Clinical Symptom Responses to Atypical Antipsychotic Medications in Alzheimer’s Disease: Phase 1 Outcomes From the CATIE-AD Effectiveness Trial
Abstract
Objective: The study measured the effects of atypical antipsychotics on psychiatric and behavioral symptoms in patients with Alzheimer’s disease and psychosis or agitated behavior. Method: The Clinical Antipsychotic Trials of Intervention Effectiveness—Alzheimer’s Disease (CATIE-AD) Alzheimer’s disease effectiveness study included 421 outpatients with Alzheimer’s disease and psychosis or agitated/aggressive behavior. Patients were assigned randomly to masked, flexible-dose treatment with olanzapine, quetiapine, risperidone, or placebo for up to 36 weeks. Patients could be randomly reassigned to a different medication at the clinician’s discretion, which ended phase 1. Psychiatric and behavioral symptoms, functioning, cognition, care needs, and quality of life were measured at regular intervals. Results: In relation to placebo, the last observation in phase 1 showed greater improvement with olanzapine or risperidone on the Neuropsychiatric Inventory total score, risperidone on the Clinical Global Impression of Changes, olanzapine and risperidone on the Brief Psychiatric Rating Scale (BPRS) hostile suspiciousness factor, and risperidone on the BPRS psychosis factor. There was worsening with olanzapine on the BPRS withdrawn depression factor. Among patients continuing phase 1 treatment at 12 weeks, there were no significant differences between antipsychotics and placebo on cognition, functioning, care needs, or quality of life, except for worsened functioning with olanzapine compared to placebo. Conclusion: In this descriptive analysis of outpatients with Alzheimer’s disease in usual care settings, some clinical symptoms improved with atypical antipsychotics. Antipsychotics may be more effective for particular symptoms, such as anger, aggression, and paranoid ideas. They do not appear to improve functioning, care needs, or quality of life.
Psychiatric and behavioral symptoms are common in patients with Alzheimer’s disease and contribute substantially to morbidity (1 – 3) . Delusions or hallucinations appear in 30%–50%, and as many as 70% demonstrate agitated or aggressive behaviors. These symptoms contribute to patient and caregiver distress (4 , 5) , can compromise safety or lead to institutionalization (6 , 7) , and add to health care costs (8 , 9) .
Optimal treatments for psychosis and agitated behavior have been elusive. Interventions that address environmental or interpersonal factors can be helpful (10 , 11) and should be attempted in all cases, although their overall effectiveness and applicability are not entirely clear. Cholinesterase inhibitor drugs and memantine may have some beneficial impact on noncognitive symptoms in Alzheimer’s disease (12 , 13) , although effects may not be significant in those with active behavioral disturbances (14) . The majority of controlled medication trials for psychosis and behavioral disturbances in Alzheimer’s disease have examined the efficacy of atypical antipsychotic medications over 6 to 12 weeks in patients with advanced disease. Several trials found modest efficacy of individual atypical antipsychotic medications in relation to placebo (15) . However, efficacy is not seen in all trials or for all symptoms, adverse events can occur, comparative efficacy is not known, and overall effectiveness in usual clinical populations is uncertain.
The National Institute of Mental Health (NIMH) Clinical Antipsychotic Trials of Intervention Effectiveness—Alzheimer’s Disease (CATIE-AD) project was designed to compare the effectiveness of antipsychotic medications and placebo in patients with Alzheimer’s disease and psychosis or agitated/aggressive behavior (16) . In contrast to typical efficacy trials, CATIE-AD included outpatients in usual care settings and assessed treatment effectiveness on several clinical outcome measures over a 9-month intervention period. Initial CATIE-AD treatment (olanzapine, quetiapine, risperidone, or placebo) was randomized and masked, yet the protocol allowed medication dose adjustments or a switch to a different treatment on the basis of the clinician’s judgment. The primary CATIE-AD phase 1 outcome was time to discontinuation of the initially assigned medication for any reason, which was intended as an overall measure of effectiveness that incorporated the judgments of patients, caregivers, and clinicians about therapeutic benefits in relation to undesirable effects. The two primary hypotheses were that 1) there would be pairwise differences between the three antipsychotic treatment groups and the placebo group in the time to discontinuation for any reason and 2) among the antipsychotics that were different from placebo, none would be inferior to the others. The results revealed no pairwise treatment differences in all-cause discontinuation. The benefits of olanzapine and risperidone, i.e., longer times to discontinuation for lack of efficacy in comparison to placebo (Kaplan-Meier estimate of median time: olanzapine, 22.1 weeks; quetiapine, 9.1 weeks; risperidone, 26.7 weeks; placebo, 9.0 weeks), were offset by shorter times to discontinuation due to adverse effects in the antipsychotic groups (discontinuations due to intolerability: olanzapine, 24%; quetiapine, 16%; risperidone, 18%; placebo, 5%) (17) .
This report presents results on the CATIE-AD phase 1 clinical symptom measures. Here, the analysis examined pairwise differences between antipsychotic treatment groups and the placebo group on change in clinical symptoms at the last observation during the initially assigned phase 1 treatment. Additional analyses explored clinical symptom changes in the four treatment groups among patients who continued the phase 1 treatment for up to 12 weeks. This approach can help to characterize treatment effects on psychosis and behavioral symptoms, and it also allows the principal CATIE-AD effectiveness outcome, treatment discontinuation, to be viewed in the context of symptom change.
Method
Study Design and Participants
The CATIE-AD research design and methods have been described previously (16 , 18) ; study group members are listed in the online data supplement. Forty-two clinical sites enrolled 421 patients, who were randomly assigned initially to masked treatment with olanzapine, quetiapine, risperidone, or placebo in a 2:2:2:3 ratio. The treating physician could adjust the dose, as indicated clinically, over the 36 weeks of the trial. At any time after the first 2 weeks of treatment, the clinician could discontinue the initially assigned (phase 1) medication for insufficient efficacy, adverse effects, or other reason. At that point, phase 1 ended and the patient could enter phase 2 and be assigned randomly to masked treatment with an atypical antipsychotic medication that had not been assigned in phase 1 or with citalopram. Patients could also go directly to an open-choice treatment.
To participate in the trial, patients had to meet the DSM-IV criteria for dementia of the Alzheimer’s type or meet the criteria for probable Alzheimer’s disease of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (19) after a thorough history, physical and cognitive examinations, and laboratory assessments were completed. Each enrolled patient was an outpatient living at home or in an assisted living facility, had a knowledgeable informant, and was ambulatory. Patients in skilled nursing homes were not included. The Mini-Mental State Examination (MMSE) (20) score was 5 to 26. The entry criteria required that delusions, hallucinations, agitation, or aggression had occurred nearly every day over the previous week or intermittently over 4 weeks. The symptoms had to have been rated at least moderate in severity on the Brief Psychiatric Rating Scale (BPRS) conceptual disorganization, suspiciousness, or hallucinatory behavior item (21) or had to have occurred at least weekly with moderate severity or greater on the delusion, hallucination, agitation, or aberrant motor behavior item of the Neuropsychiatric Inventory (22) .
Patients could be taking stable cholinesterase inhibitor medication; memantine had not been approved in the United States during the enrollment period. Patients were excluded if they were taking antidepressants or anticonvulsants for mood stabilization.
The study was reviewed and approved by the institutional review board at each site. Consent was documented according to institutional review board guidelines.
Treatments
Medications were prepared in identically appearing low-dose and high-dose capsules that contained olanzapine (2.5 mg or 5.0 mg), quetiapine (25 mg or 50 mg), risperidone (0.5 mg or 1.0 mg), or placebo. The clinician selected the number of low- or high-dose capsules for initial treatment and could subsequently adjust the number of capsules prescribed according to clinical judgment. If acute difficult behaviors emerged, the clinician could increase the study medication, prescribe a benzodiazepine for short-term use, or prescribe parenteral haloperidol for behavioral emergency.
Patients and caregivers received basic information and education about Alzheimer’s disease, behavioral symptoms, and behavioral management strategies. Caregivers were offered two counseling sessions and could speak with clinical staff as needed.
Clinical Outcome Measures: Psychiatric and Behavioral Symptoms
The following instruments were administered at study baseline and after 2 weeks, 4 weeks, 8 weeks, 12 weeks, 24 weeks, and 36 weeks of treatment.
Neuropsychiatric Inventory
The Neuropsychiatric Inventory measures the frequency and severity of 12 psychiatric symptoms on the basis of caregiver report (22) . The items are delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep disturbance, and appetite and eating disorder.
BPRS
The BPRS measures the severity of 18 psychiatric and behavioral symptoms (21) . Individual items were scored by a trained clinician after a semistructured patient interview and collection of additional information from the caregiver. Factor analysis in a geropsychiatry sample (23) revealed a five-factor structure: 1) agitation (anxiety, tension, excitement); 2) hostile suspiciousness (hostility, suspiciousness, uncooperativeness); 3) psychosis (unusual thought content, hallucinations); 4) withdrawn depression (emotional withdrawal, depressed mood, motor retardation, blunted affect); and 5) cognitive dysfunction (disorientation, conceptual disorganization).
Cornell Scale for Depression in Dementia
The Cornell scale is a 19-item instrument that measures mood symptoms, ideational disturbances of depression, and neurovegetative signs in patients with dementia (24) . The items are rated on the basis of patient report, caregiver report, and the rater’s observations of the patient.
Alzheimer’s Disease Cooperative Study—Clinical Global Impression of Change
The Alzheimer’s Disease Cooperative Study—Clinical Global Impression of Change (CGIC) is a global assessment of clinical change over time, based on the clinician’s overall impression of change in cognitive, behavioral, and functional symptoms (25) . Change over time is rated on a 7-point scale from “very much improved” to “very much worse.”
Clinical Outcome Measures: Cognitive Skills, Functional Abilities, Care Needs, and Quality of Life
The following instruments were administered at baseline and after 12 weeks, 24 weeks, and 36 weeks of treatment.
Alzheimer’s Disease Assessment Scale cognitive subscale and MMSE
The 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale assesses memory, language, visuoconstructive skill, and ideational praxis (26) . The MMSE is a brief 30-item measure of global cognitive ability (20) .
Alzheimer’s Disease Cooperative Study—Activities of Daily Living Scale
The Alzheimer’s Disease Cooperative Study—Activities of Daily Living Scale is a 23-item, hierarchically scaled inventory of basic (e.g., eating, toileting, bathing) and instrumental (e.g., using the telephone, going shopping) functional skills and abilities (27) . Ratings are based on an informant’s observations.
Dependence Scale
The Dependence Scale is a 13-item measure of the amount of caregiver assistance needed by the patient to accomplish daily activities (28) and has been used as a predictor of nursing home placement (7) . On the basis of the Dependence Scale interview with an informant, the clinician rates the patient’s level of “equivalent institutional care.” Level 1 indicates limited home care (needs some help with activities such as shopping or housekeeping), level 2 represents supervised adult home care (supervised setting with constant companionship and regular help with cooking and housekeeping), and level 3 indicates a health-related facility (24-hour supervision for personal care and safety).
Caregiver Activity Survey
The Caregiver Activity Survey measures the time that caregivers spend providing care for a patient with Alzheimer’s disease (29) . The time spent by care providers over the past 24 hours is recorded for each of five domains of care needs: using transportation, dressing, eating, looking after one’s appearance, and general supervision. Caregiving time was the sum of time for the four specific domains, excluding general supervision.
Alzheimer’s Disease Related Quality of Life
This instrument measures health-related quality of life in patients with Alzheimer’s disease on the basis of a structured interview with the caregiver (30) . Its items assess behaviors that reflect social interaction, maintained interests, participation in activities, cheerfulness, and freedom from distress. Forty-seven items in five domains (social interaction, awareness of self, feelings and mood, enjoyment of activities, response to surroundings) are rated, and the total score is a weighted sum of the item scores.
Data Analysis
The intent-to-treat group included the patients who were assigned randomly to a treatment group (N=421) and received at least one dose of study medication (N=416). Discontinuation of phase 1 occurred when the study physician judged that the patient’s response to the assigned medication was not adequate because of insufficient efficacy, adverse effects, or other reason or when the patient or family chose to discontinue the treatment. This report presents the clinical outcome measures during phase 1 treatment.
Psychiatric and behavioral symptoms
The primary analysis for these measures was the difference in change scores between the antipsychotic treatment groups and the placebo treatment group at the last observation in phase 1. The last-observation analysis was chosen because of the substantial percentage of patients who discontinued phase 1 treatment, in order to provide a concise summary of patient status immediately before discontinuation. We performed pairwise comparisons of each antipsychotic with placebo by using an analysis of covariance (ANCOVA), adjusting for the site and for the baseline rating scale score. Pairwise comparisons used a Hochberg modification of the Bonferroni adjustment for multiple comparisons (31) , in which the largest of the p values is compared to 0.05 and the smallest is compared to 0.05/3=0.017. An additional descriptive analysis also compared the active treatments to one another. If a test with 2 degrees of freedom comparing the three antipsychotic treatment groups had p<0.05, then pairwise comparisons among the active treatments were performed, also using the Hochberg adjustment for multiple comparisons. A further sensitivity analysis for key outcomes also adjusted for the duration of treatment in phase 1, since patients discontinued phase 1 at various times, and the Neuropsychiatric Inventory baseline score, which was found to be different across the groups at baseline. ANCOVA analyses for the CGIC were confirmed by an analysis of the distribution of ordinal scores at the last observation using a Mantel-Haenszel mean score chi-square test for ordinal data.
A confirmatory mixed-model repeated-measures analysis was also performed for change from baseline on two measures: the Neuropsychiatric Inventory total score and BPRS total score. This strategy compared treatment groups across all time points together; it assumed that missing data from subjects who discontinued could be represented by subjects who remained in the phase at that visit. The model had fixed effects for treatment, baseline value, classification of time (weeks 2, 4, 8, and 12), and the interactions between baseline value and time and between treatment and time, if significant, and it used a random patient intercept and spatial power covariance structure to account for the correlation of the repeated measurements within a subject. The mixed model excluded visits after week 12 because the group sizes declined substantially at later visits.
Finally, a secondary set of ANCOVA analyses compared treatment groups at each of weeks 2, 4, 8, and 12 (observed cases), in order to further characterize the response patterns for the patients who had not yet discontinued by that visit. The distribution of ordinal scores on the CGIC across the treatment groups for observed cases at week 12 was also assessed by using a Mantel-Haenszel mean score chi-square test for ordinal data. For descriptive purposes, the proportions of responders in the treatment groups were calculated; those with CGIC designations of “much improved” or “very much improved” at week 12 were considered responders. This approach is different from the previous analysis (17) , which included the entire intent-to-treat group, defined response as at least “minimal improvement” on the CGIC, and considered all patients who ended phase 1 before week 12 to be nonresponders. The current analysis of observed cases of patients who continued with phase 1 treatment at week 12 used the higher threshold for response because this group tended to have fewer symptoms, since those with more symptoms were more likely to have ended phase 1 before week 12.
Cognition, functional skills, and care needs
These measures were completed only at weeks 12, 24, and 36. Change scores were examined with ANCOVA only for patients who were continuing the phase 1 assigned treatment at week 12. Scores on these measures at the last observation during phase 1 were often not available because a high proportion of patients discontinued phase 1 before week 12 and thus had no postbaseline assessment.
Results
Patient Characteristics and Treatment
Patient characteristics, treatments prescribed, time to phase 1 discontinuation, and adverse events were reported previously (17 , 32) . The mean age of the participants was 77.9 years (SD=7.5); 56% were female, and 21% were nonwhite. Overall, 77%–85% of the patients discontinued the phase 1 medication before the end of the 36-week study period. The median duration of phase 1 treatment was 7.1 weeks and did not differ significantly across the treatment groups (median duration ranged from 5.3 to 8.1 weeks in the four groups). The mean last prescribed medication dose was 5.5 mg/day of olanzapine, 56.5 mg/day of quetiapine, and 1.0 mg/day of risperidone. There was no difference in the numbers or sizes of capsules prescribed across treatments. Rescue medications were prescribed rarely, and there were no significant differences among treatment groups: of the 421 patients, three received a conventional antipsychotic and 21 received a benzodiazepine (olanzapine, N=3; quetiapine, N=8; risperidone, N=3; placebo, N=7).
Clinical Symptoms at Baseline
The baseline scores on clinical symptom measures are shown in Table 1 . The only difference in baseline symptom scores across the treatment groups was for the total score on the Neuropsychiatric Inventory.
Change From Baseline to Last Observation
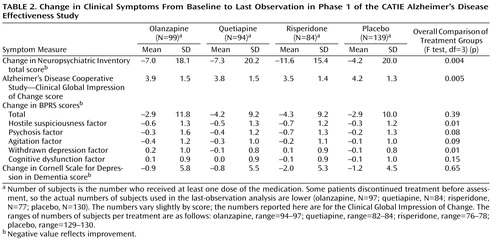
Global symptoms
Symptom changes from baseline to the last phase 1 observation are shown in Table 2 . Differences between placebo and the active drugs appear in Table 3 . On the Neuropsychiatric Inventory total score, patients treated with olanzapine or risperidone showed greater improvement from baseline to the last phase 1 observation than those treated with placebo. On the CGIC, the patients treated with risperidone had greater global improvement than those in the placebo group. Overall group differences were confirmed in an analysis of the distribution of ordinal scores on the CGIC at the last observation in phase 1 (Mantel-Haenszel mean score chi-square test for ordinal data: χ 2 =12, 11, df=3, p=0.007). Improvement on the BPRS total score at last observation was not significantly different between those treated with antipsychotic medication and those treated with placebo. Including baseline Neuropsychiatric Inventory total score and the duration of phase 1 treatment as additional covariates in the ANCOVA model did not affect these results.
Individual symptoms
Patients treated with olanzapine or risperidone showed greater improvement on the BPRS hostile suspiciousness factor at the end of phase 1 than those treated with placebo ( Table 3 ). Patients treated with risperidone had greater improvement on the BPRS psychosis factor than those treated with placebo. On the BPRS withdrawn depression factor, patients treated with olanzapine showed worsening of symptoms compared to placebo. There was no significant difference between those treated with antipsychotic medication and those receiving placebo on the BPRS cognitive dysfunction factor or the Cornell Scale for Depression in Dementia.
Repeated-Measures Analysis
The mixed-model analysis of change in both the Neuropsychiatric Inventory and BPRS total scores for time points between baseline and week 12 showed no overall time-by-treatment interaction. The reduced model without this interaction term, which compared the treatment groups across the average of all time points, revealed an overall effect of treatment group (mixed-model, repeated-measures analyses—Neuropsychiatric Inventory: F=3.15, df=3, 349, p=0.03; BPRS: F=2.80, df=3, 377, p=0.04), with significant pairwise differences between risperidone and placebo treatment on the Neuropsychiatric Inventory (estimated mean difference=5.7, SE=1.9; t=3.03, df=345, p=0.003) and on the BPRS (estimated mean difference=2.9, SE=1.1; t=2.71, df=373, p=0.007).
Change From Baseline to Week 12 for Observed Cases
Mean change scores on the Neuropsychiatric Inventory total, BPRS total, and BPRS factors over 12 weeks of phase 1 treatment are shown in Figure 1 . Only patients continuing the assigned phase 1 treatment were included at each time point, so the group sizes declined over the 12-week period. Change over time among the observed cases seen on these curves generally supports the results of the last-observation and mixed-model analyses.
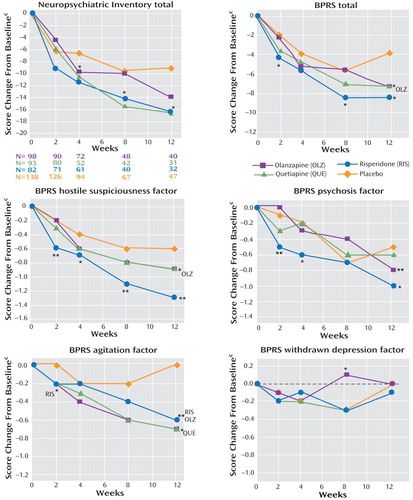
a For patients continuing to take the assigned phase 1 medication at each time point.
b Pairwise comparisons of least-squares mean change scores for each antipsychotic and placebo at each time point are shown here for descriptive purposes (ANCOVA, adjusted for baseline score and site, no adjustment for multiple comparisons). Significant pairwise comparisons with the antipsychotic treatment groups are provided in the text. Discrepancies between the magnitude of between-group differences shown in the graphs and the results of antipsychotic-placebo pairwise comparisons are due to differences between observed means and least-squares means, which adjust for baseline score and site variations.
c Change scores less than 0 reflect clinical improvement.
*p<0.05. **p<0.01.
The distribution of scores on the CGIC in observed cases after 12 weeks of treatment ( Figure 2 ) did not significantly differ among the groups (p=0.14, Mantel-Haenszel mean score chi-square test for ordinal data). The proportions of patients with a rating of “much improved” or “very much improved” were 45%, 52%, 61%, and 40% in the olanzapine, quetiapine, risperidone, and placebo groups, respectively.

a For patients continuing to take the assigned phase 1 medication at 12 weeks.
Structured ratings of functional abilities, care needs, cognitive skills, and quality of life were assessed only at baseline and at week 12 during the CATIE-AD early treatment period. On the Activities of Daily Living Scale, patients in the olanzapine treatment group showed worsening of functional ability compared to those in the placebo group at week 12 ( Table 4 ). We found no significant differences between patients treated with an antipsychotic medication and those treated with placebo in change scores at week 12 on the Alzheimer’s Disease Assessment Scale cognitive subscale, MMSE, Alzheimer’s Disease Related Quality of Life, or Caregiver Activity Survey. On the rating of equivalent institutional care, there was no overall difference among the treatment groups in change in the level of care from baseline to week 12.
Active Drug Comparisons
At the last observation in phase 1, there were no differences among the three antipsychotic treatment groups on any clinical outcome measure except the BPRS withdrawn depression factor (overall comparison: F=3.52, df=2, 376, p=0.04). In the pairwise comparisons, the olanzapine group showed worsening of symptoms compared to the quetiapine group (least-squares mean difference=0.3, 95% CI=0.1 to 0.5, p=0.009).
In the analysis of observed cases at weeks 2, 4, 8, and 12, there were only two significant differences among the three antipsychotic treatment groups on the clinical outcome measures. First, on the BPRS withdrawn depression factor at week 8 there was a significant overall group effect (F=4.51, df=2, 176, p=0.02), and the olanzapine group showed worsening of symptoms compared to the quetiapine group (least-squares mean difference=0.4, 95% CI=0.1 to 0.8, p=0.01) and the risperidone group (least-squares mean difference=0.4, 95% CI=0.1 to 0.8, p=0.02). Second, on the Activities of Daily Living scale at week 12 there was a significant overall group effect (F=4.36, df=2, 130, p=0.02), and the olanzapine group showed a greater decline (worsening) than the quetiapine group (least-squares mean difference=–5.2, 95% CI=–9.3 to –1.0, p=0.02) and the risperidone group (least-squares mean difference=–5.4, 95% CI=–9.5 to –1.2, p=0.02).
Discussion
Several clinical outcome measures in CATIE-AD indicate that patients may benefit symptomatically from treatment with atypical antipsychotic medications. Beneficial effects of olanzapine and risperidone were apparent on the Neuropsychiatric Inventory total score, a summary measure of psychiatric and behavioral symptoms. Improvement on the clinician-rated CGIC in the risperidone group provides convergent support for the improvements in symptom scores.
The magnitude of the difference in mean global score improvements between active medication and placebo was usually small. Significant group differences were not seen on the BPRS total score at the last observation, although the mixed-model analysis revealed a significantly greater effect with risperidone than with placebo, with a larger estimated mean difference in the mixed model than was measured at the last phase 1 observation. Some analyses revealed pairwise group differences that were near the statistical threshold for significance after adjustment for multiple comparisons, suggesting that while group differences cannot be excluded, their magnitude is small. In contrast, other group differences were probably clinically meaningful. For example, the baseline total score on the Neuropsychiatric Inventory (overall mean=36.9) was improved at the last observation in phase 1 by 11.6 points in the risperidone group and 7.0 points in the olanzapine group, compared to 4.2 points in the placebo group. Also, the CGIC ratings at the last observation in phase 1 and at 12 weeks indicate that some individuals were likely benefiting from antipsychotic treatment. Greater benefit from quetiapine than from placebo did not appear on most CATIE-AD symptom outcomes, although Figure 1 indicates that a quetiapine effect cannot be entirely ruled out. Limited quetiapine impact on symptoms may have been due to the low dose of quetiapine prescribed. However, sedation occurred in this group at rates comparable to those for the other drugs (17) , suggesting at least some medication effect. Use of rescue medication (haloperidol, lorazepam) likely did not contribute to clinical outcomes, since these medications were prescribed rarely and equally across the treatment groups (17) .
These clinical symptom ratings in CATIE-AD complement and extend the findings from the primary effectiveness analysis, which showed benefits of olanzapine and risperidone in terms of longer times to discontinuation due to lack of efficacy, which were offset by discontinuations due to adverse effects (17) . The clinical symptom ratings reported here also show some antipsychotic efficacy during phase 1, yet these improved last-observation ratings occurred at or very near the time when the clinician decided that phase 1 treatment was not optimal and intended to change the treatment, except for the 19% of patients who completed the entire study while taking the phase 1 medication. Notably, phase 1 discontinuations were due predominantly to insufficient efficacy. Thus, the clinical symptom ratings reveal some beneficial antipsychotic effects, despite the frequent coincident decision to change treatment. This distinction suggests that clinicians were seeking a level of clinical improvement that was greater than the change detected on the clinical scales, were mindful of possible placebo assignment in phase 1, or were balancing symptom change with other clinical considerations, such as adverse effects. Treatment expectations and patient circumstances likely contributed to the perception of effectiveness. As applied to clinical practice, these findings support an individualized approach, where the value of an individual patient’s symptomatic improvement needs to be assessed in the context of that person’s particular clinical circumstances.
The analyses that used the last clinical rating in phase 1 are conceptually different from the descriptive analyses that used scores from observed cases of continued phase 1 treatment through week 12. The last-observation analysis includes a preponderance of data from patients who, for a variety of reasons, were not doing particularly well while taking the assigned treatment. By contrast, the results from observed patients continuing phase 1 treatment apply to those who were doing reasonably well with their first prescribed medication, because phase 1 would have ended earlier for these patients had the clinicians felt the assigned treatment was not effective overall. This is reflected in the larger improvement from baseline generally seen in all treatment groups among observed cases at week 12 compared to the last-observation study group. Despite this important difference, the results from using the two analytic approaches are not substantially different.
The BPRS factor scores provide a view of antipsychotic treatment effects on distinct symptoms. Patients treated with risperidone or olanzapine showed improvement on the BPRS hostile suspiciousness factor, which assesses hostility, aggression, mistrust, and uncooperativeness. This subscale had the highest mean score at baseline among noncognitive measures, and the mean score improved by more than 50% among risperidone-treated patients continuing phase 1 treatment at week 12. The BPRS psychosis factor score also improved with risperidone treatment. In contrast, antipsychotic treatment effects on the BPRS agitation factor score, which includes excitability, tension, and anxiety, were not as robust. This observation conforms to some clinical opinion that antipsychotics might be particularly useful for suspicious thoughts, paranoid delusions, and hostile or aggressive behaviors, which occur commonly in Alzheimer’s disease.
On measures of depression or cognition, the magnitude of changes was small and there were no differences across treatments except for increased scores in the olanzapine group on the BPRS withdrawn depression factor at the last observation in phase 1. Taken together, these findings suggest that antipsychotic medication may have differential effects on specific symptoms in Alzheimer’s disease. Better understanding of specific effects can help clinicians to select optimal pharmacotherapy.
Beneficial effects were not apparent on measures of functional abilities, quality of life, or caregiving time needed. Thus, improved clinical symptoms with antipsychotic treatment did not translate into functional benefits or improved quality of life in the context of the CATIE-AD effectiveness design. This may be due to additional factors that contribute to functional disability and poor life quality, such as progression of dementia, caregiver interactions, environmental factors, and perhaps adverse effects of the drugs. Nonetheless, improved psychiatric and behavioral symptoms may be clinically meaningful for individual patients without affecting function.
There are several limitations to the study design and analyses. CATIE-AD did not follow a usual clinical efficacy trial design, and while the effectiveness design has several advantages, the results of these analyses should be interpreted carefully. For example, the end of phase 1 was defined not by a specific time point but by the clinician’s judgment that continued treatment with the assigned medication was not optimal. The protocol invited medication switches based on clinical judgment and, in fact, phase 1 treatment was often discontinued relatively quickly. In this respect, CATIE-AD results reflect clinician expectations and behaviors as well as pharmacologic efficacy. Also, the brief treatment exposure for many patients may not have captured all beneficial or adverse medication effects. Moreover, mild sedation may have contributed to behavioral improvement with antipsychotic treatment, but its specific role cannot be defined. Finally, the data analyses included many measures and comparisons, and there was no adjustment to statistical testing for the number of variables evaluated overall.
The results presented here are predominantly group mean scores on clinical rating instruments, and this approach can obscure important treatment effects in individual patients. Subgroups of participants may respond to individual treatments particularly well or particularly poorly, as a result of distinct neurobiological factors (33) , baseline symptom patterns, or sensitivity to treatment. Additional research is needed to clarify patient- or symptom-specific responses in order to maximize benefit and minimize harm of any available treatment.
The clinical outcomes in CATIE-AD contribute to the evolving understanding of psychopharmacological interventions for psychiatric symptoms in Alzheimer’s disease. Some antipsychotic efficacy trials and the analyses reported here indicate that clinical symptoms may improve. However, the extent of mean improvement on rating scales is modest, it is not apparent for all symptoms or in all treatment studies, and beneficial effects on mean ratings of functional ability, quality of life, or cost-effectiveness (34) were not seen in CATIE-AD. Because of the unique design, the results of CATIE-AD are probably more generalizable to usual outpatient clinical settings than are results from efficacy trials. Whether the potentially beneficial effects of symptom reduction with antipsychotic treatment outweigh other undesirable clinical or adverse effects depends on an individual patient’s circumstances, including severity of symptoms, vulnerability to adverse effects, and the effectiveness or opportunity for behavioral interventions. Additional studies to understand better the risk-benefit profile and effectiveness of specific treatments in individual patients will be valuable.
1. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS: Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157:708–714Google Scholar
2. Ropacki SA, Jeste DV: Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry 2005; 162:2022–2030Google Scholar
3. Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, Habeych M, DeKosky ST: Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2003; 15:346–353Google Scholar
4. Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP: A cross sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry 2005; 13:460–468Google Scholar
5. Schulz R, O’Brien AT, Bookwala J, Fleissner K: Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates and causes. Gerontologist 1995; 35:771–791Google Scholar
6. Magni E, Binetti G, Bianchetti A, Trabucchi M: Risk of mortality and institutionalization in demented patients with delusions. J Geriatr Psychiatry Neurol 1996; 9:123–126Google Scholar
7. Stern Y, Tang M-X, Albert MS, Brandt J, Jacobs DM, Bell K, Marder K, Sano M, Devanand D, Albert SM, Bylsma F, Tsai W-Y: Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997; 227:806–812Google Scholar
8. Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC: The incremental direct costs associated with behavioral symptoms in Alzheimer’s disease. Neurology 2002; 59:1721–1729Google Scholar
9. Ryu S, Katoma C, Rive B, Livingston G: Persistence of and changes in neuropsychiatric symptoms in Alzheimer’s disease over 6 months. Am J Geriatr Psychiatry 2005; 13:976–983Google Scholar
10. Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG: Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005; 162:1996–2021Google Scholar
11. Ayalon L, Gum AM, Feliciano L, Arean PA: Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med 2006; 166:2182–2188Google Scholar
12. Trinh NH, Hoblyn J, Mohanty S, Yaffe K: Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216Google Scholar
13. Cummings JL, Schneider E, Tariot PN, Graham SM: Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63Google Scholar
14. Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, Burns AS, Holmes C, Jacoby R, Johnson T, Knapp MJ, Lindesay J, O’Brien JT, Wilcock GK, Katona C, Jones RW, DeCesare J, Rodger M, CALM-AD Trial Group: Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007; 357:1382–1392Google Scholar
15. Schneider LS, Dagerman K, Insel PS: Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006; 14:190–209Google Scholar
16. Schneider LS, Ismail MS, Dagerman K, Davis S, Olin J, McManus D, Pfeiffer E, Ryan JM, Sultzer DL, Tariot PN: Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophr Bull 2003; 29:57–72Google Scholar
17. Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail S, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA: Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538Google Scholar
18. Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, Hsiao JK, Jeste DV, Katz IR, Olin JT, Pollock BG, Rabins PV, Rosenheck RA, Small GW, Lebowitz B, Lieberman JA: National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001; 9:346–360Google Scholar
19. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Google Scholar
20. Folstein M, Folstein S, McHugh P: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
21. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull 1988; 24:97–99Google Scholar
22. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Google Scholar
23. Overall JE, Beller SA: The Brief Psychiatric Rating Scale (BPRS) in geropsychiatric research, I: factor structure on an inpatient unit. J Gerontol 1984; 39:187–193Google Scholar
24. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA: Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Google Scholar
25. Schneider LS, Olin JT, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH: Validity and reliability of the Alzheimer’s Disease Cooperative Study—Clinical Global Impression of Change. Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S22–S32Google Scholar
26. Rosen WG, Mohs RC, Davis KL: A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141:1356–1364Google Scholar
27. Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S: An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S33–S39Google Scholar
28. Stern Y, Albert SM, Sano M, Richards M, Miller L, Folstein M, Albert M, Bylsma F, Lafleche G: Assessing patient dependence in Alzheimer’s disease. J Gerontol 1994; 49:M216–M222Google Scholar
29. Davis KL, Marin DB, Kane R, Patrick D, Peskind ER, Raskind MA, Puder KL: The Caregiver Activity Survey (CAS): development and validation of a new measure for caregivers of persons with Alzheimer’s disease. Int J Geriatr Psychiatry 1997; 12:978–988Google Scholar
30. Rabins PV, Kasper JD, Kleinman L, Black BS, Patrick DL: Concepts and methods in the development of the ADRQL: an instrument for assessing health-related quality of life in persons with Alzheimer disease. J Ment Health Aging 1999; 5:33–48Google Scholar
31. Hochberg YA: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802Google Scholar
32. Ismail MS, Dagerman K, Tariot PN, Abbott S, Kavanagh S, Schneider LS: National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness—Alzheimer’s Disease (CATIE-AD): baseline characteristics. Curr Alzheimer Res 2007; 4:325–335Google Scholar
33. Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, Cummings JL: Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry 2003; 160:341–349Google Scholar
34. Rosenheck RA, Leslie DL, Sindelar J, Miller EA, Tariot PN, Dagerman KS, Davis S, Lebowitz BD, Rabins P, Hsiao JK, Lieberman JA, Schneider LS, Clinical Antipsychotic Trial of Intervention Effectiveness—Alzheimer"s Disease (CATIE-AD) investigators: A randomized trial of the cost-effectiveness of second generation antipsychotics and placebo in the treatment of psychosis and aggression in Alzheimer’s disease. Arch Gen Psychiatry 2007; 64:1259–1268Google Scholar