and a cohort of typically developing children without ADHD (9) . All data thus lay within a period of cortical development predominately characterized by thinning. To our knowledge, this is the first prospective study examining whether cortical development reflects differing treatment with psychostimulants.
Method
Participants
Patients with ADHD were drawn from our cohort of 223 youths with ADHD. Diagnosis was based on the Parent Diagnostic Interview for Children and Adolescents (10) , Conners’ Teacher Rating Scales (11) , and the Teacher Report Form (for more details on the entire cohort, see references 6 , 8 , 12 , 13) . Inclusion criteria for the present study were the availability of at least two neuroanatomic scans (leading to exclusion of 112 subjects) and of treatment histories from research case notes (leading to exclusion of another 32 subjects). This left 79 eligible participants with ADHD. Nineteen of them were not treated with medication between the two scans. Most of the scans in this group were acquired between ages 9 and 20, and data beyond this age range were sparse. We therefore confined the study to this range, which had the greatest data density. From the patients with ADHD who were treated with medication between the two scans, we selected an age-matched group of 24 subjects (the remaining subjects had data lying outside our selected age range). Thus the groups on and off medication between scans did not differ significantly in age. All children selected for this study had combined-type ADHD.
Baseline data for the study were collected during an initial day hospital assessment phase. Participants were then discharged to their treating physicians in the community. Decisions regarding psychostimulant treatment after that point were the joint responsibility of the patients, their families, and their physicians.
The cortical development of the ADHD groups was compared against a template of cortical development derived from 294 typically developing comparison subjects who contributed 620 neuroanatomic MRI scans, reported previously (9) . These children were matched in IQ and gender composition with the ADHD group, since both these variables have an impact on cortical development (9 , 14) . The institutional review board of the National Institutes of Health approved the research protocol. Written informed consent was obtained from parents, and assent to participate in the study was obtained from children.
Neuroimaging
T 1 -weighted images with contiguous 1.5-mm slices in the axial plane and 2.0-mm slices in the coronal plane were obtained using three-dimensional spoiled gradient recalled echo in the steady state on the same 1.5-T GE Signa scanner (General Electric, Milwaukee) (echo time=5 msec, repetition time=24 msec, flip angle=45 degrees, acquisition matrix=256×192, number of excitations=1, field of view=24 cm). Native MRI scans were registered into standardized stereotaxic space using a linear transformation and corrected for nonuniformity artifacts (15) . Registered and corrected volumes were segmented into white matter, gray matter, CSF, and background using an advanced neural net classifier (16) . To determine cortical thickness, a surface deformation algorithm was applied that first fits the white matter surface, then expands outward to find the gray matter-CSF intersection, defining a known relationship between each vertex of the white matter surface and its gray matter surface counterpart. Cortical thickness can thus be defined as the distance between these linked vertices (a total of 40,962 such vertices are calculated) (17) . White and gray matter surfaces were resampled into native space by inverting the initial stereotaxic transformation. Cortical thickness was then computed in native space. In order to improve the ability to detect population changes, each subject’s cortical thickness map was blurred using a 30-mm surface-based blurring kernel (18) . A 30-mm-bandwidth blurring kernel was chosen on the basis of population simulations indicating that this bandwidth maximized statistical power while minimizing false positives (18) . This kernel also preserves the capacity for anatomical localization as 30-mm blurring along the surface using a diffusion smoothing operator represents considerably less cortex than the equivalent volumetric Gaussian blurring kernel because it preserves cortical topologic features (18) .
Statistical Analyses
The primary variable of interest was the rate of change in raw cortical thickness, calculated as
rate of change=(CT 2– CT 1 )/(age 2– age 1 ),
where CT is the thickness (in mm) of each cortical point at the first (age 1 ) or second scan (age 2 ). The results of the cortical thickness analyses were visualized through projection onto a standard brain template, showing regions where treatment group differences in the rate of change of cortical thickness differed significantly in a t test for independent samples at an uncorrected p threshold of 0.05. Such visualization showed clustering of the cortical points with group differences; further analyses retained those clusters with a spatial extent of more than 50 vertices, and the mean cortical thickness of each cluster was used in further analyses.
Cortical thickness values for the ADHD groups were then contrasted with a template of typical cortical development, whose derivation has been described in detail elsewhere (9) . From this template, we estimated the expected cortical thickness for a typically developing child at the mean ages at the first and second scans.
Results
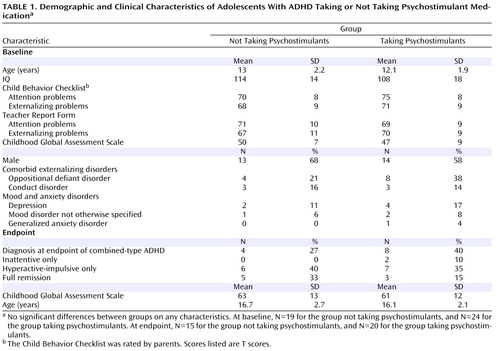
Demographic and Clinical Characteristics
The two ADHD groups—those taking and those not taking psychostimulants—did not differ significantly with respect to age, gender composition, IQ, or clinical characteristics ( Table 1 ). The mean age at the first scan was 12.5 years (SD=2.1), and at the second scan 16.4 years (SD=2.4). Outcome data at the time of the second scan, were available for 35 of the 43 patients. Neither the proportion of patients retaining a diagnosis of combined-type ADHD at follow-up nor a measure of global functioning differed significantly between groups.
At baseline, 40 of the 43 patients with ADHD (93%) were taking psychostimulants; 23 of the 24 (95.8%) in the group that took psychostimulants during the study were on stimulants at baseline, and 17 of the 19 (89.5%) who then went off psychostimulants during the study were taking stimulants at baseline. At follow-up, 11 of the 24 patients on psychostimulants were taking methylphenidate preparations, 11 were taking amphetamine preparations, and two were taking pemoline. The mean daily dose in methylphenidate equivalents was 35 mg (SD=22; range=5–85 mg). Four patients in the group taking psychostimulants were treated with second-line agents for ADHD (three with clonidine and one with guanfacine), and one patient in the group not taking psychostimulants was treated for several months with guanfacine. In the group taking psychostimulants, four patients were treated for depression (with desipramine, venlafaxine, sertraline, and nefazodone), one for generalized anxiety disorder (with fluvoxamine), and two for mood disorders not otherwise specified (both with sodium valproate). In the group not taking psychostimulants, two patients were treated for depression (with imipramine and bupropion) and one for a mood disorder not otherwise specified (with sodium valproate). The rates of comorbidity did not differ significantly between groups ( Table 1 ).
Neuroanatomic Analysis
The medication-defined ADHD groups differed significantly in the rate of change of cortical thickness in the left middle/inferior frontal gyrus (t=2.5, df=41, p=0.02), the medial and inferolateral aspect of the right precentral gyrus (t=2.5, p=0.02), and the right parieto-occipital region (t=2.3, p=0.02). Averaging the cortical thickness across all these regions, the significant difference (t=2.8, p=0.009) arose from more rapid cortical thinning in the group not taking psychostimulants, at a mean rate of loss of 0.15 mm/year (SD=0.17), compared with 0.03 mm/year (SD=0.11) for the group taking psychostimulants. The impact of these different rates of change on cortical thickness values at baseline and the endpoint are summarized in Figure 1 . At baseline there were no significant group differences, and by endpoint the off-psychostimulants group had a significantly thinner cortex than the on-psychostimulants group. All results held when gender and IQ were entered as covariates. The differential rates of cortical change in the left frontal and right medial prefrontal/motor but not the right posterior parieto-occipital region held after entering medication history prior to the baseline scan (as lifetime total dose in methylphenidate equivalents) as a covariate.

a Brain templates on the left show the regions where the two groups had a significantly different rate of cortical growth. The middle column shows the rate of change in raw cortical thickness in these regions, and the right-hand column shows the baseline and endpoint raw cortical thickness for each group and the age-expected values for a typically developing adolescent.
We compared the effects of different classes of psychostimulants, contrasting rates of cortical growth in those taking methylphenidate preparations against those taking amphetamine-based medication. The two patients taking pemoline were excluded from this analysis. Examining the mean rates of cortical change across the regions shown in Figure 1 , we found that the rate of cortical thinning was 0.03 mm/year (SD=0.09) in patients taking methylphenidate and 0.05 mm/year (SD=0.13) in those taking amphetamine, compared with 0.16 mm/year (SD=0.15) in those who were not taking psychostimulants (F=3.2, df=2, 38, p=0.05). The amphetamine and methylphenidate groups did not differ significantly from each other in rate of cortical change.
Discussion
Our results show no evidence that psychostimulants were associated with “slowing” of overall growth of the cortical mantle—a notable finding given reports of possible psychostimulant-related slowing of height and weight gain in children and adolescents (3 , 4) . Adolescents with ADHD who were not taking psychostimulants showed regional decreases in cortical thickness relative to both age-matched patients with ADHD who took psychostimulants and a typically developing cohort. The functional significance of this finding is unclear, however. We did not collect cognitive data at both time points in most participants. Also, the increased cortical thinning in the group that stopped taking psychostimulants was not associated with any difference in clinical outcome.
With these caveats in mind, it is worthwhile considering some possible interpretations. Psychostimulants tend to normalize goal-directed activity (19 – 22) and cognitive processes, including planning, cognitive flexibility, vigilance, and response inhibition (23) . In healthy adults, methylphenidate-induced improvement in working memory is associated with alterations of cerebral blood flow in the left dorsolateral prefrontal, supplementary motor, and posterior parietal cortex—overlapping in part with the regions we found to be differentially sensitive to psychostimulants (24) . In children with ADHD, psychostimulant-induced improvement in the ability to inhibit prepotent responses is associated with increased frontal (and striatal) activity as assayed by functional MRI (25) . In adults with ADHD, correction of executive deficits by psychostimulants is associated with altered prefrontal cortical activity—with increased activation of the premotor and decreased activation of the middle and medial prefrontal cortex (26) . Thus, psychostimulant-induced increases in age-appropriate levels of cognition and action, and perhaps underlying localized frontoparietal neural activity, might foster cortical development within the normative range. In this regard, psychostimulant effects on the developing brain in ADHD can be conceptualized as an example of activity-dependent neuroplasticity. Additionally, it is possible that psychostimulants have a direct trophic effect on the cortex, particularly in view of the growing evidence for the role of catecholaminergic neurotransmitters in cortical development (27 , 28) , although this explanation does not account for the highly regional effects detected in our study.
This study extends a previous demonstration of more normative white matter volumes in youths with a history of psychostimulant use by demonstrating effects on gray matter morphology (6) . Our longitudinal approach enabled detection of correlates of psychostimulant treatment on the rate of cortical development; this was not possible in our earlier cross-sectional analysis comparing cortical thickness at study entry in groups with differing psychostimulant histories (13) . Additionally, by using the metric of cortical thickness, determined at over 40,000 cortical points, we were able to detect more localized changes missed by lobar volumetric studies. The on- and off-psychostimulant groups were age-matched to ensure that any differences in cortical trajectories were not confounded by age effects.
In a recent study (8) , we demonstrated a delay in cortical maturation in most of the frontal (excluding the sensorimotor region) and temporal cortex using the age at peak cortical thickness as a developmental marker. Our ability to assess whether psychostimulant treatment contributes to this phenomenon is limited, because in the present study we focused on the adolescent phase of cortical thinning and did not examine the childhood phase of increase in cortical thickness. However, some considerations argue against psychostimulants being a major factor in the altered timing of maturation. The regions that were sensitive to medication were highly focal (unlike the disturbance in timing of maturation, which involved most of the cortex) and encompassed areas with both late (the dorsolateral prefrontal regions) and early maturation (the motor regions).
At the time of the first scan (around age 12) the ADHD medication groups did not differ significantly from each other in cortical thickness in the regions shown in Figure 1 , perhaps reflecting their similar history of medication exposure prior to the first scan. In prefrontal regions, the typically developing group attained peak cortical thickness earlier and thus entered the phase of cortical thinning earlier than did those with ADHD (8) . However, the typically developing group also reached a higher peak (i.e., a thicker cortex) and thus started thinning from a higher baseline. By age 12, the ADHD and typically developing cohorts we reported on previously (8) did not differ significantly in estimated cortical thickness in the prefrontal regions shown in Figure 1 (top left image).
In this observational study, it is important to consider the possibility that the group differences in cortical trajectories are attributable to other dimensions on which the groups differ. The groups did not differ in initial clinical characteristics or clinical outcome, so the findings were not related to differences in the severity of the disorder or clinical course. The groups also did not differ significantly on other variables known to affect cortical trajectories, such as gender and intelligence. Of course, the ideal study design for this question would be a randomized trial comparing cortical growth in children on psychostimulants against an unmedicated comparison group—but this would be both logistically and ethically challenging. Other limitations of the present study include the lack of external validation of treatment histories, which were based purely on patient and parent report. It is impossible to exclude neuroanatomic effects of the nonpsychostimulant medication received by the ADHD groups, although the prevalence of nonpsychostimulant medication use was low and did not differ between groups at the time of final assessment.
1. Centers for Disease Control and Prevention: Mental Health in the United States: Prevalence of Diagnosis and Medication Treatment for Attention-Deficit/Hyperactivity Disorder—United States, 2003. MMWR Morb Mortal Wkly Rep 2005; 54:842–847Google Scholar
2. Hyman SE: Methylphenidate-induced plasticity: what should we be looking for? Biol Psychiatry 2003; 54:1310–1311Google Scholar
3. Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M, Chuang S, Vitiello B, Skrobala A, Posner K, Abikoff H, Oatis M, McCracken J, McGough J, Riddle M, Ghuman J, Cunningham C, Wigal S: Stimulant-related reductions of growth rates in the PATS. J Am Acad Child Adolesc Psychiatry 2006; 45:1304–1313Google Scholar
4. MTA Cooperative Group: National Institute of Mental Health Multimodal Treatment Study of ADHD Follow-Up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004; 113:762–769Google Scholar
5. Valera EM, Faraone SV, Murray KE, Seidman LJ: Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:1361–1369Google Scholar
6. Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL: Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 2002; 288:1740–1748Google Scholar
7. Pliszka SR, Lancaster J, Liotti M, Semrud-Clikeman M: Volumetric MRI differences in treatment-naive vs chronically treated children with ADHD. Neurology 2006; 67:1023–1027Google Scholar
8. Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL: Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 2007; 104:19649–19654Google Scholar
9. Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J: Intellectual ability and cortical development in children and adolescents. Nature 2006; 440:676–679Google Scholar
10. Reich W: Diagnostic Interview for Children and Adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39:59–66Google Scholar
11. Werry JS, Sprague RL, Cohen MN: Conners’ teacher rating scale for use in drug studies with children: an empirical study. J Abnorm Child Psychol 1975; 3:217–229Google Scholar
12. Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF III, Sharp WS, Giedd JN, Rapoport JL: Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 2007; 164:647–655Google Scholar
13. Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J: Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63:540–549Google Scholar
14. Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN: Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007; 36:1065–1073Google Scholar
15. Sled JG, Zijdenbos AP, Evans AC: A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998; 17:87–97Google Scholar
16. Zijdenbos AP, Forghani R, Evans AC: Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging 2002; 21:1280–1291Google Scholar
17. MacDonald D, Kabani N, Avis D, Evans AC: Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 2000; 12:340–356Google Scholar
18. Lerch JP, Evans AC: Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 2005; 24:163–173Google Scholar
19. Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S: Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. J Am Acad Child Adolesc Psychiatry 2002; 41:1306–1314Google Scholar
20. Elia J, Welsh PA, Gullotta CS, Rapoport JL: Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. J Child Psychol Psychiatry 1993; 34:785–804Google Scholar
21. Borcherding BG, Keysor CS, Cooper TB, Rapoport JL: Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology 1989; 2:255–263Google Scholar
22. Porrino LJ, Rapoport JL, Behar D, Ismond DR, Bunney WE Jr: A naturalistic assessment of the motor activity of hyperactive boys, II: stimulant drug effects. Arch Gen Psychiatry 1983; 40:688–693Google Scholar
23. Pietrzak RH, Mollica CM, Maruff P, Snyder PJ: Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 2006; 30:1225–1245Google Scholar
24. Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW: Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 2000; 20:RC65Google Scholar
25. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Google Scholar
26. Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA: The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry 2004; 161:1990–1997Google Scholar
27. Kim SY, Choi KC, Chang MS, Kim MH, Kim SY, Na YS, Lee JE, Jin BK, Lee BH, Baik JH: The dopamine D 2 receptor regulates the development of dopaminergic neurons via extracellular signal-regulated kinase and Nurr1 activation. J Neurosci 2006; 26:4567–4576 Google Scholar
28. Todd RD: Neural development is regulated by classical neurotransmitters: dopamine D 2 receptor stimulation enhances neurite outgrowth. Biol Psychiatry 1992; 31:794–807 Google Scholar