Paralimbic and Medial Prefrontal Cortical Involvement in Neuroendocrine Responses to Traumatic Stimuli
Abstract
Objective: Hypothalamic-pituitary-adrenal axis activity and cortisol release are consequences of central stress system activation, but they may also influence cognitive and emotional processes within the brain. Despite the importance of central stress response systems, little is known about the specific brain circuits through which psychosocial stimuli activate the hypothalamic-pituitary-adrenal axis and through which cortisol feedback modulates central processing. The authors used [ 15 O]H 2 O positron emission tomography (PET) on subjects with posttraumatic stress disorder (PTSD) to study these circuits. Method: Participants were combat-PTSD patients, combat-exposed healthy comparison subjects, and noncombat-exposed healthy comparison subjects. Participants were scanned using [ 15 O]H 2 O PET while they experienced a series of emotional-induction conditions, which included aversive pictures and autobiographic narratives. Blood samples were obtained 2 minutes before and 5 minutes after each activation scan in order to measure the subjects’ plasma adrenocorticotropic hormone and cortisol levels. Results: In voxel-wise analyses, the authors found that adrenocorticotropic hormone responses were covaried with regional cerebral blood flow (rCBF) in the dorsal medial prefrontal cortex, rostral anterior cingulate cortex, and right insula, with some differences between PTSD patients and comparison subjects. Prestimulus cortisol levels covaried with rCBF responses in the rostral anterior cingulate cortex. In combat-PTSD patients only, prestimulus cortisol levels covaried with rCBF in the subgenual anterior cingulate cortex. Conclusions: These findings provide evidence of cortical involvement in hypothalamic-pituitary-adrenal responses to psychological stimuli, specifically implicating the insula, dorsal medial prefrontal cortex, and rostral anterior cingulate cortex. These findings also show, for the first time, that cortisol may modulate activity in specific brain areas such as the rostral and subgenual anterior cingulate cortices. Differential patterns of covariation between combat veterans with and without PTSD potentially implicate the dorsal medial prefrontal cortex and subgenual anterior cingulate cortex as areas of dysregulation in PTSD.
The hypothalamic-pituitary-adrenal axis is a major stress response system that is critical for survival and adaptation and is dysregulated in psychiatric disorders such as depression and posttraumatic stress disorder (PTSD) (1) . The “downstream” brain circuits of the hypothalamus have been well mapped, but the “upstream” brain circuits that are responsible for psychological activation of the hypothalamic-pituitary-adrenal axis in humans remain largely unknown. Relevant pathways likely involve cortical-limbic circuits that detect stimulus salience (e.g., novelty, uncontrollability, social threat) and interpret meaning based on past experience (2) . Disruptions in cortical-limbic interactions are likely involved in the pathophysiology of psychiatric disorders that are accompanied by hypothalamic-pituitary-adrenal axis abnormalities (3 , 4) . Thus, paradigms in which cortical control of hypothalamic-pituitary-adrenal activity can be studied may illuminate the neural dysregulation underlying these disorders.
Existing evidence shows that the prefrontal cortex, amygdala, hippocampus, and bed nucleus of the stria terminalis are critical cortical-limbic structures that control the hypothalamic-pituitary-adrenal axis (2) . In previous studies on rodents and primates, the prefrontal cortical regions (e.g., anterior cingulate, prelimbic and infralimbic cortices) have been implicated in the inhibition of hypothalamic-pituitary-adrenal axis activity (5) , modulation of amygdala responses, and facilitation of extinction (6) . However, these studies did not provide extensive insight into the cortical control of stress systems in humans, since the cortical homology between rodents and primates is limited. Human data utilizing pathological states have also supported the role of hypothalamic-pituitary-adrenal activation in the human amygdala (7) and an inhibitory role of the frontal cortex (8) . Additionally, there is growing realization that the hypothalamic-pituitary-adrenal axis and its “end-product” cortisol play important modulatory roles in cortical functions. Cortisol modulates cognitive and emotional processing, social appraisal, and decision making (9) and could directly contribute to symptom formation in psychiatric disorders. Thus, hypothalamic-pituitary-adrenal axis activity may be shaped by psychological inputs, and its products may in turn alter cognitive/emotional processing of subsequent inputs. Such rapid, dynamic modulation of neural activity would likely occur through rapid (likely membranous) effects of glucocorticoids (10) , since established genomic effects are too slow to modulate immediate behavior. However, there is little human data examining specific cortical regions that are modulated by hypothalamic-pituitary-adrenal axis activation and few existing paradigms in which this can be studied.
Neuroimaging offers opportunities to examine the relationships between cortical activity and hypothalamic-pituitary-adrenal axis function in vivo in humans. However, emotion-induction tasks, which are easily imaged, do not reliably activate the hypothalamic-pituitary-adrenal axis (11) or produce only modest responses (12) , and robust human hypothalamic-pituitary-adrenal/stress models (11) are difficult to adapt for neuroimaging. Recent reports have linked the plasma adrenocorticotropic hormone to regional cerebral blood flow (rCBF) in the right insula and anterior cingulate cortex (12) , cortisol levels to amygdala metabolism (13) and to rCBF in the medial frontal cortex (14) , cortisol variability to blood-oxygen-level-dependent signal in the medial prefrontal cortex and amygdala (15) , and salivary cortisol response to rCBF in the medial prefrontal cortex (16) . However, these studies were limited by between-subject designs and reliance on correlations that involved single scans or a single hypothalamic-pituitary-adrenal measure and did not disentangle the neural activity underlying hypothalamic-pituitary-adrenal activation from the neural activity associated with cortisol feedback.
Patients with sensitized stress responses may offer a unique opportunity for imaging cortical-limbic control of the hypothalamic-pituitary-adrenal axis, potentially illuminating both normal neurocircuitry and pathophysiological processes. In PTSD patients, altered rCBF has been reported in brain regions (amygdala [ 3 , 17 ], medial prefrontal cortex [3] , and rostral anterior cingulate [18] ) that have been implicated in hypothalamic-pituitary-adrenal axis regulation. These patients showed exaggerated emotional responses (19) , altered glucocorticoid sensitivity (20) , and altered hypothalamic-pituitary-adrenal responses (19 , 21) , and thus provide a potential model for studying the neural correlates of hypothalamic-pituitary-adrenal axis activation and cortisol action.
To examine the neural substrates underlying hypothalamic-pituitary-adrenal axis activity, we analyzed the temporal links between cortical activation and subsequent hypothalamic-pituitary-adrenal axis response and between circulating cortisol levels and subsequent brain activation. To our knowledge, this is the first such report on neuroendocrine covariation data. Group differences in patterns of regional brain activations have been reported previously (22 , 23) . In response to aversive pictures (aversive versus neutral), healthy comparison subjects activated the amygdala, bilateral insula, dorsal and ventral medial prefrontal cortices, and dorsal and rostral anterior cingulate cortices. It has been previously reported that PTSD and combat comparison subjects activated only the dorsal anterior cingulate cortex, and PTSD patients showed less activation of the rostral anterior cingulate cortex than healthy comparison subjects (22 , 23) . In response to traumatic/stressful scripts (traumatic versus neutral), all subjects were reported to have activated the right insula. Healthy comparison subjects also activated the amygdala and deactivated the ventral medial prefrontal cortex. Combat comparison subjects deactivated both the amygdala and ventral medial prefrontal cortex, and PTSD patients deactivated the dorsal rostral anterior cingulate cortex (22 , 23) .
In the present study, we covaried rCBF responses with postscan plasma adrenocorticotropic hormone responses following a series of emotional challenges in voxel-wise analyses in order to investigate cortical-limbic circuits that influence hypothalamic-pituitary-adrenal axis activation (and subsequent plasma adrenocorticotropic hormone) ( Figure 1 ). To investigate cortical regions through which cortisol might modulate subsequent cognitive/emotional processing, we measured the subjects’ plasma cortisol concentrations prior to emotional activation and covaried the data with the subsequent rCBF responses to emotional stimuli. Additionally, we identified a nonoverlapping set of cortical regions potentially involved in hypothalamic-pituitary-adrenal activation or responsive to circulating cortisol levels.
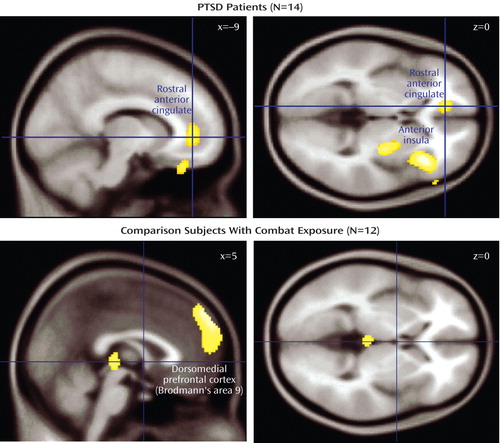
a Regions of covariation in the rostral anterior cingulate, anterior insula, putamen, and dorsal medial prefrontal cortex (Brodmann"s area 9). Covarying voxels (p<0.005, uncorrected) are projected onto a canonical Montreal Neurological Institute brain.
Method
Participants
After complete description of the study to the participants was provided, written informed consent was obtained. The study was approved by the University of Michigan and Ann Arbor Veterans Affairs Healthcare System. Three groups of right-handed, male participants were recruited via newspaper advertisements. The three study groups were Vietnam combat veterans with PTSD (N=16); age-matched, comparison Vietnam combat veterans with similar combat exposure but without PTSD (N=15); and healthy comparison subjects without combat experience (N=15). PTSD diagnosis was established using the Structured Clinical Interview for DSM-IV and Clinician Administered PTSD Scale. Details of the subjects’ characteristics, including psychiatric history and symptom severity, are available in the data supplement (Part A), which accompanies the online version of this article. The subjects’ urine toxicology screens were negative, and no structural abnormalities were present on magnetic resonance imaging (MRI) scans (1.5 T Signa scanner, GE, Milwaukee). Adrenocorticotropic hormone samples were available from 15 PTSD patients, eight noncombat comparison subjects, and 11 combat comparison subjects. Cortisol level samples were available from 14 PTSD patients, 12 noncombat comparison subjects, and 12 combat comparison subjects.
Neuroimaging Procedures
Positron emission tomography (PET) [ 15 O]H 2 O neuroimaging procedures were performed using Siemens ECAT EXACT and HR+ scanners as previously described (22 , 23) . Two types of emotional challenge stimuli were used during the imaging experiment: emotional pictures from the International Affective Picture System (24) and autobiographical script-driven imagery. The autobiographical script-driven imagery was administered as recommended by Pitman et al. (25) , describing either traumatic/stressful or neutral events. A series of 10 emotional challenges were presented during the 2-hour experiment, which were counterbalanced by stimulus type. PET scans (10 to 15 mCi of [ 15 O]H 2 O PET given as an intravenous bolus, three-dimensional data acquisition mode in a 60-second frame) were performed immediately after each stimulus presentation and separated by 12 minutes. Subjective emotional responses were assessed after each scan using the modified Positive and Negative Affect Schedule, and blood samples (3 ml) were collected from an indwelling catheter 5 and 10 minutes after each scan (2 minutes preceding the subsequent scan). Plasma adrenocorticotropic hormone and cortisol levels were collected before and after each scan and were assayed using commercial radioimmunoassay kits (PET procedures, emotional-challenge stimuli, and presentation schedules are described in detail in the data supplement [Part A] accompanying the online version of this article).
Data Analyses
PET image preprocessing and analyses were performed using Statistical Parametric Mapping (SPM) 99 (Wellcome Department of Cognitive Neurology, London). The primary analyses involved voxel-wise analyses of covariance (ANCOVAs) of [ 15 O]H 2 O PET activity (“multisubject covariates-only” module in SPM99), with plasma adrenocorticotropic hormone and cortisol used as separate regressors. We examined 1) links between brain activation patterns and acute (5-minute postscan) plasma adrenocorticotropic hormone response as well as 2) the impact of circulating cortisol levels (2-minute prescan) on rCBF responses. Separate analyses of variance (ANOVAs) were conducted using Statistical Package for the Social Sciences to assess whether the emotional challenges produced changes in plasma adrenocorticotropic hormone or cortisol levels.
Results
Subjective Responses and Neuroendocrine Findings
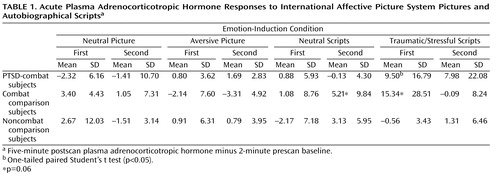
All three study groups reported greater negative emotional response on the Positive and Negative Affect Schedule for aversive versus neutral pictures (F=231.4, df=1, 43, p<0.001) and traumatic/stressful versus neutral scripts (F=121.1, df=1, 41, p<0.001). None of the groups differed in baseline adrenocorticotropic hormone or cortisol levels. Combat-PTSD patients showed significant (p=0.03) acute plasma adrenocorticotropic hormone responses to trauma scripts but not to pictures ( Table 1 ). After removal of a high adrenocorticotropic hormone responder, the combat-exposed healthy comparison group also showed significant (p<0.01) response to trauma scripts. Collection timing was not designed to test the effects of individual stimuli on plasma cortisol; however, cortisol levels were significantly higher during the presentation of scripts compared with the presentation of pictures in combat-PTSD patients (10.3 mg/dl versus 8.2 mg/dl [F=8.19, df=1, 11, p=0.01]) and combat-exposed healthy comparison subjects (9.7 mg/dl versus 8.1mg/dl [F=7.55, df=1, 10, p=0.02]), with no difference in noncombat healthy comparison subjects (see the data supplement [Part B], which accompanies the online version of this article).
Covariation Between Plasma Adrenocorticotropic Hormone and rCBF
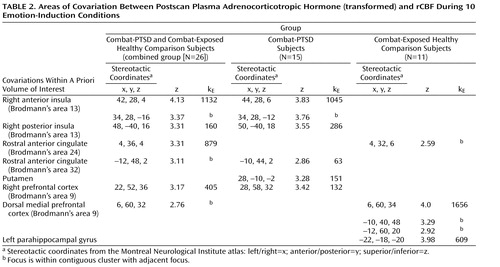
To identify patterns of neural activity reflecting the normative relationship between rCBF responses and subsequent adrenocorticotropic hormone levels, we covaried activity in regions of interest with 5-minute postscan adrenocorticotropic hormone levels. All 10 emotional-challenge scans were used to provide a maximum range of within-subject variance in adrenocorticotropic hormone levels ( Table 2 ). When all three study groups were combined (N=34), a single large cluster (591 voxels) of positive covariation with adrenocorticotropic hormone levels was found within the a priori area of interest in the right insula ([ 38 , 28 , 2 ] z=3.97), which was significant after small-volume correction (small volume correction, p<0.005) using false discovery rate. A subthreshold peak of covariation was also observed in the dorsal medial prefrontal cortex ([4, 54, 40] z=2.50). No areas of negative covariation were found.
Covariations between brain activity and adrenocorticotropic hormone levels of each study group were then examined separately. In noncombat-exposed healthy comparison subjects (N=8), no significant regions of covariation were found. A detailed presentation of all significant loci of positive adrenocorticotropic hormone covariation among the combat-exposed healthy comparison group is shown in Table 2 . Since both the combat-PTSD and combat-exposed healthy comparison groups showed meaningful adrenocorticotropic hormone responses to traumatic scripts, they were first analyzed together (N=26) in order to examine the general relationship between cortical activation and rCBF response. Large clusters of covariation were found in the right anterior and posterior insula (Brodmann’s area 13), rostral anterior cingulate cortex (Brodmann’s areas 24, 32) and right and medial prefrontal cortex (Brodmann’s area 9). When the combat-PTSD and combat-exposed healthy comparison groups were analyzed separately in order to identify disease-specific patterns, the combat-PTSD group (N=15) showed adrenocorticotropic hormone covariation in the right anterior and posterior insula (Brodmann’s area 13), rostral anterior cingulate cortex (Brodmann’s area 32), and right prefrontal cortex. The combat-exposed healthy comparison subjects (N=11) showed a large cluster of adrenocorticotropic hormone covariation in the dorsal medial prefrontal cortex (Brodmann’s area 9) and in the parahippocampal gyrus. Because script conditions were the main contributors to the adrenocorticotropic hormone response, we performed a subanalysis for script conditions only to ensure that inclusion of all conditions did not produce spurious results. Despite fewer data points (four scripts versus 10 total challenges), similar patterns of adrenocorticotropic hormone covariation were confirmed in combat-PTSD patients (right anterior insula/inferior frontal gyrus: [36, 38, –14] z=3.99, 402 voxels; right prefrontal cortex: [26, 58, 34] z=3.97, 279 voxels) and in combat-exposed healthy comparison subjects (dorsal medial prefrontal cortex: [10, 62, 22] z=3.40, 94 voxels). Negative covariations were not found in either group.
Covariation Between Plasma Cortisol and rCBF
To identify patterns of covariation that might reflect the normative relationship between circulating cortisol and cortical responses, all three groups of subjects were combined in a single analysis of covariation ( Table 3 ). Prestimulus plasma cortisol levels covaried with rCBF in the rostral anterior cingulate cortex ([12, 50, –2] z=3.12) and superior temporal lobe ([46, –32, 8] z=3.45). To examine potential differences in covariation patterns among combat-PTSD and comparison subjects, similar analyses were performed separately for each group. Negative covariations were not found within the volume of interest. Healthy comparison subjects showed covariation in the dorsal medial prefrontal cortex (Brodmann’s area 8) and outside the volume of interest in the posterior cingulate gyrus (Brodmann’s area 31). Combat-PTSD patients showed covariation spanning the rostral anterior cingulate cortex (Brodmann’s area 32) and subgenual anterior cingulate (Brodmann’s area 25); in the bilateral superior temporal gyrus (Brodmann’s areas 21, 41); and outside the volume of interest in the right temporal pole (Brodmann’s area 38). Combat-exposed healthy comparison subjects showed covariation in the rostral anterior cingulate cortex and outside the volume of interest in the medial and left precuneus (Brodmann’s areas 7, 31). Adding depression severity (Beck Depression Inventory) as a nuisance covariate did not affect any of our peaks.
Discussion
The data presented in the present study demonstrate that adrenocorticotropic hormone responses to emotional stimuli may be modulated by paralimbic and prefrontal activity and that activity in the subgenual anterior cingulate may, in turn, be sensitive to prestimulus plasma cortisol levels. Autobiographic trauma scripts elicited adrenocorticotropic hormone responses in trauma-exposed individuals (with or without PTSD), which were positively correlated with the severity of symptoms in combat-PTSD subjects. In trauma-exposed subjects, adrenocorticotropic hormone covaried with activity in the right insula, rostral anterior cingulate cortex (Brodmann’s areas 25, 32), and dorsal medial prefrontal cortex (Brodmann’s areas 9, 10), with differential patterns in combat-PTSD patients and combat-exposed healthy comparison subjects. This suggests involvement of the insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex in “top down” cortical activation or modulation of neuroendocrine stress responses. In turn, preactivation cortisol levels covaried with rCBF responses in the rostral anterior cingulate cortex and right superior temporal gyrus (Brodmann’s area 42) in the combined group (combat-PTSD and combat-exposed and nonexposed healthy comparison groups) and in the subgenual anterior cingulate cortex (Brodmann’s area 25) in combat-PTSD patients, which suggests that activation of these regions may be modulated by circulating levels of cortisol. To our knowledge, these data provide the first demonstration of a methodology that allows in vivo identification of the human neurocircuitry controlling hypothalamic-pituitary-adrenal axis activity and those brain regions modulated by the neuroendocrine milieu.
Cortico-Limbic Circuitry and Adrenocorticotropic Hormone Response
Both general aversive (International Affective Picture System pictures) and trauma-specific (autobiographical scripts) challenges elicited strong emotional responses in all participants, but acute adrenocorticotropic hormone responses were observed only in combat-exposed subjects and only in response to trauma scripts, which supports the notion that the hypothalamic-pituitary-adrenal axis responds to specific, personally salient psychological stimuli rather than to general distress (26) . Adrenocorticotropic hormone responses were found in both combat-exposed healthy comparison subjects and combat-PTSD patients, suggesting that hypothalamic-pituitary-adrenal sensitization does not necessarily reflect pathological processes. Voxel-by-voxel analyses revealed significant covariation of the right anterior insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex activity with subsequent adrenocorticotropic hormone responses in the combat-exposed groups. Trauma-script activation of the insula has been previously reported (18) , but our finding of a dynamic association between insula activity and adrenocorticotropic hormone response is novel and consistent with a previous single photon emission computed tomography study that reported higher insular activity in subjects with higher adrenocorticotropic hormone levels (12) . This finding is also consistent with the role of the insula in orchestrating negative emotional responses, since the insular cortex has been implicated in processing of aversive emotional stimuli (27) , recall of emotional material (4 , 28) , and integration of visceral sensory information and emotional and autonomic responses (29) . The extensive interconnections of the insula with the amygdala, temporal pole, superior temporal gyrus, and orbitofrontal lobe are also consistent with a role in influencing psychological activation of the hypothalamic-pituitary-adrenal axis (30) .
The covariation observed between the rostral anterior cingulate cortex activation and adrenocorticotropic hormone responses is consistent with evidence linking rostral anterior cingulate cortex to processing of aversive emotional stimuli (27 , 31) . The rostral anterior cingulate cortex is connected with the amygdala, periaqueductal gray matter, nucleus accumbens, and ventral striatum, and it has also been associated with emotional behavior and autonomic responses in animals (32) . Additionally, lesions of the anterior cingulate cortex lead to attenuated autonomic responses to conditioned stimuli. The association of activity in the anterior insula and rostral anterior cingulate cortex with subsequent adrenocorticotropic hormone responses is particularly intriguing in light of a recent study that linked both the insula and anterior cingulate cortex responses with subsequent inflammatory measures in asthma patients (33) . Our findings suggest the possibility that psychogenic, cortically modulated hypothalamic-pituitary-adrenal axis activation may mediate the functional coupling of activity in emotional circuits with peripheral immune function.
Adrenocorticotropic hormone responses were strongly associated with activity in the dorsal medial prefrontal cortex in combat-exposed healthy comparison subjects but not in combat-PTSD patients. In humans, the medial prefrontal cortex has been implicated in emotion regulation and autonomic arousal (4) and in modulating amygdala responses (3 , 34) , and decreased medial prefrontal cortex activity has been reported in PTSD patients (3) . In rats, the medial prefrontal cortex homologues modulate hypothalamic-pituitary-adrenal axis responses to psychogenic stressors, provide inhibitory inputs to the PVN (5) , and are involved in extinction recall (6) . If the dorsal medial prefrontal cortex is indeed involved in inhibitory modulation of integrated response to threat (including hypothalamic-pituitary-adrenal axis activation) and these “modulatory” regions act in concert with the insula and rostral anterior cingulate cortex, the covariation of adrenocorticotropic hormone responses with the dorsal medial prefrontal cortex may reflect this compensatory/modulation role of the dorsal medial prefrontal cortex. Thus, initial activation in modulatory circuits could correlate positively with the magnitude of acute adrenocorticotropic hormone response, while the inhibitory effects might be seen in a shortened duration of response. Follow-up studies with a longer time course of adrenocorticotropic hormone monitoring are needed to test this hypothesis. If our interpretation is correct, this (to the best of our knowledge) is the most direct evidence to date of dorsal medial prefrontal cortex regulation of the hypothalamic-pituitary-adrenal axis in humans, which is consistent with medial prefrontal cortex regulation of the hypothalamic-pituitary-adrenal axis in animals (5) . In this scenario, hypothalamic-pituitary-adrenal hyper-reactivity may be a result of a failure to recruit medial prefrontal cortex modulatory circuits in concert with hypothalamic-pituitary-adrenal activating regions, which, in the present study, is reflected in the apparent lack of functional coupling of adrenocorticotropic hormone responses with dorsal medial prefrontal cortex activity in combat-PTSD patients.
Cortisol Modulation of Cortical Regions
The data presented in the present study also suggest that circulating cortisol levels modulate paralimbic activity ( Figure 2 ). Voxel-wise analysis of prescan cortisol levels revealed covariation with subsequent rCBF responses in specific paralimbic regions. Effects of cortisol on brain and emotional processing have traditionally been conceptualized in terms of genomic effects of chronically elevated glucocorticoids. However, stress-level glucocorticoids also have rapid nongenomic effects (within minutes) on mammalian neurons, including effects on membrane excitability (35) , which provided a mechanism for rapid, dynamic modulation of brain activity by cortisol. Our data suggest that circulating cortisol may modulate affective/cognitive processing of subsequent stimuli by modulating limbic and/or paralimbic activity in humans.

a Covariations were found in the posterior cingulate, rostral anterior cingulate cortex, subgenual anterior cingulate cortex, and superior temporal gyrus. Covarying voxels (p<0.005, uncorrected) are projected onto a canonical Montreal Neurological Institute brain.
The covariation of cortisol with activity in the rostral anterior cingulate cortex was found in all of the study groups, whereas cortisol covariation with the subgenual anterior cingulate cortex was observed only in combat-PTSD patients. If the rostral anterior cingulate cortex is directly involved in hypothalamic-pituitary-adrenal axis activation, as suggested in this study, it is not surprising that it might also be responsive to circulating cortisol levels. However, the rostral anterior cingulate cortex has also been implicated in other psychological functions (self-relevance, social emotions) (36) . It is possible that circulating cortisol modulates rostral anterior cingulate cortex activity subserving these processes. This is consistent with evidence suggesting that the hypothalamic-pituitary-adrenal axis is particularly sensitive to social threat (11) and with our finding of cortisol covariation with the superior temporal gyrus, which has been implicated in social aspects of emotion (37) . Activity in the subgenual anterior cingulate cortex has been associated with the induction of sadness (4 , 38) , and reduced metabolism in this region has been observed with remission of major depression (4) . Our finding that prestimulus cortisol covaried with subgenual anterior cingulate cortex activity only in combat-PTSD patients may suggest a PTSD-specific sensitivity to cortisol in regions linked to mood regulation. The combat-PTSD group also showed covariation between cortisol and rCBF in the right temporal polar cortex and bilateral superior temporal cortex. The temporal poles have also been implicated in the processing of aversive emotional stimuli and anticipatory and lactate-induced anxiety. The temporal poles receive auditory inputs from the superior temporal cortex and are reciprocally connected with the medial prefrontal cortex in macaque (39) , which suggests that these temporal cortical regions may participate in circuits that communicate emotional auditory information to the medial frontal network. Although speculative, our finding of cortisol-rCBF covariation in the superior temporal cortex, temporal pole, and subgenual anterior cingulate cortex in combat-PTSD patients suggests that cortisol may potentially “prime” the circuits that detect emotional cues in auditory stimuli.
Interestingly, we did not find covariations of cortisol with the hippocampus, which is perhaps because of our use of an emotion-induction paradigm. Previous studies have reported negative between-subject correlations of baseline cortisol and resting hippocampal rCBF (14) and decreased hippocampus activity following cortisol administration during explicit memory tasks (40) . In contrast, we examined brain regions in which activity varied with endogenous cortisol levels. Furthermore, while cortisol administration may decrease hippocampal rCBF during declarative memory tasks, there is no evidence for similar effects in emotional-induction paradigms. Future work is needed to study the links between cortisol and the hippocampus during emotion processing tasks.
There are several limitations to the present study. The results are covariations, and do not demonstrate causality. However, the temporal linkage of emotional challenge, simultaneous brain activity, and adrenocorticotropic hormone levels obtained 5 minutes later suggests meaningful physiological relationships between rCBF and subsequent adrenocorticotropic hormone levels. Cortisol responses to individual challenges could not be resolved because of the sampling schedule, and thus correlations of brain activity with poststimulus cortisol were not examined. Future studies using administration of exogenous cortisol will be helpful in verifying and expanding the present findings. The absence of adrenocorticotropic hormone covariation with any brain regions in the noncombat-exposed healthy comparison subjects should be interpreted with caution. Personalized scripts in this group recounted sad/stressful but not traumatic/threatening events, and thus lower threat salience may explain smaller emotional and endocrine responses. Although this limited our ability to generalize some of our covariation findings to a healthy, nontrauma exposed population, it also highlights the value of using a sensitized population in this type of study.
In conclusion, our study provides evidence of in vivo involvement of specific cortical regions in dynamic hypothalamic-pituitary-adrenal axis responses to emotional stimuli in humans. Acute adrenocorticotropic hormone responses to autobiographical trauma scripts in combat veterans suggest that the experience of personal threat may sensitize the hypothalamic-pituitary-adrenal axis to subsequent recall. Covariation of plasma adrenocorticotropic hormone with activity in the anterior insula, rostral anterior cingulate cortex, and dorsal medial prefrontal cortex implicates these regions in activation and/or modulation of hypothalamic-pituitary-adrenal axis responses in humans, which is consistent with evidence in animal models. The covariation of cortisol levels with activity in the rostral and subgenual anterior cingulate cortex, which are regions associated with self-induced sadness/depression, suggests that activity in the anterior cingulate cortex may be modulated by circulating cortisol during emotional processing, potentially sensitizing limbic structures toward aversive stimuli.
1. Yehuda R: Biology of posttraumatic stress disorder. J Clin Psychiatry 2001; 62(suppl) 17:41–46Google Scholar
2. Herman JP, Cullinan WE: Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997; 20:78–84Google Scholar
3. Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK: Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61:168–176Google Scholar
4. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Google Scholar
5. Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP: The medial prefrontal cortex differentially regulates stress-induced c-FOS expression in the forebrain depending on type of stressor. Eur J Neurosci 2003; 18:2357–2364Google Scholar
6. Milad MR, Quirk GJ: Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420:70–74Google Scholar
7. Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J: Behavioral and neuroendocrine responses in shy children. Dev Psychobiol 1997; 30:127–140Google Scholar
8. Tchiteya BM, Lecours AR, Elie R, Lupien SJ: Impact of a unilateral brain lesion on cortisol secretion and emotional state: anterior/posterior dissociation in humans. Psychoneuroendocrinology 2003; 28:674–686Google Scholar
9. Erickson K, Drevets W, Schulkin J: Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev 2003; 27:233–246Google Scholar
10. Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M: Multiple actions of steroid hormones: a focus on rapid, nongenomic effects. Pharmacol Rev 2000; 52:513–556Google Scholar
11. Dickerson SS, Kemeny ME: Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004; 130:355–391Google Scholar
12. Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA: Neural and endocrine correlates of sadness in women: implications for neural network regulation of HPA activity. J Neuropsychiatry Clin Neurosci 2004; 16:446–455Google Scholar
13. Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME: Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav 2002; 71:431–447Google Scholar
14. Bonne O, Gilboa A, Louzoun Y, Brandes D, Yona I, Lester H, Barkai G, Freedman N, Chisin R, Shalev AY: Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol Psychiatry 2003; 54:1077–1086Google Scholar
15. Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ: Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 2006; 26:4415–4425Google Scholar
16. Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA: Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A 2005; 102:17804–17809Google Scholar
17. Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM: Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 1999; 45:817–826Google Scholar
18. Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL: An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50:932–942Google Scholar
19. Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA: Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology 1999; 21:40–50Google Scholar
20. Yehuda R: Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25:341–368, viiGoogle Scholar
21. Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB: Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 2001; 158:575–581Google Scholar
22. Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I: Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry 2005; 57:832–840Google Scholar
23. Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I: Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry 2006; 63:184–192Google Scholar
24. Lang PJ BM, Cuthbert BN: International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, Fla, University of Florida, NIMH Center for the Study of Emotion and Attention, 1997Google Scholar
25. Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM: Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry 1987; 44:970–975Google Scholar
26. Abelson JL, Liberzon I, Young EA, Khan S: Cognitive modulation of the endocrine stress response to a pharmacological challenge in normal and panic disorder subjects. Arch Gen Psychiatry 2005; 62:668–675Google Scholar
27. Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry 2003; 54:504–514Google Scholar
28. Phan KL, Wager T, Taylor SF, Liberzon I: Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Google Scholar
29. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ: Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Google Scholar
30. Augustine JR: Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 1996; 22:229–244Google Scholar
31. Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, Fredrikson M: Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur J Neurosci 2003; 18:1325–1331Google Scholar
32. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001; 2:417–424Google Scholar
33. Rosenkranz JA, Grace AA: Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 1999; 19:11027–11039Google Scholar
34. Taylor SF, Phan KL, Decker LR, Liberzon I: Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 2003; 18:650–659Google Scholar
35. Lupien SJ, McEwen BS: The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev 1997; 24:1–27Google Scholar
36. Lawrence EJ, Shaw P, Giampietro VP, Surguladze S, Brammer MJ, David AS: The role of ‘shared representations’ in social perception and empathy: an fMRI study. Neuroimage 2006; 29:1173–1184Google Scholar
37. Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I: Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage 2006; 31:397–409Google Scholar
38. Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P: Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry 2002; 159:1830–1840Google Scholar
39. Kondo H, Saleem KS, Price JL: Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 2003; 465:499–523Google Scholar
40. de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C: Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci 2003; 17:1296–1302Google Scholar